During embryogenesis, endothelial cells (ECs) induce organogenesis before the development of circulation1-4. These findings suggest that ECs not only form passive conduits to deliver nutrients and oxygen, but also establish an instructive vascular niche, which through elaboration of paracrine trophogens stimulate organ regeneration, in a manner similar to EC-derived angiocrine factors that support hematopoiesis5-7. However, the precise mechanism by which tissue-specific subsets of ECs promote organogenesis in the adults is unknown. Here, we demonstrate that liver sinusoidal endothelial cells (LSECs) constitute a unique population of phenotypically and functionally defined VEGFR3+CD34−VEGFR2+VE-cadherin+FactorVIII+CD45− ECs, which through the release of angiocrine trophogens initiate and sustain liver regeneration induced by 70% partial hepatectomy (PH). After PH, residual liver vasculature remains intact without experiencing hypoxia or structural damage, which allows for studying physiological liver regeneration. Employing this model, we show that inducible genetic ablation of VEGF-A receptor-2 (VEGFR2) in the LSECs impairs the initial burst of hepatocyte proliferation (days 1-3 after PH) and subsequent reconstitution of the hepato-vascular mass (days 4-8 after PH) by inhibiting upregulation of the EC-specific transcription factor Id1. Accordingly, Id1-deficient mice also manifest defects throughout liver regeneration, due to diminished expression of LSEC-derived angiocrine factors, including hepatocyte growth factor (HGF) and Wnt2. Notably, in in vitro co-cultures, VEGFR2-Id1 activation in LSECs stimulates hepatocyte proliferation. Indeed, intrasplenic transplantation of Id1+/+ or Id1−/− LSECs transduced with Wnt2 and HGF (Id1−/−Wnt2+HGF+ LSECs) re-establishes an inductive vascular niche in the liver sinusoids of the Id1−/− mice, initiating and restoring hepato-vascular regeneration. Therefore, in the early phases of physiological liver regeneration, VEGFR2-Id1-mediated inductive angiogenesis in LSECs through release of angiocrine factors Wnt2 and HGF provokes hepatic proliferation. Subsequently, VEGFR2-Id1 dependent proliferative angiogenesis reconstitutes liver mass. Therapeutic co-transplantation of inductive VEGFR2+Id1+Wnt2+HGF+ LSECs with hepatocytes provides for an effective strategy to achieve durable liver regeneration.
Sinusoidal ECs (SECs) compose a structurally and functionally unique capillary network that vascularizes specific organs, including bone marrow (BM) and liver. In adult mice, BM SECs, via expression of specific angiocrine trophogens, such as Notch ligands, support hematopoietic regeneration5-7. Similarly, the hepatic circulation is predominantly lined by liver SECs (LSECs)8-10, with each hepatocyte residing in cellular proximity to LSECs. However, the lack of phenotypic and operational definition of liver ECs and paucity of relevant mouse angiogenic genetic models11-13 have handicapped studies of the role of LSECs in regulation of hepatic regeneration14-18.
Here, we use a physiologically relevant PH model to elucidate the instructive role of LSECs in mediating hepatic regeneration (supplementary Fig. 1). In contrast to the administration of hepatotoxic chemicals, which impairs the organization of LSECs and causes tissue hypoxia, cell death, and inflammation (supplementary Fig. 2)8,13,19, in the PH model, resection of 70% of the liver mass without perturbing the integrity of the residual liver vasculature11 activates hepatocyte regeneration15-17. As such, this model provides an instructive model for interrogating the role of structurally and functionally intact LSECs in supporting liver regeneration.
