Abstract
Purpose
Melanoma is relatively resistant to chemotherapy; improved targeting of molecules critical for cell proliferation and survival are needed. PI3K is an important target in melanoma, however activity of PI3K inhibitors (PI3KIs) is limited. Our purpose was to assess mTOR as a co-target for PI3K.
Methods
Using a method of quantitative immunofluorescence to measure mTOR expression in a large melanoma cohort, we studied associations with PI3K subunits, p85 and p110α. We assessed addition of the mTOR inhibitor rapamycin to two PI3KIs, NVP-BKM120 and LY294002. We studied in vitro activity of a novel dual PI3K/mTOR inhibitor NVP-BEZ235 and activity of the combination of NVP-BEZ235 and the MEK inhibitor AZD6244.
Results
Strong co-expression of mTOR and p110α was observed (ρ=0.658; P<0.0001). Less co-expression was seen with p85 (ρ =0.239; P<0.0001). Strong synergism was demonstrated between rapamycin and both PI3KIs. Activity of both PI3KIs was similarly enhanced with all rapamycin concentrations used. The dual PI3K/mTOR inhibitor effectively inhibited viability in 23 melanoma cell lines (IC50s in the nanomolar range), regardless of B-Raf mutation status, with resultant reduction in clonogenicity and down-regulation of pAkt and pP70S6K. Synergism was seen when combining NVP-BEZ235 and AZD6244, with resultant increases in PARP and caspase-2 cleavage.
Conclusions
mTOR and p110α are co-expressed in melanoma. Rapamycin concentrations as low as 1ηM enhance activity of PI3KIs. The dual PI3K/mTOR inhibitor NVP-BEZ235 is highly active in melanoma cells in vitro, suggesting that concurrent PI3K and mTOR targeting in melanoma warrants further investigation, alone and in combination with MEK inhibitors.
Keywords: PI3K pathway, mTOR, melanoma
Introduction
Melanoma is the malignancy with the highest rise in incidence in the past decades (1). Over 68,700 new diagnoses were estimated in 2009 in the United States, and 8,650 patients were estimated to have died (2). Once melanoma has metastasized, systemic therapeutic options are limited (3). A small subset (less than 10%) of patients have durable responses to immune/cytokine based therapies, such as high dose interleukin-2 and ipilimumab (4–5). Response rates to chemotherapy are somewhat higher, but responses are typically more transient (3). In the past years improved understanding of cellular processes essential for cell growth, metastases, proliferation and survival have led to development of targeted therapies for this disease (6–7), and additional studies are needed to expand these approaches.
Phosphatidylinositol-3 kinases (PI3Ks) are a family of intracellular signaling intermediary proteins that are essential for inhibition of apoptosis. These kinases are active in human cancers and are critical for malignant progression (8–10). Class IA PI3Ks, which consist of a p85 regulatory subunit and a p110 catalytic subunit, are the most widely implicated in cancer and are primarily activated by receptor tyrosine kinases (11). PI3K activity is inhibited by a number of molecules, including PTEN, which can be mutated and inactivated in malignant cells (11). PI3K activation results in phosphorylation of Akt and subsequent activation of a number of proteins including GSK3, GSK3ß, FOXO transcription factors, MDM2, and BAD, which in turn result in cell survival and promote cell cycle entry (9). In addition, Akt phosphorylation results in activation of the mTOR (mammalian target of rapamycin)/raptor complex, which in turn activates downstream mediators including pP70S6K, resulting in regulation of protein synthesis and cell growth (12). Activation of this pathway in malignant cells can occur due to multiple mechanisms, including activating mutations, decreased expression of pathway suppressers such as PTEN, amplification of PI3K, amplification of Akt and activation of receptors or oncogenes upstream of PI3K. Given the critical role of the PI3K/Akt/mTOR pathway in human cancer, targeting this pathway is the focus of intense research, and drugs that target members of this pathway are in pre-clinical and clinical development.
There are a number of lines of evidence that support the importance of the PI3K pathway in melanoma in clinical and pre-clinical models, as detailed (11, 13). Overexpression of Akt can convert radial growth melanoma to vertical growth melanoma (14). Drugs that target PI3K have demonstrated activity in melanoma cells in pre-clinical models. For example, inhibitors of the p110α PI3K subunit, result in growth inhibition in melanoma cells (15). A highly specific PI3K inhibitor, ZSTK474, was shown to be very active in a B16 melanoma mouse model as a single agent, with minimal associated toxicity (16). In our previous work, we showed that PI3K expression was up-regulated in melanomas compared to nevi, and that expression was significantly higher in metastastic than primary specimens (11). Taken together, these findings strongly support further clinical development of PI3K inhibitors for melanoma.
One of the possible limitations of specific PI3K inhibition as a single modality for treatment of melanoma cells is development of escape mechanisms via activation of parallel pathways, particularly the Ras-Raf-MAPK pathway. This pathway is constitutively active in approximately 70% of melanomas due to activating mutations in B-Raf or N-Ras (17–18). In our prior work we showed that the degree of sensitivity to the PI3K inhibitor LY294002 is unrelated to B-Raf mutational status (11); however, the MAPK pathway can also be activated by other mechanisms in melanoma, and members of this pathway such as ERK and RSK inhibit TSC2, resulting in mTOR activation and PI3K pathway activation that bypasses PI3K and Akt (19). Another potential mechanism of resistance to specific PI3K inhibition is down-regulation of S6, a negative regulator of PI3K through inhibition of insulin receptor substrate 1, causing a negative feedback loop (20). Down-stream mediators, including mTOR, can activate Akt via PDK2 (21).
mTOR kinases are key downstream components of the PI3K pathway and include mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2). mTORC1 activates p70S6K resulting in protein synthesis, as reviewed (21). mTORC2 includes the rapamycin insensitive companion of mTOR (Rictor) and other proteins. mTORC2 phosphorylates Akt, resulting in further PI3K pathway activation (21). The role of mTOR in melanoma has not been widely studied. A phase II trial of the rapamycin analogue temsirolimus was conducted in metastatic melanoma, and although some disease stabilization and shrinkage were seen, activity of the drug was insufficient to warrant further testing of this drug as a single agent (22). As with other targeted therapies, it is possible that mTOR inhibitors might be more active in patients whose tumors the target is either overexpressed or highly activated. The frequency of mTOR over-expression and activation in melanoma has not been well studied in human specimens. A relatively small study of 107 melanomas reported S6 phosphorylation as a surrogate for mTOR activity and showed that S6 was phosphorylated in 73% of melanomas (23), but no published studies assess expression of mTOR on large cohorts of melanomas and nevi.
