Abstract
Tim8 and Tim13 are non-essential, conserved proteins of the mitochondrial intermembrane space, which are organized in a hetero-oligomeric complex. They are structurally related to Tim9 and Tim10, essential components of the import machinery for mitochondrial carrier proteins. Here we show that the TIM8–13 complex interacts with translocation intermediates of Tim23, which are partially translocated across the outer membrane but not with fully imported or assembled Tim23. The TIM8–13 complex binds to the N-terminal or intermediate domain of Tim23. It traps the incoming precursor in the intermembrane space thereby preventing retrograde translocation. The TIM8–13 complex is strictly required for import of Tim23 under conditions when a low membrane potential exists in the mitochondria. The human homologue of Tim8 is encoded by the DDP1 (deafness/dystonia peptide 1) gene, which is associated with the Mohr–Tranebjaerg syndrome (MTS), a progressive neurodegenerative disorder leading to deafness. It is demonstrated that import of human Tim23 is dependent on a high membrane potential. A mechanism to explain the pathology of MTS is discussed.
Keywords: membrane insertion/mitochondria/protein translocation/TIM8–13 complex/Tim23
Introduction
Nuclear-encoded mitochondrial precursor proteins are imported and sorted to mitochondrial subcompartments by preprotein translocases that are located in the outer and inner membrane. The outer membrane contains one general preprotein translocase, the TOM complex, which is used by virtually all nuclear-encoded preproteins (the import pathway of apo-cytochrome c is not clear) (Ryan and Jensen, 1995; Schatz, 1996; Pfanner and Meijer, 1997; Bauer et al., 2000). The inner membrane contains two distinct preprotein import systems, the TIM23 complex and the TIM22 complex, which differ in their specificity for preprotein substrates (Sirrenberg et al., 1996, 1998; Kerscher et al., 1997; Koehler et al., 1998a,b; Bauer et al., 2000). The TIM23 complex imports precursors with a matrix targeting signal into the matrix space and into the inner membrane (Bauer et al., 1996; Sirrenberg et al., 1996). Initiation of translocation through the TIM23 complex requires an inner membrane potential, Δψ (Bauer et al., 1996). Further translocation of a preprotein into the matrix is then driven by ATP-dependent reaction cycles of the mitochondrial heat shock protein 70 (mt-Hsp70), its co-chaperone Mge1p and the peripheral membrane protein Tim44, which is associated with the outlet of the import channel (Bauer et al., 2000).
The TIM22 complex imports members of the mitochondrial carrier family and a number of other integral inner membrane proteins that are synthesized without a matrix targeting signal (Sirrenberg et al., 1996, 1998; Kerscher et al., 1997; Koehler et al., 1998a,b; Adam et al., 1999; Endres et al., 1999; Leuenberger et al., 1999). Insertion of the precursors into the inner membrane is strictly dependent on Δψ (Pfanner and Neupert, 1985; Káldi et al., 1998).
Both TIM complexes co-operate with the TOM complex to form so-called translocation contact sites, which mediate passage of precursors across the aqueous intermembrane space (Pfanner, 1998; Bauer et al., 2000). Translocation contact sites between the TOM complex and the TIM23 complex are facilitated by the N-terminal domain of Tim23 (amino acid residues 1–50), which is tethered to the outer membrane (Donzeau et al., 2000). When TOM complexes and TIM23 complexes come into contact by lateral diffusion the precursor is directly transferred from the trans-site of the TOM complex to the presequence receptor domain of Tim23 (amino acid residues 50–100), which is exposed in the intermembrane space (Bauer et al., 1996).
Precursors of carrier proteins are transferred from the TOM complex to the TIM22 complex with the help of the TIM9–10 complex of the intermembrane space (Sirrenberg et al., 1998; Endres et al., 1999; Leuenberger et al., 1999). Tim9 and Tim10 are zinc-finger proteins, which interact with translocation intermediates of carrier proteins that are partially translocated across the TOM complex. The TIM9–10 complex delivers the TOM-bound precursor to the TIM9–10–12 complex, which is peripherally associated with the TIM22 complex and thereby triggers the formation of translocation contact sites (Endres et al., 1999).