As the VEGF family plays a critical role in the regeneration of the BM SECs6, we hypothesized that VEGF-receptors20-22, including VEGFR2 or VEGFR3 also modulate LSEC function. Using VEGFR2-GFP mice in which the GFP expression is driven by the native promoter of VEGFR2, we demonstrate that VEGFR2 and VEGFR3 are exclusively expressed in the liver ECs but not other liver cell types, including hepatocyte nuclear factor 4α (HNF4A)+ hepatocytes (Fig. 1a, supplementary Fig. 3). Notably, distribution of VEGFR3 expression is restricted to VEGFR2+ LSECs that branch out from CD34+VEGFR3− large vessels (Fig. 1b). Polyvariate flow cytometric analysis on nonparenchymal cells (NPCs) demonstrates the expression of EC-specific marker VE-cadherin on non-hematopoietic VEGFR3+VEGFR2+CD45− LSECs, 97.6% of which are non-lymphatic (Prox1−CD34−)22 ECs expressing Coagulation Factor VIII (Fig. 1c, d). Thus, we have designated a unique phenotypic and operational signature for LSECs of the adult mice as VEGFR3+CD34−VEGFR2+VE-cadherin+FactorVIII+Prox-1−CD45− vessels, distinguishing them from VEGFR3−CD34+VEGFR2+VE-cadherin+CD45− non-sinusoidal ECs and VEGFR3+CD34+Prox-1+FactorVIII−CD45− lymphatic ECs. Identification of LSECs as VEGFR3+CD34− and non-sinusoidal ECs as VEGFR3−CD34+ is sufficient for quantification, purification and molecular profiling of LSECs.
Figure 1. Phenotypic signature and contribution of LSECs to physiological liver regeneration induced by 70% partial hepatectomy (PH).
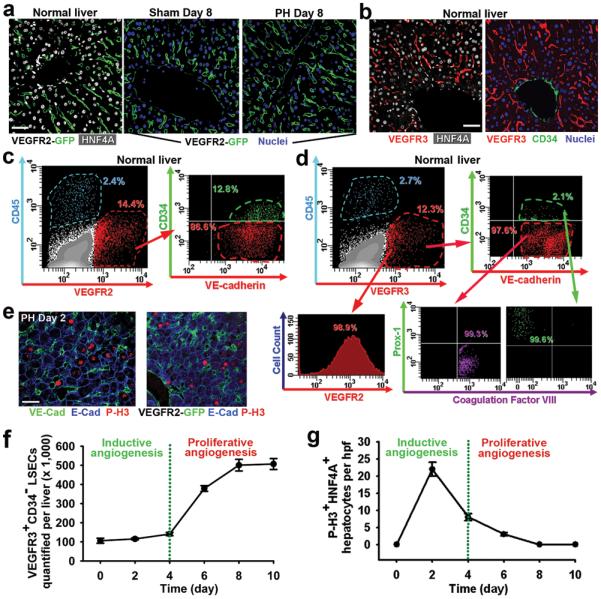
a) Liver sections obtained from VEGFR2-GFP reporter mice6. During liver regeneration VEGFR2 is exclusively expressed on the liver ECs. b) Restricted expression of VEGFR3 on LSECs, but not CD34+ large vessels or hepatocytes. c) Polyvariate flow cytometric analysis of the liver nonparenchymal cells. VEGFR2+ cells that are CD45−, express EC-specific VE-cadherin. d) Specific expression of VEGFR3 on VEGFR2+VE-cadherin+CD45− LSECs, with a predominant fraction being CD34−FactorVIII+Prox-1−. Thus, LSECs could be identified as VEGFR3+CD34− cells. e) 48 hours after PH, E-cadherin+P-H3+ mitotic hepatocytes are localized adjacent to VE-cadherin+ and VEGFR2+ ECs. f, g) Kinetics of LSECs expansion (f) and hepatocyte mitosis (g) during liver regeneration (n = 4). Hpf, high power field. Scale bars, 50 μm. Error bars, s.e.m.