A number of inhibitors of mTOR are in clinical development, some of which have been approved by the Federal Drug Administration for other malignancies (24). We therefore hypothesized that although single-agent mTOR inhibitors have limited efficacy in melanoma, co-targeting mTOR might be a valuable strategy for overcoming the escape mechanisms that can limit the activity of PI3K inhibitors in melanoma. This approach has been explored by Marone et al and Werzowa et al (25). Our purpose was to expand the studies of Marone and Werzowa by utilizing early passage, patient derived, melanoma cell strains studying clinical quality PI3K inhibitors in addition to LY294002, and assess co-expression of PI3K subunits and mTOR in melanoma tumors. To obtain objective, quantitative measures of mTOR expression we employed a new method of automated, quantitative analysis (AQUA) of in situ protein expression, which has been validated and used in a number of previous melanoma studies (11, 26). We found that mTOR and the p110α subunit of PI3K were strongly co-expressed in human melanoma specimens and that co-targeting mTOR and p110α was highly synergistic. A novel dual PI3K/mTOR inhibitor was also studied alone and in combination with a MEK inhibitor.
Materials and methods
TMA Construction
TMAs were constructed as previously described (11). Cohorts of 230 primary melanomas, each measuring 0.6mm in diameter, were spaced 0.8mm apart on glass slides. For comparison of expression, specimens from a series of 293 metastatic patients were included in the array. Specimens and clinical information were collected with approval of a Yale Institutional Review Board. Specimens were resected from 1959 to 2000. The cohort has been described and validated in numerous publications (11). Pellets of 15 melanoma cell lines were embedded as described (27), for normalization across slides. The benign nevus array contained 540 nevi as well as 40 melanomas and cell lines that were also present on the tumor array, used for controls and for normalization.
Immunohistochemistry
Staining was performed for automated analysis of melanoma specimens as previously described (11). Slides were incubated at 4°C overnight in a humidity tray with a primary antibody cocktail containing rabbit anti-human mTOR, (Cell Signaling Technology, Danvers, MA) at a dilution of 1:100 with goat anti-mouse IgG conjugated to Alexa 546 (Molecular Probes, Eugene, OR) to identify the S100 mask. Goat anti-rabbit HRP decorated polymer (Envision; Dako Corporation, Carpinteria, CA) was used as a secondary reagent. The target was visualized with Cy5-tyramide (Perkin Elmer, Boston, MA). Coverslips were mounted using ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA).
Automated Image Acquisition and Analysis
Images were acquired using our previously described automated method (11). S100 conjugated Alexa 546 defines the tumor compartment from stroma. Coalescence of S100 at the cell surface was used to localize cell membranes, and DAPI was used to identify nuclei. mTOR was visualized with Cy5. The mTOR signal pixels within the cytoplasm was normalized to the area of tumor mask and scored on a scale of 0–255 (the AQUA score).
Statistical Analysis
JMP version 5 and Statview were used to perform data analysis (SAS Institute Inc., Cary, NC). Associations with clinical and pathological parameters were assessed by ANOVA. Associations between mTOR and PI3K subunits were assessed using Spearman's rank correlation.
Human Cell Lines
Nineteen low-passage patient derived melanoma cell lines were obtained from the Cell Culture Facility of the Yale Skin Disease Research Core Center. Metastatic cell lines: YUMAC (locally recurrent metastasis), YUSAC2, YULAC, YUROB, YUKSI, YUVON, YURIF, YUSIV, YUSTE, YUCAS, YUROL (distant soft-tissue metastases), YUFIC, YUKIM, YUHOIN, YUSIK (lymph node metastases), YUGEN8 (brain metastasis), YUSOC (in-transit cutaneous metastasis), YUHEF (lung metastasis), YUPLA (in-transit cutaneous metastasis) were maintained in 15 cm dishes and OptiMEM media (Invitrogen) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Invitrogen) and 1% antibiotic-antimycotic (penicillin, streptomycin, amphotericin B) (Invitrogen). The human primary melanoma cells, WW165 were maintained in OptiMEM supplemented with 10% heat inactivated FBS, 0.1 mmol/L 3-isobutyl-1-methyl-xanthine (IBMX) (Sigma Aldrich, St. Louis, MO) and 1% antibiotic-antimycotic. Established cell lines mel 501, mel 928 and mel 624 were obtained from Dr. Steven Rosenberg, Surgery Branch, National Cancer Institute (Bethesda, MD) and were maintained in RPMI 164 (Invitrogen) supplemented with 10% FBS and 1% antibiotic-antimycotic. Cells were incubated at 37°C in a humidified atmosphere of 95% air/ 5% CO2. V600K or V600E mutations in B-Raf were found in YUMAC, YUSAC, YULAC, YUGEN, YUKSI, YUSIK, YURIF, YUSTE, WW165, mel 501, mel 928 and mel 624. All remaining cell lines were wild type for B-Raf. One (YUFIC) was found to harbor an N-Ras mutation.
