Abstract
Objectives
Sleep disturbance is a common co-morbidity of chronic pain. Inflammatory processes are dysregulated in sleep disturbance and also contribute to pain sensitivity. Thus, inflammation may play an important role in bi-directional associations between pain and sleep. Little is known about concurrent relationships among chronic pain, sleep, and inflammation. The aim of our study was to examine associations among sleep disturbance and circulating levels of the inflammatory cytokine, interleukin-6 (IL-6), in individuals with and without chronic low back pain.
Methods
Gender and age-matched adults with chronic low back pain (CLBP; n = 25) or without chronic pain (controls; n = 25) completed measures of sleep quality in the past month and depressive symptoms in the past week, and provided a blood draw for IL-6. The next morning, participants reported their sleep quality the previous night and their current experience of morning pain.
Results
Individuals with CLBP had more sleep disturbance than controls. Circulating IL-6 levels were similar for the two groups; however, in adults with CLBP, poorer sleep quality was associated with higher IL-6 levels, and both sleep and IL-6 related to pain reports. Unlike CLBP participants, controls showed normal, age-related increases in IL-6 levels, whereas sleep quality was unrelated to IL-6 levels. Depressive symptoms could not fully explain the observed associations.
Discussion
Inflammatory processes may play a significant role in cycles of pain and sleep disturbance. Clinical interventions that improve sleep and reduce concomitant inflammatory dysregulation hold promise for chronic pain management.
Keywords: chronic back pain, sleep disturbance, inflammatory cytokines, interleukin-6
Introduction
The association between pain and sleep disturbance is bi-directional: pain contributes to disrupted sleep, and sleep disturbance can likewise enhance pain perception.1-5 The neurobiological mechanisms linking pain and sleep remain unclear. Recent attention to interactions between inflammatory processes in pain, on the one hand, and sleep, on the other, points to neuroimmunological pathways that may contribute to the strong association between pain and sleep.
Proinflammatory cytokines, including interleukin-6 (IL-6), may be involved in pain processes.6-8 Inflammatory mediators can modulate nociception9 and contribute to the amplification and persistence of pain.10-13 In clinical studies, higher levels of IL-6 have been associated with greater pain severity in individuals with rheumatoid arthritis14 and fibromyalgia,15 as well as with greater post-operative pain.16 Although the nature of relationship between IL-6 and pain is not fully understood, sleep disruption may be an important contributor to proinflammatory activity in those with chronic and recurrent pain. For example, IL-6 normally peaks approximately 2.5 hours after sleep onset and typically shows higher circulating levels at night.19, 20 However, reductions in sleep quality and quantity have each been associated with higher circulating levels of IL-6 during the day. 20 21, 22.23, 24 To the authors' knowledge, only one study to date has simultaneously examined sleep, IL-6, and pain. Haack and colleagues randomly assigned 18 young women without sleep disorders to either a 50% reduction in usual sleep time (i.e., 4 hours per night) or an 8 hour sleep condition. Over a period of ten days the women in the sleep reduction condition showed increased IL-6 levels and greater daily pain ratings compared to women sleeping 8 hours per night.23 Although this study provides suggestive evidence for an association between sleep restriction, IL-6, and pain reports, it is difficult to generalize these findings to the potential relationship between naturally-occurring sleep disturbance and clinical pain.
In the current study, we assessed self-reported sleep quality and circulating IL-6 levels in a community-based sample of adults with and without chronic low back pain (CLBP). Intervertebral discs from CLBP patients have been shown to produce high levels of proinflammatory cytokines, including IL-6,25 suggesting inflammatory contributions to back pain. Whether IL-6 levels in circulation are associated with sleep quality in adults with CLBP, however, is unknown. Because clinical observations indicate that inflammatory back pain tends to be greatest in the morning, in the present study our focus was on morning pain after awakening. We predicted that individuals with CLBP would report poorer sleep quality, evidence more clinical sleep disturbance, have higher IL-6 levels, and demonstrate stronger associations among IL-6 and sleep quality compared to healthy controls. We also expected that poorer sleep quality and IL-6 levels would be associated with more morning pain among CLBP. Finally, given the interrelationships between depression, pain, and sleep disturbance,3, 5, 26 we also examined the potential influence of depressive symptoms on associations among pain, sleep and IL-6.