To determine the mechanism by which LSECs regulate hepatic proliferation, we studied the regenerative kinetics of hepatocytes and LSECs after PH. Two days after PH, staining with VE-cadherin, hepatocyte marker Epithelial (E)-cadherin, and mitotic marker phosphorylated-histone-3 (P-H3) revealed that P-H3+E-cadherin+ mitotic hepatocytes were positioned in the proximity of non-proliferating LSECs (Fig. 1e). However, proliferation of LSECs starts at day 4 and plateaus by day 8 after PH (Fig. 1f, supplementary Fig. 4). In comparison, quantification of P-H3+HNF4A+ hepatocytes showed that the rate of hepatocyte proliferation peaks during the first 4 days, while leveling off by day 8 (Fig. 1g). These results suggest a chronologically biphasic contribution of LSECs in mediating hepatic reconstitution. At the early phases of PH (days 1-3 after PH), inductive angiogenesis in the non-proliferative LSECs stimulates hepatic regeneration possibly by releasing angiocrine factors, while 4 days after PH, the increased demand of blood supply for the regenerating liver is met via proliferative angiogenesis of LSECs.
To investigate the significance of VEGF-receptors during LSEC-driven hepatic regeneration, we designed experiments to conditionally delete the VEGFR2 gene by crossing VEGFR2loxP/loxP mice withROSA-CreERT2 mice, generating inducible VEGFR2-deficient, VEGFR2flox/flox (VEGFR2fl/fl) mice (supplementary Fig. 5)6. Due to the EC-specific expression of VEGFR2 in the liver, in VEGFR2fl/fl mice only liver ECs but not non-EC cells, will manifest functional defects. As control, we used mice with heterozygous deletion of the VEGFR2 gene (VEGFR2fl/+). Forty eight hours after PH, bromodeoxyuridine+ hepatocyte proliferation (BrdU+HNF4A+ cell number) was decreased by 67% in VEGFR2fl/fl mice (Fig. 2a, b). Notably, despite the patency of the VE-cadherin+isolectin+ perfused vessels at this early phase, the regeneration of liver mass was attenuated in VEGFR2fl/fl mice (Fig. 2c). Therefore, in the early phases (PH days 1-3) of the liver regeneration, targeting VEGFR2 primarily impairs the effect of EC-derived angiocrine factors to induce hepatocyte regeneration, but not vascular perfusion capacity.
Figure 2. VEGFR2-Id1 activation in LSECs mediates PH-induced liver regeneration.
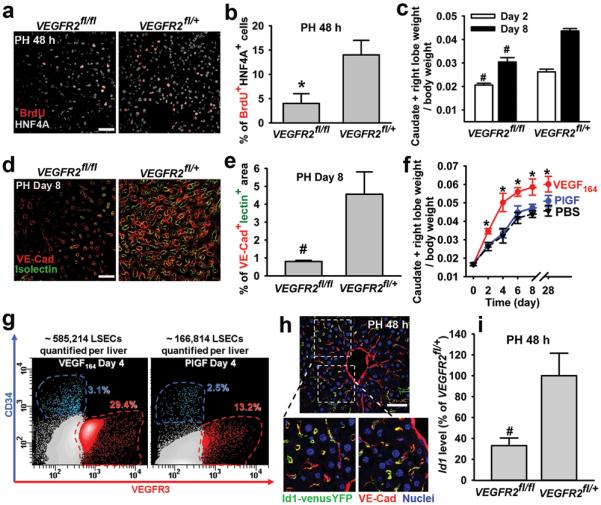
a, b) Hepatocyte proliferation after PH is impaired in VEGFR2fl/fl mice (n = 5). c-e) Inhibition of liver mass regeneration (c) and functional VE-cadherin+isolectin+ vessel formation (d, e) in VEGFR2fl/fl mice after PH (n = 4-6). f, g) Injection of VEGF-A164, but not VEGFR1-specific ligand PlGF, accelerates the regeneration of liver mass (f), associated with an incremental increase in VEGFR3+CD34− LSEC number (g) (n = 4). h) Regenerative liver section of Id1VenusYFP mouse24. Id1 is selectively upregulated by PH in VE-cadherin+ vessels. i) VEGFR2 deletion diminishes Id1 upregulation in the regenerative liver (n = 5). *P < 0.05; #P < 0.01, versus VEGFR2fl/+ (b-e, i), versus PlGF-treated group (f). Scale bar, 50 μm. Error bars, s.e.m.