Synergism Studies
At a density of 103, cells were plated in triplicate in 96 well plates with growth medium and allowed to adhere overnight. Two PI3K inhibitors, LY294002 (LC Laboratories, Woburn MA) and NVP-BKM120 (Novartis Pharmaceuticals, Basel, Switzerland) were used alone and in combination with the mTORC1 inhibitor, Rapamycin (LC Laboratories) at concentrations of 5–50 μmol/L, 0.313–2.5 μmol/L and 0.001–1 μmol/L, respectively for 48 hours. Combinations of NVP-BEZ235 and the dual MEK inhibitor, AZD6244 (Selleck Chemicals, Houston, TX) were studied at concentrations of 1–50 ηmol/L and 0.05–5 μmol/L, respectively. The relative number of viable cells was assessed by the luminometric Cell-Titer Glo assay (Promega), and luminescent quantification was measured using a Viktor plate reader (Perkin Elmer). Using CalcuSyn software (Biosoft, Ferguson, MO), results were analyzed for synergistic, additive, or antagonistic effects. Synergism is indicated by a Combination Index (CI) of < 0.9, additivity by CI values between 0.9 and 1.1, and antagonism by CI of > 1.1 (28).
Cell Viability Assays
Cells were exposed to NVP-BEZ235 (Novartis Pharmaceuticals, Basel, Switzerland), for 48 hours at concentrations ranging from 1 to 500 nmol/L. DMSO was used in the control wells. Relative number of viable cells was assessed by the luminometric Cell-Titer Glo assay (Promega, Madison, WI). Luminescent quantification of present ATP was determined using a Viktor plate reader (Perkin Elemer, Waltham, MA). To determine the IC50, we used XLfit statistical software (IDBS, Surrey, UK).
Immunoblot Assays
After treatment with LY294002, Rapamycin, NVP-BEZ235 and AZD6244, cells were lysed using standard methods (11). DMSO was used alone for the control cells. The following primary rabbit anti-human antibodies were used: PARP, phosporylated AKT Ser473, phosphorylated p70S6K Thr389 at 1:1000 (Cell Signaling Technologies, Danvers, MA). Mouse anti-human anti-caspase-2 (BD Pharmigen, Franklin Lakes, NJ) was used at a concentration of 1:1000. A monoclonal mouse anti-β-actin antibody (Sigma Aldrich) was used at 1:10000 for normalization of protein gel loading.
Clonogenic assays
Cells were seeded at a density of 500 cells per well in 6 well plates, and allowed to adhere overnight. Cells were incubated with NVP-BEZ235 at concentrations of 1, 10 and 40 ηM for seven days. Combination studies were done using NVP-BEZ235 at 5, 20, and 50 ηM and AZD6244 at 50, 500, and 5000 ηM. Medium and drugs were then replaced on the eighth day. On day 11, cell colonies were fixed with 25% glutaraldehyde (Sigma Aldrich) and 1% methanol, stained with 0.5% crystal violet (Sigma Aldrich), and colonies composed of over 50 cells were counted. Experiments were done in triplicate and results averaged. Cytotoxity was determined relative to the control (untreated) colonies.
Results
mTOR expression in human melanoma tumors and co-expression with PI3K
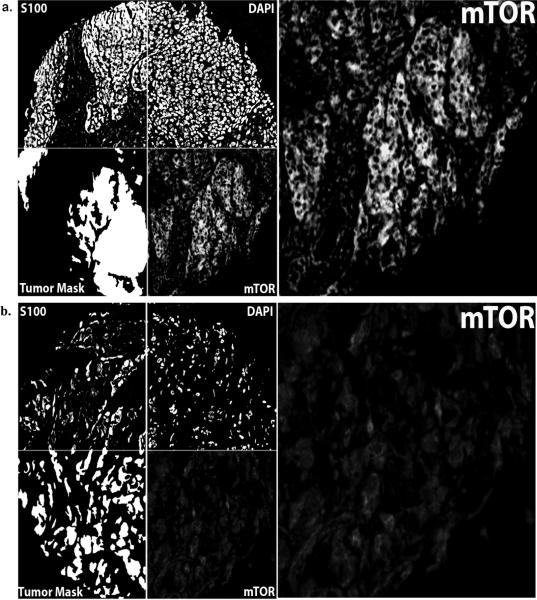
To assess patterns of mTOR expression in melanoma specimens, we stained 230 primary and 293 metastatic melanomas with an anti-mTOR antibody. To account for intratumor heterogeneity in mTOR expression, two separate melanoma TMAs, each containing a core from a different area of tumor for each patient, were stained. Figures 1A and 1B respectively show examples of strong and weak immunoreactivity of mTOR, which was only expressed in the cytoplasmic region, and not the nuclear compartment. Expression levels in the two arrays were tightly correlated (p < 0.0001), and scores were averaged for each case to generate a composite score used for the analyses. AQUA scores ranged between 11.12 and 69.36. mTOR expression was not associated with survival among either the primary or metastatic patients (data not shown). We assessed the association between mTOR expression and previously published expression of the p85 and p110α subunits of PI3K (11). Using the Spearman's nonparametric rank correlation coefficient, a strong association was found between mTOR expression and p110α expression (ρ = 0.658, p < 0.0001), while a weaker association was found between levels of mTOR and p85 (ρ = 0.239, p < 0.0001).
Figure 1.
Panel A: Automated Quantitative Analysis (AQUA) of in situ protein levels for mTOR: AQUA uses S100 to create a tumor mask (two upper left quadrants at 10×). S100 staining was both nuclear and cytoplasmic and the mask is made by filling in holes (lower left quadrants on left). 4', 6-diamidino-2-phenylindole (DAPI) defines the nuclear compartment within the tumor mask, which is then subtracted from the tumor mask to create a cytoplasmic compartment within the tumor mask. mTOR expression is measured within the cytoplasmic compartments, within the tumor mask (lower right quadrants), and each clinical case is assigned a score based on pixel intensity per unit area within the tumor mask. Panel A shows an example of a histospot with strong mTOR staining and panel B shows a histospot with weak mTOR staining.