Tim8 and Tim13 are structurally related to Tim9 and Tim10 and are organized in a hetero-oligomeric complex in the intermembrane space, the TIM8–13 complex (Adam et al., 1999; Bauer et al., 1999b; Koehler et al., 1999). They are, in contrast to Tim9 and Tim10, not essential for the viability of yeast. The TIM8–13 complex interacts with translocation intermediates of Tim23 but is not required for import of Tim23 under normal growth conditions (Leuenberger et al., 1999). Thus, the function of the TIM8–13 complex is not fully understood.
Tim8 and Tim13 are evolutionarily conserved (Bauer et al., 1999b). Mutations in the DDP1 (deafness/dystonia peptide 1) gene, which encodes the human homologue of Tim8, are associated with Mohr–Tranebjaerg syndrome (MTS) (Jin et al., 1996, 1999; Koehler et al., 1999; Bauer et al., 2000). MTS is a neurodegenerative disorder characterized by progressive sensorineural hearing loss, dystonia, mental retardation and blindness.
In the present study we demonstrate that the TIM8–13 complex interacts in a metal-dependent manner with Tim23 precursors that are partially translocated across the outer membrane while it does not bind to fully imported or assembled Tim23. Tim8 and Tim13 bind to the intermediate domain of Tim23 (amino acid residues 30–90). Binding of the TIM8–13 complex facilitates translocation of this domain across the outer membrane and prevents retrograde translocation. The function of the TIM8–13 complex becomes essential in vivo and in vitro at a low mitochondrial membrane potential. Import of human Tim23 into yeast and mammalian mitochondria requires a high membrane potential. Mutations in DDP1 may thus affect the biogenesis of human Tim23 when mitochondria generate only a low membrane potential.
Results
Tim8 and Tim13 facilitate translocation of Tim23 across the outer membrane
Tim8 and Tim13 are non-essential components, which are localized in the mitochondrial intermembrane space (Koehler et al., 1999). The TIM8–13 complex is not required for import of Tim23 but increases slightly the import efficiency in vitro (Leuenberger et al., 1999). We constructed a yeast strain, Δ8/Δ13, where the TIM8 and TIM13 genes were deleted in order to investigate the role of the TIM8–13 complex in import of Tim23 (see Materials and methods).
Radiolabelled Tim23 precursor was synthesized in reticulocyte lysate in the presence of [35S]methionine and incubated with energized mitochondria from Δ8/Δ13 cells and from wild-type (WT) control cells. When mitochondria from WT cells were subsequently treated with trypsin, the radiolabelled Tim23 was protected from degradation, indicating that the precursor was imported into the mitochondria (Figure 1A, left panel). Upon treatment with proteinase K (PK), Tim23 was clipped and a fragment, Tim23*, was produced which indicates that the N-terminus of Tim23 was sorted into the outer membrane and exposed on the surface of the mitochondria (Donzeau et al., 2000). When mitoplasts were generated and treated with trypsin, a 16 kDa fragment, Tim23′, was formed, which included most of the intermediate domain and the membrane-integrated C-terminal portion of Tim23 (Figure 1B, upper left panel) (Káldi et al., 1998). PK treatment led to the formation of a 14 kDa fragment, Tim23′′, which corresponded to the membrane-integrated portion (Donzeau et al., 2000). This indicates that the Tim23 precursor was inserted into the inner membrane. Tim23 was also imported into mitochondria from Δ8/Δ13 cells and inserted into the inner membrane (Figure 1A, right panel and 1B, lower left panel), demonstrating that Tim8 and Tim13 are not required for the import of Tim23. The import efficiency was reduced to ∼75%, while import of pSu9-DHFR into the matrix and also import of ADP/ATP carrier into the inner membrane was not affected, supporting the view that the TIM8–13 complex may assist the import process (Leuenberger et al., 1999).