However, in VEGFR2fl/fl mice at the later stages of liver regeneration (PH days 4-8), proliferative angiogenesis was also defective (Fig. 2c), interfering with the assembly of patent VE-cadherin+isolectin+ vasculature (Fig. 2d, e), thereby blunting restoration of the liver mass for at least 28 days (supplementary Fig. 5). Furthermore, in VEGFR2fl/fl mice, liver function after PH was abnormal as manifested by elevated plasma bilirubin levels. To corroborate the EC-specific VEGFR2 function in mediating liver regeneration, VEGFR2loxP/loxP mice were also crossed with VE-cadherin-CreERT2 mice to induce EC-selective deletion of VEGFR2 (supplementary Fig. 5). Both the liver mass and formation of perfused vessels in the VE-cadherin-CreERT2VEGFR2fl/fl mice were decreased after PH, underlining the significance of VEGFR2 in mediating liver regeneration. Indeed, if the VEGF-A/VEGFR2 pathway promotes the LSEC-driven hepatic regeneration, then VEGF-A should enhance liver regeneration. Hence, we compared the effect of VEGF-A164, to placental growth factor (PlGF), with the latter selectively activates only VEGFR121. After PH, VEGF164, but not PlGF, accelerated the regeneration of both liver mass and the number of VEGFR3+CD34−LSECs, which were sustained for at least 28 days (Fig. 2f, g). Therefore, after PH, the activation of VEGF-A/VEGFR2, but not PlGF/VEGFR1, is crucial for priming LSECs to initiate and maintain hepatic proliferation.
To identify the angiocrine signals that stimulate liver regeneration, we employed microarray analysis (supplementary Fig. 6, supplementary table 1). Among the EC-specific genes, the transcription factor Id1 was specifically upregulated in the PH-activated ECs23. Using Id1venusYFP reporter mice in which the venusYFP expression is driven by the Id1 promoter24, we found exclusive Id1 upregulation in LSECs 48 hours after PH (Fig. 2h), which was significantly blunted in VEGFR2fl/fl mice (Fig. 2i). Remarkably, the liver mass recovery in Id1-deficient (Id1−/−) mice after PH was impaired for 28 days and remained unchanged upon VEGF-A164 administration (Fig. 3a, supplementary Fig. 7). Furthermore, after PH, Id1−/− mice exhibited significant decrease in mitotic BrdU+HNF4A+ hepatocyte number, disrupted formation of functional VE-cadherin+isolectin+ vessels, diminished proliferation of VEGFR3+CD34− LSECs, and abnormal liver function, as evidenced by an increase in plasma bilirubin levels (Fig. 3b, c, supplementary Fig. 7). Thus, activation of the VEGF-A/VEGFR2 pathway through upregulation of Id1 drives liver regeneration.
Figure 3. Id1 upregulation in LSECs is essential for liver regeneration.
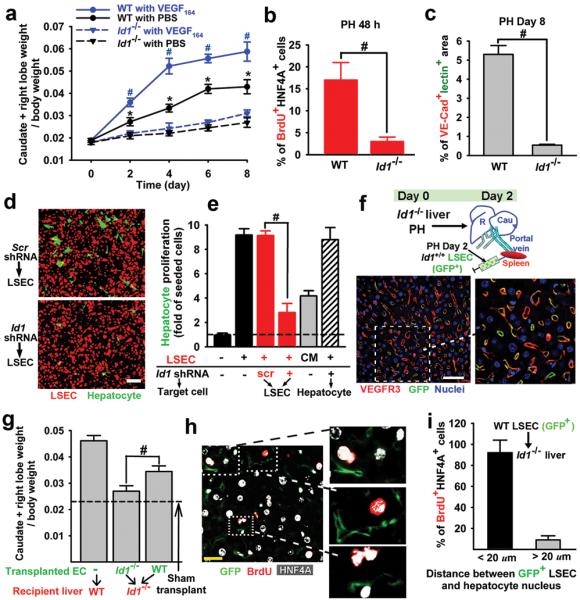
a) Compared to their wild type littermates (WT), Id1−/− mice manifest impaired regeneration in liver mass, which fails to be rescued by VEGF-A164 administration (n = 5). b, c) Impaired hepatocyte proliferation (b) and assembly of VE-cadherin+isolectin+ vessels (c) in the Id1−/− mice after PH (n = 5). d, e) The LSEC-dependent stimulation of hepatocyte proliferation was specifically inhibited by Id1 gene knockdown. Scr, scrambled. CM, LSEC-conditioned medium (n = 4). f) Intrasplenic transplantation of GFP-marked LSECs incorporates into the lumen of VEGFR3+ sinusoidal vasculature in the Id1−/− liver25.