Synergism between PI3K and mTOR inhibition
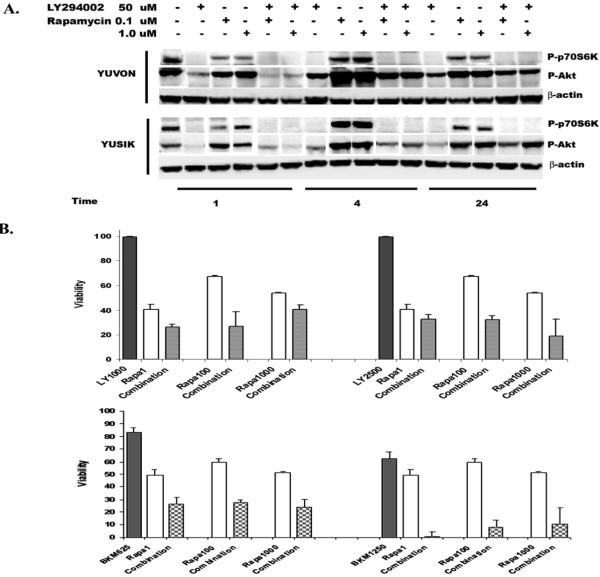
Using concentrations of 5, 25 and 50μM of the PI3K inhibitor, LY294002, we studied synergism with a range of concentrations of rapamycin (1, 100 and 1000ηM) in five melanoma cell lines (3 B-Raf mutant and 2 wild type). Synergism was seen in all five cell lines at a concentration of 5μM LY294002 with all three concentrations of rapamycin (Table 1). No synergism was seen at the higher concentrations of LY294002. Interestingly, the decrease in viability seen with the addition of all concentrations of rapamycin was similar; viability seen with adding 1ηM rapamycin to LY294002 was similar to that of 1μM. In one B-Raf mutant and one B-Raf wild type cell line we assessed the effects of LY294002 and rapamycin, alone and in combination, on the downstream PI3K targets, pAkt and pS70S6K. While treatment with rapamycin resulted in an increase in pAkt compared to untreated cells, treatment with LY294002 both alone and in combination with rapamycin resulted in down-regulation of pAkt and pP70S6K, as shown in Figure 2A. We note that the degree of decrease in pAkt and pP70S6K was the same or better with the lower concentration of rapamycin compared with the higher concentrations (100ηM and 1000ηM).
Table 1.
Combination Indexes (CI) assessing synergism (syn)/additivity (add)/antagonism (ant) between rapamycin and LY294002 and NVP-BKM120. Synergism is indicated by a CI of < 0.9, additivity by CI values between 0.9 and 1.1, and antagonism by CI of > 1.1.
| PI3K Inhibitor (nM) | Rapamycin (nM) | YUCAS (CI) | YULAC (CI) | YUKSI (CI) | YUSIK (CI) | YUVON (CI) |
|---|---|---|---|---|---|---|
| LY294002 (5000) | 1 | 0.71, syn | 0.56, syn | 0.25, syn | 0.19, syn | 0.33, syn |
| LY294002 (5000) | 100 | 0.60, syn | 0.39, syn | 0.20, syn | 0.11, syn | 0.27, syn |
| LY294002 (5000) | 1000 | 0.57, syn | 0.28, syn | 0.14, syn | 0.10, syn | 0.22, syn |
|
| ||||||
| BKM120 (313) | 1 | 0.46 syn | 0.20, syn | 0.27, syn | 0.29, syn | 0.29, syn |
| BKM120 (313) | 100 | 0.47, syn | 0.21,syn | 0.26, syn | 0.28, syn | 0.33, syn |
| BKM120 (313) | 1000 | 0.48, syn | 0.23, syn | 0.38, syn | 0.63, syn | 0.32, syn |
|
| ||||||
| BKM120 (625) | 1 | 0.85, syn | 0.40, syn | 0.57, syn | 0.58, syn | 0.59, syn |
| BKM120 (625) | 100 | 0.85, syn | 0.41, syn | 0.53, syn | 0.54, syn | 0.62, syn |
| BKM120 (625) | 1000 | 0.83, syn | 0.39, syn | 0.59, syn | 0.54, syn | 0.57, syn |
|
| ||||||
| BKM120 (1250) | 1 | 1.30, ant | 0.35, syn | 0.92, add | 0.80, syn | 0.94, add |
| BKM120 (1250) | 100 | 1.18, ant | 0.58, syn | 0.83, syn | 0.82, syn | 0.86, syn |
| BKM120 (1250) | 1000 | 0.80, syn | 0.63, syn | 0.86, syn | 0.68, syn | 0.82, syn |
Figure 2.
Panel A: Western blots showing levels of pAkt and pP70S6K in two cell lines with and without exposure to 50μM LY294002, 0.1μM rapamycin and 1μM rapamycin for 1, 4 and 24 hours, and the combination of LY294002 and the two concentrations of rapamycin at 1, 4 and 24 hours. β-actin is shown as a loading control. Levels of pAkt and pP70S6K decreased in all treatments that contained LY294002, while pAkt increased with rapamycin alone. Panel B: Cell viability assays with rapamycin and LY294002 or NVP-BKM120 alone and in combination in YULAC cells, showing similar enhancement of LY294002 and NVP-BKM120 activity with adding 1ηM, 100ηM and 1000ηM rapamycin.
Given the poor pharmacologic qualities of LY294002, we then studied synergism between a clinical quality PI3K inhibitor being developed by Novartis, NVP-BKM120, and rapamycin. The IC50s for the panel of five cell lines for NVP-BKM120 alone ranged from 1.06μM to 2.28μM. In all five cell lines synergism was seen at lower concentrations of NVP-BKM120 (313 and 625ηM), as shown in Table 1, and at the highest concentration (1250 ηM) YULAC and YUSIK remained synergistic. YUKSI and YUVON were additive with 1 ηM of rapamycin, but synergistic with higher concentrations (100 and 1000ηM). YUCAS remained synergistic with NVP-BKM120 at 1000 ηM, but at 1 and 100 ηM of rapamycin, antagonistic. As shown in Figure 2B, the combination of LY294002 and rapamycin (1, 100, and 1000 ηM) and that of NVP-BKM120 and at all concentrations of rapamycin resulted in comparable decreases in cell viability, using YULAC as an example.