Materials and Methods
Participants
Twenty-five men and women with chronic low back pain (defined as low back pain for 6 months or more) were recruited, along with 25 age- and gender-matched individuals without chronic pain. Participants were recruited primarily through newspaper and posted advertisements; we also invited participants who had completed another study of chronic low back pain27 to participate in the current study. Eligibility criteria paralleled the Trost et al. study and were as follows: no history of spinal surgeries; no orthopedic injuries to the upper or lower extremities that restrict motions of these segments; no lower extremity weakness or neurological signs that reflect brain or nerve injury (e.g., seizures, cerebral palsy); no prescription narcotics for symptom relief; no chronic diseases that may restrict movement (e.g., severe arthritis). Additional eligibility criteria for the current study included: no current infectious, autoimmune or inflammatory diseases or other diseases with obvious immunological consequences (e.g., rheumatoid arthritis, cardiovascular disease, cancer within the past 5 years); no diabetes or thyroid conditions; no heavy smoking (i.e., > 15 cigarettes daily); no reported needle or blood phobias; no current use of psychotropic medication (e.g., anti-depressants); no fibromyalgia. Participants in the current study were 19-55 years of age (M = 30.82, SD = 11.38) and primarily White, non-Hispanic (90%).
Procedure
Eligible participants were enrolled in a two-part study. Participants completed informed consent, a series of self-report questionnaires, and provided a blood sample at a morning study session (Time 1); all blood samples were obtained by a research nurse between 8:30 am and 10:30 am. Participants were asked to refrain from alcohol and the use of anti-inflammatory medications (for example, ibuprofen) for 24 hours prior to their blood draw. At home the next morning, participants completed questionnaires regarding sleep during the previous night and their current morning pain (Time 2). Participants received $20 for participation. All recruitment and study procedures were reviewed, approved, and monitored by appropriate institutional review boards.
Key Measures
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a 24 item scale that measures sleep disturbances along 7 dimensions, including subjective sleep quality, sleep latency (time it takes to fall asleep), sleep duration (time asleep while in bed), sleep efficiency (percentage of time asleep while in bed), sleep disturbance (e.g., trouble sleeping because of bad dreams, need to use bathroom, coughing or snoring, pain etc), use of sleep medication, and daytime dysfunction. Scores from these 7 dimensions (ranging from 0-3) are individually reported as component scores and summed to derive a global sleep quality score (0-21).28 In a prior reliability and validity study, a cutoff of >6 resulted in the best sensitivity and specificity with respect to insomnia,29 a convention that we adopt in this study. At Time 1, participants completed the PSQI with regard to sleep in the past month; at Time 2 (the next morning after awakening), participants completed a single-night PSQI with regard to sleep during the previous night only. We derived component and global scores from PSQI assessment at Time 1 (reflecting sleep quality in the past month) and Time 2 (single-night PSQI, reflecting sleep quality the previous night). Participants were categorized as having or not having clinical sleep disturbance (i.e., a PSQI global score > 6 and ≤ 6, respectively) from the PSQI assessment at Time 1. We also report two single items from the scale that are useful self-report measures of sleep continuity disturbance observed in insomnia samples: total sleep duration (in hours) and sleep efficiency, which is the ratio of time spent in bed to total sleep duration (expressed as a percentage).
The single-night PSQI completed at Time 2 differed from the original PSQI in two ways. First, 2 items assessing daytime dysfunction in the past month were modified to assess expectations of daytime fatigue. Second, response options for these 2 items and for 9 items assessing types of sleep disturbances were modified from 0 (not during the past month) to 3 (three or more times per week) scales to Yes or No, equaling 3 or 0, respectively. The original and single-night PSQI global scores were significantly correlated (r = .54, p = .01).