g, h) Transplantation of Id1+/+ LSECs restores the regeneration of mass (g) and hepatocyte proliferation (h) in the Id1−/− liver (n = 4). Dashed line, level of Id1−/− liver without EC transplantation. k) Cellular proximity is essential in the stimulation of hepatocyte mitosis by the transplanted GFP+Id1+/+ vasculature. *P < 0.05, versus Id1−/− (a); #P < 0.01, versus Id1−/− with VEGF164 (a), versus WT (b, c). Scale bars, 50 (d, f) and 20 (h) μm. Error bars, s.e.m.
The role of Id1 upregulation in mediating the angiocrine function of LSECs on hepatocyte proliferation was also examined by a LSEC-hepatocyte coculture system. Co-incubation of isolated hepatocytes with primary LSECs led to a 9-fold increase in hepatocyte number, which was selectively abolished by knockdown of Id1 in LSECs (Fig. 3d, e, supplementary Fig. 8). Conditioned medium (CM) from LSECs failed to support hepatocyte growth, underlining the importance of cell-cell contact in LSEC-derived angiocrine function. Therefore, lack of Id1 results in defective inductive function of LSECs, impairing hepatocyte regeneration.
To determine whether in vivo angiocrine effects of Id1+/+ LSECs could initiate hepatocyte regeneration in Id1−/− mice, we used the intrasplenic transplantation approach on day 2 after PH to engraft LSECs into the Id1−/− liver vasculature (Fig. 3f)25. GFP-marked Id1+/+ LSECs selectively incorporated into the VEGFR3+ sinusoidal vascular lumen and restored the regeneration of liver mass and LSEC expansion (Fig. 3g, supplementary Fig. 9). In contrast, the transplanted Id1−/− LSECs failed to restore the regeneration of the Id1−/− liver. Moreover, in the Id1−/− liver, transplantation of GFP+Id1+/+ LSECs at day 2 after PH initiated the proliferation of the hepatocytes in their immediate proximity (Fig. 3h, i). Thus, partial vascular chimerism afforded by the incorporation of Id1-competent LSECs generates sufficient EC-derived inductive signals to initiate hepatic proliferation in the Id1−/− liver.