Activity of a dual PI3K-mTOR inhibitor in melanoma cell lines
Given the synergism seen between PI3K inhibitors and rapamycin in melanoma cell lines, we studied the activity of a dual PI3K-mTOR inhibitor that has been administered to solid tumor patients in phase I clinical trials, NVP-BEZ235. To verify broad activity of the dual PI3K-mTOR inhibitor in melanoma cells, we expanded our panel of cell lines to include a total of 23 cell lines; one harboring an N-Ras mutation, 12 harboring B-Raf mutations and 10 wild-type for both. In all 23 melanoma cell lines the IC50s for NVP-BEZ235 were in the ηM range, as shown in Table 2.
Table 2.
IC50s of 23 melanoma cell lines treated with NVP-BEZ235 and characterization of B-Raf and N-Ras mutation status.
| Cell Line | IC50 BEZ235 (ηM) | B-Raf | N-Ras |
|---|---|---|---|
| MEL501 | 20.0 | V600E/WT (GAG/GTG) | WT |
| MEL624 | 28.7 | V600E/WT (GAG/GTG) | WT |
| MEL928 | 45.7 | V600E/WT (GAG/GTG) | WT |
| WW165 | 39.4 | V600K/WT (AAG/GTG) | WT |
| YUCAS | 20.8 | WT (GTG) | WT |
| YUFIC | 34.7 | WT (GTG) | Q61R/WT (CGA/CAA) |
| YUGEN8 | 34.8 | V600E (GAG/GAG) | WT |
| YUHEF | 9.8 | WT (GTG) | WT |
| YUHOIN | 25.0 | WT (GTG) | WT |
| YUKIM | 13 | WT (GTG) | WT |
| YUKSI | 26.6 | V600K (AAG/AAG) | WT |
| YULAC | 14.6 | V600K (AAG/AAG) | WT |
| YUMAC | 23.9 | V600K (AAG/AAG) | WT |
| YUPLA | 5.9 | WT (GTG) | WT |
| YURIF | 44.1 | V600K (AAG/AAG) | WT |
| YUROB | 25.2 | WT (GTG) | WT |
| YUROL | 6.9 | WT (GTG) | WT |
| YUSAC2 | 35.9 | V600E (GAG/GAG) | WT |
| YUSIK | 41.2 | V600E/WT (GAG/GTG) | WT |
| YUSIV | 23.6 | WT (GTG) | WT |
| YUSOC | 27.7 | WT (GTG) | WT |
| YUSTE | 8.8 | V600E (GAG/GAG) | WT |
| YUVON | 77.4 | WT (GTG) | WT |
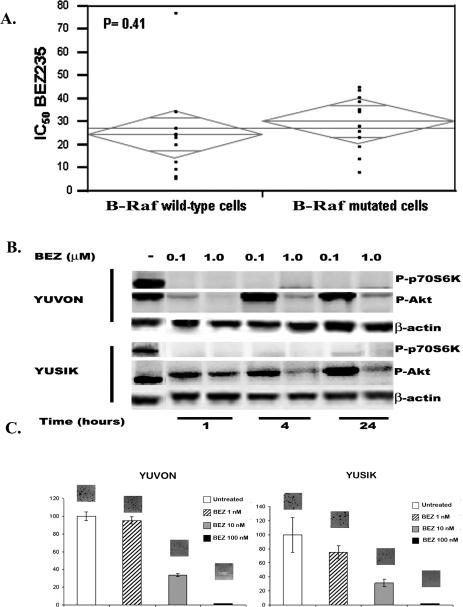
One of the proposed mechanisms of resistance to PI3KIs is mutations in the Ras-Raf pathway, which are found in over half of melanomas. By analysis of variance, no association was found between the IC50s of NVP-BEZ235 and presence or absence of B-Raf mutations (Figure 3A).
Figure 3.
Panels A: ANOVA analysis of IC50s of the dual PI3K/mTOR inhibitor NVP-BEZ-235 in 23 melanoma cell lines showing no significant difference in sensitivity to this compound in B-Raf mutant and B-Raf wild type cells. Panel B: Decreases in pAkt and pP70S6K in a dose- and time-dependant fashion in two melanoma cell lines treated with NVP-BEZ-235. pP70S6K levels are undetectable at all concentrations and time points studied, whereas levels of pAkt start rising again after 4 hours of drug exposure in a dose-dependant fashion. Panels C: Clonogenic assays in two melanoma cell lines (YUVON and YUSIK) treated with NVP-BEZ-235 at different concentrations. NVP-BEZ235 was effective in inhibiting colony formation at low nanomolar concentrations.
The targets of NVP-BEZ235, pAkt and pP70S6K were both decreased with exposure to the drug in a time- and dose-dependent fashion, as shown in Figure 3B for YUVON and YUSIK cell lines. Relative to untreated cells, pAkt, and to a greater degree, pP70S6K, were down-regulated at 1 and 4 hours, and levels of pAkt started to increase after 4 hours with 100ηM of NVP-BEZ235.
Clonogenicity was studied in YUVON and YUSIK cells with exposure to the dual PI3K/mTOR inhibitor. As shown in Figure 3C, NVP-BEZ235 effectively inhibits clonogenicity at low ηM concentrations.