McGill Pain Questionnaire-Short Form (MPQ-SF)
We used the MPQ-SF, a commonly used, well-validated, reliable instrument for the assessment of current pain for clinical and research applications.30 Respondents report their current pain intensity using a single-item rating of six pain levels, ranging from “no pain” to “excruciating.” Respondents also rate 15 pain descriptors, including sensory (11 items, e.g., throbbing, shooting) and affective (4 items, e.g., sickening, fearful) descriptors, on a scale from 0 (none) to 3 (severe). Summation of descriptors provides both sensory and affective rating scores, as well as a total pain rating score.
IL-6 levels
Following venipuncture, blood was kept on ice, centrifuged, and plasma was stored at -80° C. Plasma IL-6 concentrations were determined via assay using standard ELISA protocols and high sensitivity anti-cytokine antibody pairs (R&D Systems, Minneapolis, MN). Absorbance was measured at 405/630 nm using an automated Opsys MR Microplate Reader (Thermo Labsystems, Chantilly, VA). Sensitivity for the assay is .039 pg/mL, and intra- and inter-assay variation was < 10%.
Potential Covariates
Demographics and health questionnaire
We assessed demographics (race/ethnicity, relationship status), smoking (frequency, amount of tobacco use), exercise (frequency, amount of moderate to highly vigorous physical activity for 30 minutes or more), caffeinated beverage consumption, hypertension and other chronic conditions, current medications, and verified inclusion criteria using a form developed for the current study.
Center for Epidemiological Studies Depression Scale – Revised (CESD-R)
The CESD-R is a well-validated 20-item measure of depressive symptoms in the previous week.31 In many populations, large proportions of individuals with CESD scores greater than 15 meet diagnostic criteria for major depression.32 Responses involve a 4-point Likert scale ranging from 0 (“not at all”) to 3 (“nearly every day”). For descriptive purposes we report total CESD-R scores. For analyses, we use the total score with the sleep items removed, as has been recommended.33
Data Analysis
Characteristics of individuals with chronic low back pain (CLBP) and without chronic pain (controls) were compared via chi-square tests (categorical variables) or independent t-tests (continuous variables). The following served as the dependent variables for key group comparisons: global PSQI scores at Time 1 (past month) and Time 2 (previous night), presence of sleep disturbance (PSQI > 6), and log transformed IL-6 levels. PSQI component scores (used to derive global PSQI score), and sleep efficiency and total sleep time scores, were also examined. Pearson correlation coefficients were evaluated to determine associations between IL-6 levels and global PSQI scores at Time 1 and Time 2 for participants with and without CLBP. Among the CLBP group, we also examined correlations between MPQ-SF pain scores, and PSQI scores and IL-6. Age and CESD-R modified scores (i.e., excluding sleep items) were examined as potential covariates given their associations with IL-6 levels34, 35 and sleep.22.
Results
Characteristics of Individuals With and Without Chronic Low Back Pain (CLBP)
Participants were primarily female (60%), White, non-Hispanic (92%), young adults (age: M = 30.8 years; SD = 11.4), and approximately half were in a romantic relationship (57%); there were no differences on these variables between individuals with and without CLBP (p's > .8). Average caffeine use (M = 2.3 cups daily; SD = 2.1) and the proportion of individuals who engaged in regular exercise (64%), were smokers (12%), or had consumed caffeine prior to the blood draw (30%) were also similar for individuals with and without CLBP (all p's > .50). Two women with CLBP and 1 woman without chronic pain were postmenopausal. One CLBP participant reported hypertension and the use of a diuretic and angiotensin-converting enzyme (ACE) inhibitor. No participants reported statin use. One man with CLBP reported taking an anti-inflammatory medication (hydrocortisone tablet) 25 hours prior to his blood draw; however, excluding him from analyses did not alter results reported here.