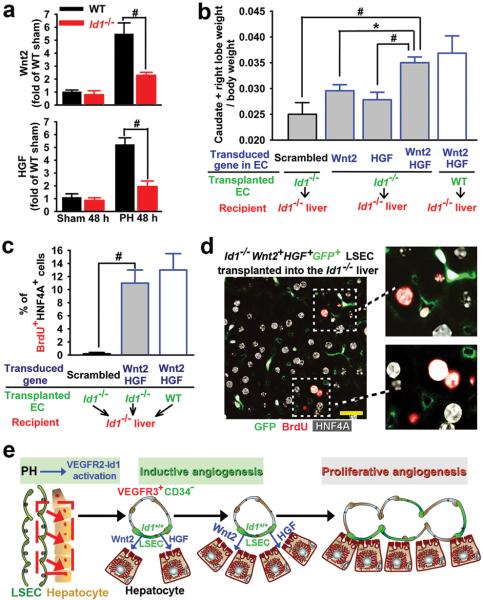
To identify EC-derived angiocrine factors that induce liver regeneration, we analyzed LSECs purified from the wild-type and Id1−/− mice 48 hours after PH. Among the known hepatic trophogens10,18,26-28, the expression of Wnt2 and HGF, but not other trophogens expressed by LSECs, such as Wnt9B and thrombomodulin, were drastically diminished in Id1−/− LSECs (Fig. 4a, supplementary Fig. 10). These results suggest that Id1 upregulation in LSECs initiates hepatocyte proliferation through inducing Wnt2 and HGF expression. To test this hypothesis, on day 2 after PH, we engrafted Id1−/− LSECs transduced with Wnt2, HGF, or both Wnt2 and HGF into the Id1−/− liver vasculature via intrasplenic transplantation. Only Id1−/− LSECs carrying both Wnt2 and HGF (Id1−/−Wnt2+HGF+) restored the regeneration of mass and LSEC expansion in the Id1−/− liver (Fig. 4b), suggesting a collaborative effect between HGF and Wnt2. Notably, transplantation of Id1−/−Wnt2+HGF+ LSECs into Id1−/− mice increased the mitotic BrdU+HNF4A+ hepatocyte number to a similar degree achieved by Id1+/+ LSEC transplantation (Fig. 4c). The mitotic hepatocytes were also found to be positioned adjacent to the transplanted Id1−/−Wnt2+HGF+GFP+ LSECs (Fig. 4d). Therefore, Id1-activated LSECs through elaboration of Wnt2 and HGF induce proliferation of juxtaposed hepatocytes (Fig. 4e).
Figure 4. Id1-mediated induction of Wnt2 and HGF in LSECs stimulates hepatic regeneration.
a) Upregulation of HGF and Wnt2 is impaired in Id1−/− LSECs after PH (n = 5). b) Intrasplenic transplantation of GFP-marked Id1−/− LSECs carrying both Wnt2 and HGF (Id1−/−Wnt2+HGF+GFP+) rescues the regeneration of Id1−/− liver mass (n = 4). c) Transplantation of Id1−/−Wnt2+HGF+ LSECs restores the impaired hepatocyte proliferation in the Id1−/− liver. (n = 4). d) The proximity between the mitotic hepatocytes and the Id1−/−Wnt2+HGF+GFP+ LSECs in the Id1−/− liver. e) Requirement for VEGFR2-Id1 pathway in LSEC-mediated liver regeneration. Intrasplenic transplantation of Id1+/+ LSECs into the Id1−/− liver sinusoids restores hepatic-vascular regeneration. Transplanted Id1+/+ or Id1−/−Wnt2+HGF+GFP+ LSECs localize to the vicinity of hepatocytes, promoting inductive and proliferative angiogenesis thereby sustaining physiological liver regeneration. *P < 0.05; #P < 0.01. Scale bar, 20 μm. Error bars, s.e.m.
Here, we have employed conditional VEGFR2 knockout, Id1−/− mice, and an EC transplantation model to identify the essential angiocrine role of a specialized organ-specific vascular niche cell, defined operationally as VEGFR3+CD34−VEGFR2+VE-cadherin+FactorVIII+Prox1−CD45− LSECs, in orchestrating PH-induced physiological liver regeneration. Similar to upregulation of Id1 in the angiogenic tumor vessels23, Id1 expression is minimal in the normal LSECs, but after PH activation of VEGFR2 induces exclusive upregulation of Id1 in the angiogenic LSECs. We demonstrate that in the first 3 days post-PH, activation of the VEGFR2-Id1 pathway switches on an inductive angiogenesis program in non-proliferative VEGFR3+CD34− VEGFR2+Id1+ LSECs, which through production of angiocrine factors Wnt2 and HGF, provokes hepatic proliferation. Subsequently, as the regenerating liver demands additional blood supply, VEGFR2-Id1 mediated proliferative angiogenesis of LSECs reconstitutes hepato-vascular mass. Therefore, we introduce the concept that LSECs support liver regeneration through a biphasic mechanism: at the early phase immediately post-PH, inductive angiogenic LSECs promote organogenesis through release of angiocrine factors, while proliferative angiogenic LSECs vascularize and sustain the expanding liver mass.