Synergism between the dual PI3K/mTOR inhibitor NVP-BEZ235 and the MEK inhibitor AZD6244 in B-Raf mutant and wild-type cell lines
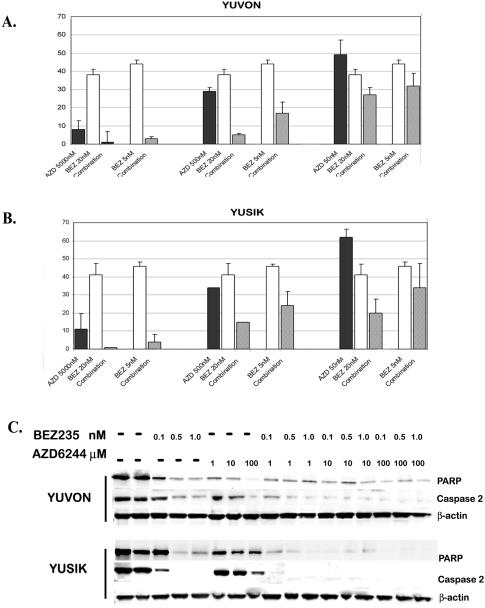
Given that mTOR blockade is necessary to enhance the inhibition of the PI3K pathway, we finally studied the efficacy of dual PI3K-mTOR inhibition and MAPK pathway inhibition in the same subset of five melanoma cell lines used for studying synergism between rapamycin and PI3K inhibitors. We added AZD6244 to NVP-BEZ235 and synergism was seen in four of the five cell lines studied, as shown in Supplemental Table 1. The only cell line in the panel for which we did not see synergism was YULAC, which was highly resistant to AZD6244. An example of cell kill with the combination of these two drugs in YUVON and YUSIK cells is shown in Supplemental Figure 1. Clonogenic assays show similar enhanced effects with the addition of AZD6244 to NVP-BEZ235 (Figure 4A–B).
Figure 4.
Panel A and B: Clonogenic assays in two melanoma cell lines (YUVON and YUSIK) treated with NVP-BEZ235 and AZD6244 alone and in combination. Combinations were more effective in inhibiting colony formation at lower concentrations than either drug alone. Panel C: Treatment of YUVON and YUSIK cells with ascending concentrations of NVP-BEZ235 resulted in dose-dependant PARP cleavage and caspase-2 induction. Treatment with AZD6244 also resulted in PARP cleavage and caspase-2 induction, but to a lesser degree. Treatment with a combination of these compounds has a greater effect on PARP and caspase-2 cleavage than either drug alone.
Induction of apoptosis
Previous studies with NVP-BEZ235 have shown that it causes PARP cleavage and induces apoptosis via activation of caspase-2, with no effect on caspases-3, -7, and -9 (29). We therefore studied the effect of NVP-BEZ235 on PARP cleavage and caspase-2 activation alone and in combination with AZD6244 in a B-Raf wild-type and a B-Raf mutant cell line. As shown in Figure 4C, NVP-BEZ235 alone results in PARP cleavage and caspase-2 activation. AZD6244 alone has a smaller effect on these two apoptotic pathway members. The two drugs in combination cause PARP and caspase-2 cleavage at lower concentrations than either drug alone.
Discussion
In this work we studied dual targeting of PI3K and mTOR in melanoma. Expression of mTOR was assessed on a large cohort of melanomas and nevi, and higher levels were found in the malignant melanocytes, which correlated with high PI3K levels, particularly the p110α subunit. We showed that the addition of rapamycin to the PI3K inhibitor LY294002 resulted in synergism at all concentrations of rapamycin used, which resulted in pAkt and pP70S6K down-regulation, with similar levels seen for the different concentrations of rapamycin used.
LY294002 is a relatively weak PI3K inhibitor, and its poor pharmacologic properties preclude its use in humans. We therefore verified the synergism between mTOR and PI3K inhibition using a clinical quality compound under development by Novartis Pharmaceuticals, NVP-BKM120. Significant synergism was seen with viability curves overlapping for 1ηM, 100ηM and 1μM of rapamycin. Finally, we studied the in vitro activity of a dual PI3K/mTOR inhibitor that is in clinical trials, also developed by Novartis, NVP-BEZ235, on a panel of 23 human melanoma cell lines. The dual PI3K/mTOR inhibitor was equally effective in vitro in B-Raf mutant and wild-type cell lines and demonstrated induction of caspase-2 and PARP cleavage. Addition of the MEK inhibitor AZD6244 to NVP-BEZ235 resulted in synergism in four of five cell lines.
PI3K has been shown to be a good therapeutic target in melanoma by our group and others using a variety of PI3K inhibitors (11, 13–14, 16, 30–32). While these agents are clearly active in pre-clinical models, they have yet to be tested in clinical trials specific for melanoma patients. As is the case with many targeted therapies, resistance to highly specific inhibitors can result from escape mechanisms such as downstream target activation, activation of parallel pathways, acquired mutations, etc. For specific PI3K inhibitors, resistance can be due to a number of possible escape mechanisms. There is ample evidence that downstream PI3K pathway activation can occur by mechanisms other than activation of PI3K itself. For example, Akt can be activated and phosphorylated by mTORC2 (33). Resistance to PI3K inhibition can also be due to the significant cross-talk between the PI3K and MAPK pathways, and MAPK pathway members can activate the PI3K pathway. For example, ERK and RSK inhibit TSC2, resulting in mTOR activation and downstream PI3K pathway activation that is independent of PI3K and Akt (19). Negative feedback loops such as activation of insulin receptor substrate 1 results in further PI3K pathway activation (20). Co-inhibition of mTOR and PI3K could overcome all of these mechanisms of resistance to PI3K inhibitors and enhance their activity.