Key Comparisons: Sleep and IL-6 Levels by Group
Descriptive statistics for PSQI measures are presented in Table 1. At Time 1, participants with CLBP had higher global PSQI scores compared to controls, indicating poorer sleep quality in the past month. CLBP participants also had a larger proportion of individuals with a global PSQI score > 6 (n = 13 (52%) compared to controls, n = 5 (20%); χ2 = 5.56, p = .04), indicating more clinically meaningful sleep disturbance among adults with CLBP. Based on PSQI component scores, individuals with CLBP reported more sleep disturbances, greater daytime dysfunction, and marginally lower habitual sleep efficiency compared to controls. No group differences were evident for total sleep time. At Time 2, single-night PSQI global scores (reflecting sleep the previous night) were higher for the CLBP group (Table 1). Outcomes for the single-night PSQI component scores were similar overall to those for the past month PSQI, but sleep efficiency was also lower among the CLBP group compared to controls. Finally, circulating IL-6 levels did not differ between participants with CLBP (M = 1.2 pg/ml; SD = 1.0) versus controls (M = 1.1 pg/ml, SD = 0.6; t(48) = .44; p = .67).
Table 1. PSQI Scores for Time 1 and Time 2 by Group.
| Time 1 | Time 2 | |||||
|---|---|---|---|---|---|---|
| Chronic low back pain (n = 25) |
Controls (n = 25) |
p | Chronic low back pain (n = 25) |
Controls (n = 25) |
p | |
| PSQI global sleep quality, M (SD) | 7.0 (3.7) | 4.2 (2.5) | .003 | 6.2 (3.9) | 3.2 (2.1) | .001 |
| PSQI Components, M (SD) | ||||||
| 1. Subjective sleep quality | 1.2 (0.8) | 0.9 (0.8) | .24 | 1.1 (0.8) | 0.8 (0.6) | .11 |
| 2. Sleep latency | 1.0 (0.9) | 0.6 (0.7) | .11 | 0.9 (1.1) | 0.4 (0.8) | .08 |
| 3. Sleep duration | 1.3 (1.1) | 0.8 (0.8) | .15 | 1.2 (1.0) | 0.8 (0.9) | .18 |
| 4. Sleep efficiency | 0.4 (0.6) | 0.2 (0.6) | .50 | 0.7 (0.6) | 0.2 (0.9) | .04 |
| 5. Sleep disturbance | 1.4 (0.7) | 0.9 (0.5) | .008 | 1.2 (0.6) | 0.8 (0.6) | .08 |
| 6. Use of sleep medication | 0.3 (0.8) | 0.1 (0.3) | .23 | 0.0 (0.0) | 0.0 (0.0) | -- |
| 7. Daytime dysfunction | 1.5 (0.8) | 0.6 (0.6) | <.001 | 1.0 (1.2) | 0.0 (0.0) | <.001 |
| PSQI Items, M (SD) | ||||||
| Total sleep time (hours) | 6.7 (1.5) | 7.1 (1.0) | .28 | 6.5 (1.3) | 7.1 (1.1) | .08 |
| Habitual sleep efficiency (%) | 87.3 (9.0) | 92.5 (9.8) | .05 | 85.0 (13.0) | 91.9 (8.9) | .04 |
PSQI = Pittsburgh Sleep Quality Index; Time 1 PSQI indexes sleep in the past month, Time 2 PSQI indexes sleep the previous night.
Age was not associated with sleep measures at either time point. With groups combined, the correlation between age and IL-6 levels was not significant (r = .24, p = .10); when we stratified by group, controls evidenced a moderate correlation between age and IL-6 (r = .34, p = .09), whereas adults with CLBP did not (r = .15, p = .48).
Associations between IL-6 and Sleep Quality
Among the CLBP group, lower sleep quality was associated with higher IL-6 levels (Time 1 PSQI: r = .39, p = .05; Time 2 PSQI: r = .52, p = .008). Among controls, IL-6 levels were not associated with PSQI scores (Time 1 PSQI: r = .22, p = .29; Time 2 PSQI: r = .18, p = .39). IL-6 level by PSQI global scores are plotted for all participants in Figure 1A (Time 1) and 1B (Time 2). Overall, individuals characterized as poor sleepers (global PSQI score > 6; n = 18/50, or 36% of participants), the majority of whom were participants with CLBP (n = 13/18, or 72.2%), had higher IL-6 levels compared to good sleepers (poor sleepers, M = 1.40 pg/mL, SD = 1.01; good sleepers, M = 0.99 pg/mL, SD = .70; F (1, 49) = 4.61, p = .04).