We show that transplantation of the Id1−/−Wnt2+HGF+ LSECs into Id1−/− mice initiates and restores liver regeneration. This finding along with the observation that hepatic proliferation is severely blunted in the VEGFR2 and Id1-deficient mice, suggest that LSECs are chartered with the responsibility to establish an inductive vascular niche to initiate hepatic proliferation by elaborating angiocrine factors. Since isolation of LSECs for therapeutic liver regeneration might encounter technical difficulties, endothelial progenitor cells (EPCs) derived from non-hepatic tissues may alternatively substitute for LSECs to initiate and restore liver regeneration29. Notably, VEGFR2+Id1+ EPCs could initiate angiogenesis through release of angiocrine factors rather than structurally incorporating into vessel wall29. As such, intrahepatic transplantation of EPCs will open up new avenues of cell therapy to promote liver regeneration.
In PH model employed in our study, the vascular integrity of the residual liver lobes is maintained with minimal inflammatory response (supplementary Fig. 2), thereby establishing an ideal model to study EC-dependent liver regeneration. However, in chemical (CCl4)-induced liver injury models, severe vascular damage and cell death might require the recruitment of other non-EC cells, including stellate cells19 and pro-angiogenic hematopoietic cells, such as CXCR4+VEGFR1+ hemangiocytes30, to support liver regeneration.
However, here is one unsolved enigma: How is removal of 70% of the liver sensed by the LSECs in the residual liver to ignite hepatic proliferation14-17. Conceivably, the mass of the liver is maintained through continuous release of as yet unrecognized inhibitory factors. Removal of the liver shifts the balance towards the predominance of vascular excitatory factors, which activate LSECs. Likewise, an increase in the mass of the liver 4 days post-PH instigates the release of factors that stimulate sprouting-angiogenesis in LSECs. Subsequently, recovery of the liver to its developmentally pre-determined baseline mass might re-establish as yet unidentified inhibitory signals that terminate the regenerative process. The rapid regeneration of the liver after PH requires collective and global proliferation of a large number of hepatocytes. Indeed, as each hepatocyte resides in close proximity to LSEC, this remarkably harmonious activation of hepatocytes is achieved by switching on angiocrine-dependent regenerative program to induce proliferation of mature hepatocytes throughout the residual liver after PH. Whether, angiocrine factors could also promote the propagation of liver progenitor cells14, in addition to the mature hepatocytes, remains to be investigated.
In our study, Wnt2 and HGF represent the predominant liver-specific angiocrine factors driving hepatic regeneration. As direct cellular contact between LSECs and hepatocytes was essential for proliferation of hepatocytes, it is conceivable that other angiocrine factors might collaborate with Wnt2 and HGF to modulate liver regeneration. For instance, EC-specific extracellular matrix components, proteases, adhesion molecules and chemokines might also participate in hepatogenesis. Our in vitro EC-hepatocyte coculture model and in vivo intrasplenic transplantation model provide for ideal model to assess the role of these unknown angiocrine factors in modulating hepatic homeostasis during recovery from chemical or traumatic injury.
Accumulating evidence suggests that in addition to LSECs, other organ-specific vascular niche plays a seminal role in organ-repair and tumorigenesis5-7. For example, stress-induced expression of Notch-ligands by the bone marrow SECs was shown to be essential for hematopoietic stem cell reconstitution5. Furthermore, elaboration of specific prototypical angiocrine factors, such as BMP2, Nitric oxide, FGF2 and PDGFβ by tumor vessels also directly provoke tumor progression and metastasis7. Collectively, these data suggest that tissue-specific expression of defined angiocrine factors may dictate heterogeneity of vasculature in regulating developmental and adult organogenesis.