Previous studies have reported synergism between PI3K and mTOR inhibitors in melanoma and other cancers (34–35). In a study by Marone et. al. synergism was demonstrated when treating melanoma cells with the combination of 100ηM of rapamycin and 1μM of the pure PI3K inhibitor ZSTK474 (25). Werzowa et. al. demonstrated synergism in two melanoma cell lines with the combination of 25ηM rapamycin and 10μM LY294002 (36). Our studies confirm and expand these findings showing synergism between LY294002 (at 5μM, but not at 50μM) and a range of concentrations of rapamycin that spans 4 logs. We found similar synergism between rapamycin and the novel, clinical quality PI3K inhibitor NVP-BKM120. No major differences in viability were seen with the different concentrations of rapamycin, indicating that minimal mTOR inhibition is sufficient to potentiate PI3K inhibition. This finding is particularly important when designing inhibitors for clinical use, as mTOR inhibitors, especially when used in combination with other molecular targeted therapies, can be associated with a fair degree of toxicity (37–38).
Dual PI3K/mTOR inhibitors have been shown to be active in a number of malignancies (39–45). Marone et. al. showed that NVP-BEZ235 is active in melanoma cell lines and mouse models, as were two other dual PI3K/mTOR inhibitors, NVP-BAG956 and NVP-BBD130 (25). In addition, they showed that NVP-BEZ235 was active in a B16 mouse model, causing a decline in tumor burden, increased tumoral necrosis and decreased tumor vasculature. These findings are fully consistent with ours, in which we demonstrated IC50s in the low nanomolar range in 23 melanoma cell lines, which included 19 early passage, patient derived cell lines. Similar to the studies by Marone et. al., we found that the NVP-BEZ235 decreases viability and clonogenicity. As demonstrated by Brachmann et. al. in breast cancer, we found PARP cleavage and caspase-2 induction in melanoma cells with exposure to NVP-BEZ235 (29).
A large number of drugs that target the PI3K pathway are currently in pre-clinical and clinical development. Pathway members that are direct targets of drugs in development include PI3K, PDK1, ILK, Akt, mTOR, P70S6K and forkhead (11, 21). The plethora of drugs that target different pathway components provides a unique opportunity for rational drug combinations. PI3K and Akt inhibitors have been assessed in phase I clinical trials for solid tumors, but they have yet to be studied in phase II trials for melanoma. mTOR inhibitors have been widely used in patients, and are available for clinical testing in combination with PI3K and Akt inhibitors. Our data suggest that small doses of mTOR inhibitors might be adequate to effectively down-regulate pAkt when given in combination with PI3K inhibitors; smaller doses are likely to be associated with less toxicity.
Mutations in c-Kit are relatively rare, and c-Kit targeting therapies have resulted in durable responses (6, 46). B-Raf mutations are much more common (approximately 60%), and PLX-4032 (Plexxikon), a specific inhibitor of mutated B-Raf, has shown dramatic activity in metastatic melanomas that harbor B-Raf mutations (47). Responses to PLX-4032 are transient, however, and one potential mechanism of acquired resistance is activation of the PI3K pathway. A number of studies have shown that co-activation of the PI3K pathway is necessary for malignant transformation and tumor growth in cells harboring mutations of the Ras-Raf pathway (48–49). Our findings also suggest that concurrent targeting of the Ras-Raf and the PI3K pathways with drugs such as AZD6244 and NVP-BEZ235 might be more effective in some (but not all) patients than targeting either one of the pathways alone.
In this work we used two novel PI3K pathway inhibitors; the dual PI3K/mTOR inhibitor NVP-BEZ235 and the PI3K inhibitor NVP-BKM120. Both are currently being tested in clinical trials for patients with solid tumors (CBEZ235A101, CBKM120A2101). The MTD of both compounds has been established and publication of the toxicity data is pending. Our results suggest that further assessment of these drugs alone and in combination with MAPK pathway inhibitors in melanoma patients is warranted. We note that no association was found in our studies between sensitivity to NVP-BEZ235 and B-Raf mutation status, further supporting the critical role of the PI3K inhibition in this disease, and suggesting that NVP-BEZ235 is effective in both B-Raf wild type and mutant phenotypes.
In summary, we previously showed that PI3K is up-regulated in melanoma. Here we demonstrated strong co-expression of the p110α catalytic subunit and mTOR, suggesting that co-targeting these two molecules might be an effective approach for treating this disease. We further showed strong synergism between two PI3K inhibitors and rapamycin, with no major differences seen between different rapamycin concentrations, indicating that minimal mTOR inhibition might be sufficient for potentiating the effects of PI3K inhibitors and might result in less toxicity than larger doses of mTOR inhibitors. A dual PI3K/mTOR inhibitor NVP-BEZ235 was highly active in vitro in a large panel of B-Raf mutant and wild type melanoma cell lines, and synergism was seen when adding AZD6244 in most, but not all, cell lines. Further evaluation of NVP-BEZ235 in melanoma is warranted. Studies to identify biomarkers predictive of sensitivity to PI3K/mTOR inhibition are ongoing in our laboratory.
Statement of translational relevance.
We previously showed that PI3K is a valuable therapeutic target in melanoma; however, mechanisms of resistance to PI3K inhibitors (PI3KIs) exist. Given the availability of drugs that target both PI3K and mTOR, we demonstrated strong co-expression of the p110α PI3K subunit and mTOR in a large cohort of melanomas. In vitro, addition of rapamycin at concentrations as low as 1ηM, sensitized melanoma cells to two PI3KIs, LY294002 and a novel clinical-grade PI3KI, NVP-BKM120. The dual PI3K/mTOR inhibitor currently in clinical trials, NVP-BEZ-235, was active in vitro in a panel of 23 B-Raf mutant and wild-type melanoma cell lines. Synergism was seen between NVP-BEZ-235 and the MEK inhibitor, AZD6244, with resultant decrease in clonogenicity and increased induction of apoptosis. Taken together, these studies strongly support further in vivo assessment of NVP-BEZ-235 alone and in combination with MAPK pathway inhibitors in melanoma. Low, intermittent dosing might be better tolerated while preserving activity.
Supplementary Material
Acknowledgments
This work was supported by the Yale SPORE in Skin Cancer, 1 P50 CA121974-01 (to R. Halaban), NIH grant CA115756-01 (to H. Kluger) and RO-1 CA 114277 (to D. Rimm).