Figure 1.
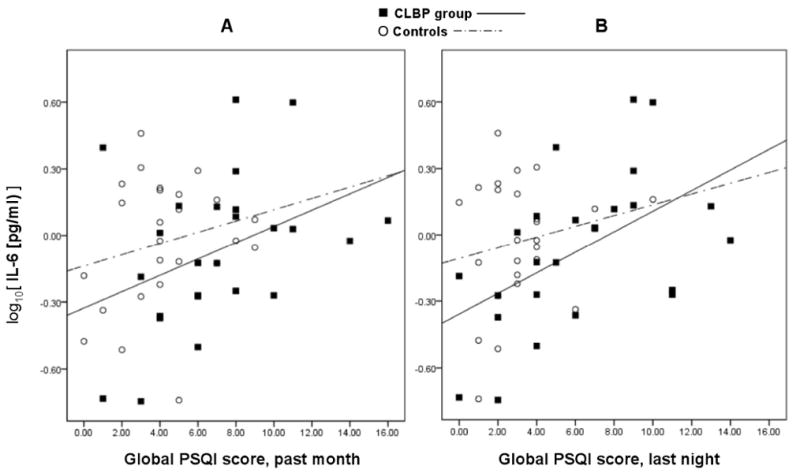
Scatterplots of IL-6 levels by Pittsburgh Sleep Quality Index (PSQI) global sleep quality score at (A) Time 1 and (B) Time 2. Time 1 (A): For CLBP group, R2= .15, B = .04, p = .05 (solid linear regression line); for controls, R2= .05, B = .03, p = .29 (dashed linear regression line).Time 2 (B): For CLBP group, R2= .27, B = .04, p = .02 (solid linear regression line); for controls, R2= .03, B = .02, p = .39 (dashed linear regression line).
Associations among Sleep Quality, IL-6 Levels, and Pain Ratings in Adults with CLBP
Among the CLBP group, PSQI global sleep quality (past month) was strongly associated with MPQ-SF assessments of morning pain, including higher MPQ-SF total scores (r = .51, p = .01), and higher sensory (r = .43, p = .03) and affective pain ratings (r = .56, p = .004). Single-night PSQI scores were also associated with MPQ-SF affective scores (r = .47, p = .02), but not with total or sensory scores. Higher IL-6 levels were also associated with higher MPQ-SF affective pain ratings (r = .46, p = .02).
We explored whether controlling for sleep quality scores would mitigate (and therefore potentially mediate) associations between affective pain ratings and IL-6. When regressing affective pain ratings onto PSQI scores and IL-6, global sleep quality remained a significant predictor of affective pain (B = .16; p = .02), while IL-6 levels were no longer significantly related to pain (B = 1.06; p = .14).
Do Depressive Symptoms Explain Associations among Sleep Disturbance, IL-6, and Pain?
CESD-R scores (minus sleep items) were significantly higher among those with CLBP (M = 17.0; SD = 12.7) compared to those without chronic pain (M = 6.9; SD = 5.3), after adjusting degrees of freedom for unequal variances (t(32.1) = -3.63, p = .001). Among the CLBP group, R coefficients indicated moderate although non-statistically significant associations between CESD-R modified scores and sleep (PSQI past month: r = .37, p = .07; single-night PSQI: r = .24, p = .25), IL-6 (r = .31. p = .13), and pain (MPQ-SF total: r = .35, p = .08), particularly affective pain ratings (affective: r = .39, p = .05; sensory: r = .30, p = .15). Thus, we next conducted separate analyses regressing sleep onto IL-6 and sleep onto pain ratings while controlling for CESD-R modified scores to evaluate whether depressed mood was a potential mediator of sleep and IL-6, and pain, relationships.