So far, attempts in liver regeneration by hepatocyte transplantation have culminated in limited success25. Our study indicates that co-transplantation of hepatocytes or their progenitor cells14 with VEGFR2+Id1+ LSECs or EPCs might permit designing effective strategies to rescue hepato-vascular function in patients inflicted with traumatic or infectious liver damage. Furthermore, the fact that physiological liver regeneration is dependent on the proper inductive and proliferative functioning of the LSECs, also calls for the assessment of the potential increased risks of anti-angiogenic therapy in clinical trials involving liver regeneration.
Supplementary Material
Acknowledgements
S.R. is supported by Howard Hughes Medical Institute; Ansary Stem Cell Institute; National Institute of Health grants HL097797, U01 HL-66592-03, RC1 AI080309; Qatar National Priorities Research Program; Anbinder and Newmans Own Foundations; Empire State Stem Cell Board and the New York State Department of Health grant NYS C024180. We are grateful to Dr. Nan Kang Hong in the University of Pennsylvania for his advice on mouse surgical procedure and Dr. Franklin Roth for his critical editing of the manuscript.
Appendix
Method Summary
Transgenic Reporter, Gene Targeted Animals and Mouse Surgery
C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). VEGFR2-GFP mice were acquired from Dr. Janet Rossant (the Hospital for Sick Children, Canada). VE-cadherin-CreERT2 mice were kindly provided by Dr. Luisa Iruela-Arispa (University of California, Los Angeles, CA). Inducible VEGFR2 knockout (generated by Dr. Thomas N. Sato) and Id1−/− mice were previously described6,23. Id1venusYFP mice were obtained from Dr. Robert Benezra (Sloan Kettering Institute, New York, NY)24. PH was performed by resecting three most anterior lobes. Hepatic engraftment of endothelial cells was adapted as previously reported25. All animal experiments were performed under the guidelines set by Institutional Animal Care and Use Committee.
Image Acquisition, Image Analysis, and Flow Cytometric Analysis
Fluorescent images were captured on AxioVert LSM510 or 710 confocal microscope (Zeiss). For flow cytometry, antibodies were conjugated to Alexa Fluorescent dyes or Qdots (Invitrogen, CA). Purified liver cells were analyzed on LSRII-SORP (BD Biosciences, CA). Doublets were excluded by FSC-W × FSC-H and SSC-W × SSC-H analysis, and single stained channels were used for compensation.
Footnotes
Supplementary information available online. A figure summarizing the main result of this paper is available.
The authors declare no competing financial interests.
References
- 1.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 2.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi TF, Sadler KC, Crosnier C, Stainier DY. Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr. Biol. 2008;18:1565–1571. doi: 10.1016/j.cub.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler JN, Vertes J, Varnum-Finney E, Kobayashi B, Hooper H, Seandel A, Shido M, White K, Kobayashi I, Witte M, May L, Shawber C, Kimura C, Kitajewski Y, Rosenwaks J, Bernstein Z, Rafii I, Endothelial S. Cells Are Essential for the Self-Renewal and Repopulation of Notch-Dependent Hematopoietic Stem Cells. Cell Stem Cell. 2010;6:1–14. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald B, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J. Exp. Med. 2008;205:915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 10.Klein D, et al. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology. 2008;47:1018–1031. doi: 10.1002/hep.22084. [DOI] [PubMed] [Google Scholar]
- 11.Greene AK, et al. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann. Surg. 2003;237:530–535. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Buren G, 2nd, et al. Effect of molecular therapeutics on liver regeneration in a murine model. J. Clin. Oncol. 2008;26:1836–1842. doi: 10.1200/JCO.2007.11.6566. [DOI] [PubMed] [Google Scholar]
- 13.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 14.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 16.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 17.Greenbaum LE, et al. CCAAT enhancer- binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J. Clin. Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh CG, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. U S A. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 21.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 22.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 23.Lyden D, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 24.Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell stem cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Follenzi A, et al. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J. Clin. Invest. 2008;118:935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 29.Rafii S, Lyden D. Cancer. A few to flip the angiogenic switch. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat. Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.