References
- 1.Rigel DS. Malignant melanoma: incidence issues and their effect on diagnosis and treatment in the 1990s. Mayo Clin Proc. 1997;72:367–71. doi: 10.4065/72.4.367. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Jilaveanu LB, Aziz SA, Kluger HM. Chemotherapy and biologic therapies for melanoma: do they work? Clin Dermatol. 2009;27:614–25. doi: 10.1016/j.clindermatol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Acquavella N, Kluger H, Rhee J, et al. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother. 2008;31:569–76. doi: 10.1097/CJI.0b013e318177a4ba. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–51. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 7.Whang T, Goldenberg G. IDrugs; American Academy of Dermatology--Summer Meeting; Boston, MA, USA. 29 July–2 August 2009; 2009. pp. 617–9. [PubMed] [Google Scholar]
- 8.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 11.Aziz SA, Davies M, Pick E, et al. Phosphatidylinositol-3-kinase as a therapeutic target in melanoma. Clin Cancer Res. 2009;15:3029–36. doi: 10.1158/1078-0432.CCR-08-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–8. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 13.Smalley KS, Herlyn M. Targeting intracellular signaling pathways as a novel strategy in melanoma therapeutics. Ann N Y Acad Sci. 2005;1059:16–25. doi: 10.1196/annals.1339.005. [DOI] [PubMed] [Google Scholar]
- 14.Govindarajan B, Sligh JE, Vincent BJ, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–29. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa M, Kaizawa H, Moritomo H, et al. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem. 2006;14:6847–58. doi: 10.1016/j.bmc.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Yaguchi S, Fukui Y, Koshimizu I, et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98:545–56. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 17.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 18.van 't Veer LJ, Burgering BM, Versteeg R, et al. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol. 1989;9:3114–6. doi: 10.1128/mcb.9.7.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 20.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 22.Margolin K, Longmate J, Baratta T, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–8. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 23.Karbowniczek M, Spittle CS, Morrison T, Wu H, Henske EP. mTOR is activated in the majority of malignant melanomas. J Invest Dermatol. 2008;128:980–7. doi: 10.1038/sj.jid.5701074. [DOI] [PubMed] [Google Scholar]
- 24.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Marone R, Erhart D, Mertz AC, et al. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res. 2009;7:601–13. doi: 10.1158/1541-7786.MCR-08-0366. [DOI] [PubMed] [Google Scholar]
- 26.Divito KA, Berger AJ, Camp RL, Dolled-Filhart M, Rimm DL, Kluger HM. Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res. 2004;64:8773–7. doi: 10.1158/0008-5472.CAN-04-1387. [DOI] [PubMed] [Google Scholar]
- 27.Kluger HM, Kluger Y, Gilmore-Hebert M, et al. cDNA microarray analysis of invasive and tumorigenic phenotypes in a breast cancer model. Lab Invest. 2004;84:320–31. doi: 10.1038/labinvest.3700044. [DOI] [PubMed] [Google Scholar]
- 28.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Brachmann SM, Hofmann I, Schnell C, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–82. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 31.Krasilnikov M, Adler V, Fuchs SY, et al. Contribution of phosphatidylinositol 3-kinase to radiation resistance in human melanoma cells. Mol Carcinog. 1999;24:64–9. doi: 10.1002/(sici)1098-2744(199901)24:1<64::aid-mc9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Meier F, Busch S, Lasithiotakis K, et al. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol. 2007 doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 33.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 34.Chiarini F, Fala F, Tazzari PL, et al. Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res. 2009;69:3520–8. doi: 10.1158/0008-5472.CAN-08-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi H, Kondo Y, Fujiwara K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 36.Werzowa J, Cejka D, Fuereder T, et al. Suppression of mTOR complex 2-dependent AKT phosphorylation in melanoma cells by combined treatment with rapamycin and LY294002. Br J Dermatol. 2009;160:955–64. doi: 10.1111/j.1365-2133.2008.08991.x. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Kluger H, Saif MW, et al. A phase I study of sunitinib in combination with sirolimus in adults with advanced refractory malignancies. J Clin Oncol. 2009;27(suppl):15s. abstr 3554. 2009. [Google Scholar]
- 38.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115:2368–75. doi: 10.1002/cncr.24234. [DOI] [PubMed] [Google Scholar]
- 39.Cao P, Maira SM, Garcia-Echeverria C, Hedley DW. Activity of a novel, dual PI3-kinase/mTor inhibitor NVP-BEZ235 against primary human pancreatic cancers grown as orthotopic xenografts. Br J Cancer. 2009;100:1267–76. doi: 10.1038/sj.bjc.6604995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268–73. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faber AC, Li D, Song Y, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–8. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konstantinidou G, Bey EA, Rabellino A, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–52. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu TJ, Koul D, LaFortune T, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–10. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMillin DW, Ooi M, Delmore J, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69:5835–42. doi: 10.1158/0008-5472.CAN-08-4285. [DOI] [PubMed] [Google Scholar]
- 45.Roccaro AM, Sacco A, Husu EN, et al. Dual targeting of the PI3K/Akt/mTOR pathway as an anti-tumor strategy in Waldenstrom's macroglobulinemia. Blood. 2009 doi: 10.1182/blood-2009-07-235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 47.McArthur GAPI, Ribas A, Chapman PB, et al. Early FDG-PET responses to PLX 4032 in BRAF-mutant advanced melanoma. J Clin Oncol. 2010;28(suppl):15s. abstr 8529. 2010. [Google Scholar]
- 48.Robinson JP, Vanbrocklin MW, Guilbeault AR, Signorelli DL, Brandner S, Holmen SL. Activated BRAF induces gliomas in mice when combined with Ink4a/Arf loss or Akt activation. Oncogene. 2009 doi: 10.1038/onc.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skeen JE, Bhaskar PT, Chen CC, et al. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–80. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.