On the contrary, controlling for depressive symptoms did not weaken the association between single-night PSQI global score and IL-6 level (B = .48, p = .02), but did render the association between past month PSQI global score and IL-6 level non-significant (B = .31, p = .14). Notably, the associations between sleep quality (past month and previous night) and pain ratings were unchanged after controlling for depressive symptoms.
Discussion
Our findings support a role for sleep disturbance and associated inflammatory processes in understanding bi-directional associations among pain and sleep. In adults with chronic low back pain, we found poorer subjective sleep quality to be associated with higher levels of the inflammatory cytokine IL-6. This association is especially noteworthy given the absence in our sample of common chronic back pain co-morbidities, such as heart disease, rheumatoid arthritis, or diabetes,36 that are characterized by inflammatory dysregulation and elevated IL-6.35, 37 Thus, we suspect our findings may underestimate the bi-directional associations among sleep, IL-6, and pain that may be particularly problematic for individuals with chronic pain co-morbidities and associated inflammatory burden.
Our evidence also suggests that sleep and IL-6 play a role in pain perceptions. Poorer sleep quality was associated with higher morning pain. IL-6 levels related only to affective pain ratings, and this association was mediated by sleep quality. To explore this relationship further, we examined associations among IL-6 and specific affective pain descriptors from the MPQ-SF. We found that higher IL-6 levels were strongly associated with higher ratings of current pain being “tiring/exhausting” (r = .58, p = .002), a relationship that was not attenuated by including sleep quality in the model. This finding suggests that individuals may attribute daytime fatigue to pain, rather than to its more likely source: sleep disturbance and concomitant increases in IL-6 levels. Inflammatory cytokines have multiple effects on the central nervous system, producing “sickness behaviors” that include both hyperalgesia and fatigue.26, 38 Misattribution of negative affect and fatigue to pain may lead to greater fear-avoidant behavior and disability,39 a prospect we did not address in this study but a possible avenue for future research.
As expected, depressive symptom scores were significantly higher among our CLBP group compared to controls.40 However, depression did not fully explain associations among IL-6 and sleep, and poor sleep quality remained a significant predictor of pain after adjusting for depressive symptoms. Given the strong associations between depression and insomnia, and depression and pain, depressed mood likely plays an important role in neurobiological pathways linking pain and sleep. Nevertheless, it is also probable that depression is neither necessary nor sufficient for persistent pain to be underpinned by sleep and inflammatory processes. Additionally, sickness behaviors, all of which can characterize clinical depression,41, 42 may be a consequence of pain and sleep-related inflammatory function. Longitudinal studies evaluating pain, sleep, mood, and inflammation are necessary to fully delineate the nature of these complex and bi-directional associations.
Our findings in the context of emerging literature on inflammation and pain, and sleep, suggest that the mechanisms underpinning increased IL-6 among chronic pain sufferers with sleep disturbance warrant scrutiny. Recent psychoneuroimmunological research suggests a role for inflammatory mediators in hyperalgesia, including exacerbated back pain,7, 11 possibly through direct effects of inflammatory cytokines on pain sensory pathways,10, 12, 43-45 These links, however, are not fully understood. Sleep disturbance, as a modulator of inflammation, may contribute to this process. Stress pathways may be involved as well.46 Notably, nociceptive stimulation can lead to inflammatory reactivity,47, 48 and, in a recent study, IL-6 responses were attenuated in individuals reporting less pain-related catastrophizing.49 Thus, pain-associated stress and anxiety may exacerbate pain through heightened inflammatory responses to nociception. Sleep disturbance, too, can modulate physiological stress reactivity.50, 51 Future research should assess both sleep disturbance and pain-related stress reactivity as a source of increased systemic inflammation in pain disorders.
Limitations of the current study include a small sample size and the inclusion of otherwise healthy and relatively young adults with chronic low back pain. Despite the small sample, we found significant associations among poorer sleep quality and higher IL-6. Further, inflammatory dysregulation increases with age, and higher IL-6 levels are associated with age-related sleep changes.22 Thus, the association we observed here between sleep disturbance and inflammation in younger to middle-aged adults with chronic pain may be particularly problematic for older adults with chronic pain. Controls showed the expected age-related increases in IL-6,34, 35 although the age and IL-6 correlation was non-significant (p = .09). In contrast, for the CLBP group, age showed no association with IL-6 (p = .48). The most parsimonious explanation is that there is no significant association within either group, which may be due to our smaller and younger sample. Alternatively, variation in sleep quality may play a more prominent role in inflammatory function among individuals with chronic pain than does chronological age.
Additional limitations include the use of a modified version of the PSQI to assess sleep quality during a single night, which has not been validated previously, and a single blood draw. Multiple assessments of IL-6 throughout the day on multiple days would allow for analysis of change and improved reliability. Whether venipuncture pain affected outcomes is also unknown. We did not have information on factors such as body mass index, estradiol levels, or 12-hour pre-blood draw diet (as this was not a fasting blood draw), any of which may have related to sleep,52, 53 IL-6,54-56 or pain57, 58 in our sample. Whether these factors can explain our findings warrants future study. Nonetheless, our exclusion criteria do reduce the likelihood that relationships observed here are due to confounding by inflammatory-related conditions. Finally, we assessed fibromyalgia and inflammatory conditions characterized by episodic pain (e.g., rheumatoid arthritis), but we did not specifically inquire about all episodic pain conditions (e.g., migraine, mennorhagia). Thus, we cannot rule out the possibility that these conditions were present and influenced our findings.
In sum, the current findings suggest important roles for sleep disturbance and associated inflammatory processes in chronic pain that require further study. Notably, we have also found that improving sleep in adults with insomnia as a secondary co-morbidity to chronic pain leads to reductions in circulating IL-6.59 Adults with non-malignant, non-site specific chronic pain and insomnia who received a cognitive behavioral treatment (CBT) that included a sleep intervention component had significantly larger declines in IL-6 levels following treatment compared to a group receiving CBT without attention to sleep. These encouraging data underscore significant clinical implications of understanding pathways linking sleep, inflammation, and pain, and support explicit treatment of sleep problems in the management of chronic pain. Given the strong associations we observed between sleep and IL-6 in our study, these implications may indeed be similar in the context of chronic low back pain.
Acknowledgments
This research was supported by National Institutes of Health, National Institute on Aging, R24AG031089-01. We would like to thank Marcia Smart, B.S.N, R.N. for her assistance with data collection for this study.
Contributor Information
Kathi L. Heffner, The Rochester Center for Mind-Body Research, Department of Psychiatry, University of Rochester Medical Center
Christopher R. France, Department of Psychology, Ohio University
Zina Trost, Department of Psychology, Ohio University
H. Mei Ng, Department of Psychology, Ohio University
Wilfred R. Pigeon, Sleep & Neurophysiology Research Laboratory, Department of Psychiatry, University of Rochester Medical Center
References
- 1.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–396. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- 3.Pigeon WR, Park H, Satiea MJ. Sleep and pain. In: Lader M, Cardinali DP, Pandi-Perumal SR, editors. Sleep and sleep disorders: A neuropsychopharmacological approach. New York: Springer; 2006. pp. 201–209. [Google Scholar]
- 4.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 5.Chiu YH, Silman AJ, Macfarlane GJ, Ray D, Gupta A, Dickens C, et al. Poor sleep and depression are independently associated with a reduced pain threshold. Results of a population based study. Pain. 2005;115:316–21. doi: 10.1016/j.pain.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 7.Solovieva S, Leino-Arjas P, Saarela J, Luoma K, Raininko R, Riihimaki H. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109:8–19. doi: 10.1016/j.pain.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Watkins LR, Maier SF. The pain of being sick: Implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 9.Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 10.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 11.Starkweather A, Witek-Janusek L, Mathews HL. Neural-immune interactions: implications for pain management in patients with low-back pain and sciatica. Biol Res Nurs. 2005;6:196–206. doi: 10.1177/1099800404272221. [DOI] [PubMed] [Google Scholar]
- 12.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96:1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. [DOI] [PubMed] [Google Scholar]
- 13.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–7. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mukai E, Nagashima M, Hirano D, Yoshino S. Comparative study of symptoms and neuroendocrine-immune network mediator levels between rheumatoid arthritis patients and healthy subjects. Clin Exp Rheumatol. 2000;18:585–90. [PubMed] [Google Scholar]
- 15.Maes M, Libbrecht I, Van Hunsel F, Lin AH, De Clerck L, Stevens W, et al. The immune-inflammatory pathophysiology of fibromyalgia: increased serum soluble gp130, the common signal transducer protein of various neurotrophic cytokines. Psychoneuroendocrinology. 1999;24:371–83. doi: 10.1016/s0306-4530(98)00087-0. [DOI] [PubMed] [Google Scholar]
- 16.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237:65–8. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 17.Krueger J, Majde J. Host defense. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 4th. Philadelphia: W.B. Saunders Company; 2005. [Google Scholar]
- 18.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 19.Bauer J, Hohagen F, Ebert T, Timmer J, Ganter U, Krieger S, et al. Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Clin Investig. 1994;72:315. doi: 10.1007/BF00180048. [DOI] [PubMed] [Google Scholar]
- 20.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165–72. doi: 10.1016/j.bbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Zoumakis M, Bixler EO, Lin HM, Prolo P, Vela-Bueno A, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 23.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 25.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 26.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trost Z, France CR, Thomas JS. Exposure to movement in chronic back pain: evidence of successful generalization across a reaching task. Pain. 2008;137:26–33. doi: 10.1016/j.pain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, Monk TH, Berman SR, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 30.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 31.Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: Review and revision (CESD and CESD-R) In: Maruish M, editor. Use of psychological testing for treatment planning and outcomes assessment. Princeton, NJ: Lawrence Erlbaum Associates; 2004. pp. 363–377. [Google Scholar]
- 32.Schulberg HC, Saul M, McClelland M, Ganguli M, Christy W, Frank R. Assessing depression in primary medical and psychiatric practices. Arch Gen Psychiatry. 1985;42:1164–70. doi: 10.1001/archpsyc.1985.01790350038008. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 34.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 35.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 36.Ritzwoller DP, Crounse L, Shetterly S, Rublee D. The association of comorbidities, utilization and costs for patients identified with low back pain. BMC Musculoskelet Disord. 2006;7:72. doi: 10.1186/1471-2474-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 39.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 40.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54:399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 41.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–21. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- 44.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–69. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins LR, Maier SF. When Good Pain Turns Bad. Curr Dir Psychol Sci. 2003;12:232–236. [Google Scholar]
- 46.Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain Behav Immun. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Lutgendorf SK, Logan H, Costanzo E, Lubaroff D. Effects of acute stress, relaxation, and a neurogenic inflammatory stimulus on interleukin-6 in humans. Brain Behav Immun. 2004;18:55–64. doi: 10.1016/s0889-1591(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 48.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–17. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–44. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meerlo P, Koehl M, van der Borght K, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 51.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–13. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 33:647–56. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 55.Yasui T, Maegawa M, Tomita J, Miyatani Y, Yamada M, Uemura H, et al. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56:396–403. doi: 10.1016/j.maturitas.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–50. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 57.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: epidemiology, mechanisms, and implications. Headache. 50:631–48. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, Vosburg SK, Comer SD. Sex differences and hormonal influences on response to mechanical pressure pain in humans. J Pain. 11:330–42. doi: 10.1016/j.jpain.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pigeon WR, Perlis ML, Swan J, Costescu S, Matteson-Rusby S, Walton J, et al. CBT for Insomnia is associated with decreased fatigue and diminished IL-6 levels in chronic pain patients. Sleep. 2008;31S:A253. [Google Scholar]