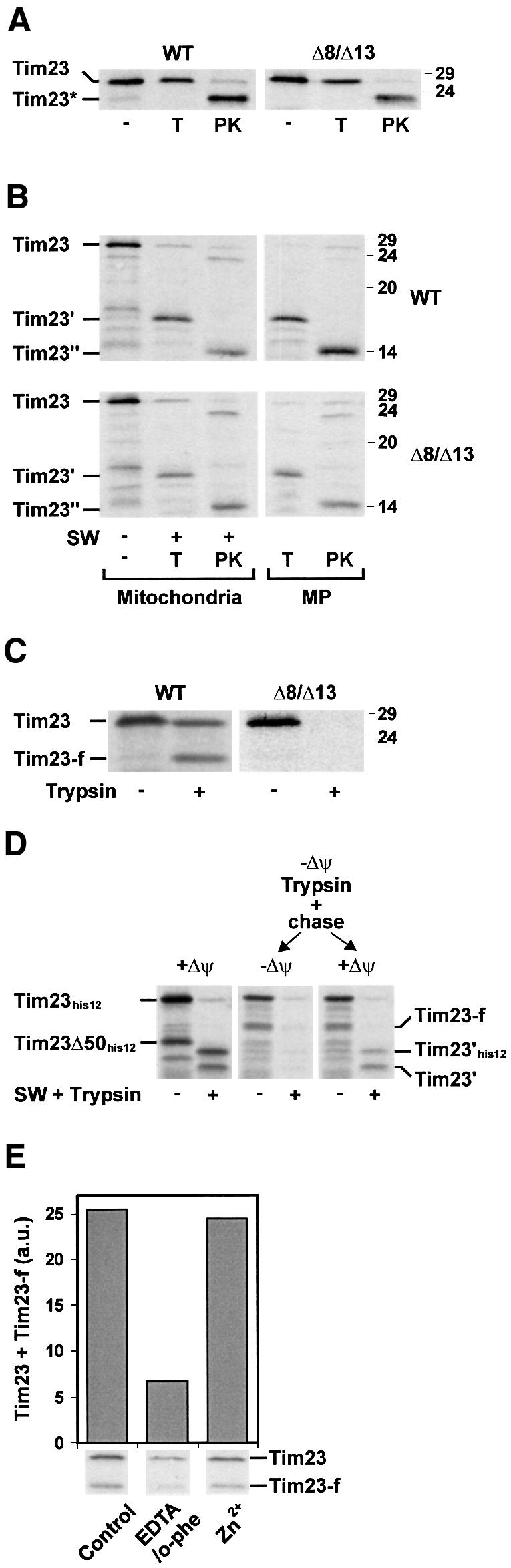
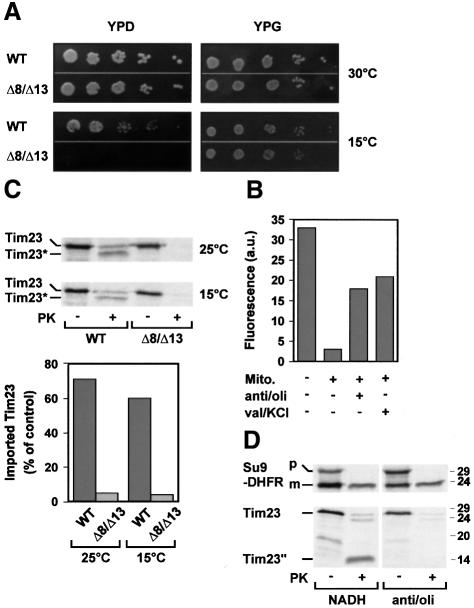
Fig. 1. Import of Tim23 into wild-type (WT) mitochondria and into mitochondria lacking Tim8 and Tim13 Δ8/Δ13. (A) Radiolabelled Tim23 precursor was incubated for 10 min at 25°C with WT and Δ8/Δ13 mitochondria that were energized with NADH. Subsequently the samples were diluted 10-fold with 0.6 M sorbitol, 20 mM HEPES–KOH pH 7.2 and treated with 100 µg/ml trypsin (T) or 200 µg/ml proteinase K (PK) when indicated. Mitochondria were reisolated and import was analysed by SDS–PAGE and autoradiography. Tim23*, PK-resistant fragment of imported Tim23. The electrophoretic mobility of molecular mass standards (in kDa) is indicated on the right. (B) Insertion of Tim23 into the inner membrane. Tim23 precursor was imported for 20 min at 25°C into mitochondria and mitoplasts (MP) from WT and Δ8/Δ13 cells. Subsequently the mitochondria (left panels) were converted into mitoplasts in the presence of 50 µg/ml trypsin (T) or 100 µg/ml PK, as indicated. Mitoplasts (right panels) were directly treated with trypsin and PK. Protease-resistant fragments of inserted Tim23 generated by trypsin (Tim23′) and PK (Tim23′′) are indicated. (C) Partial translocation of Tim23 across the outer membrane in the absence of Δψ. WT and Δ8/Δ13 mitochondria were incubated with 1 µM valinomycin and 25 µM FCCP to dissipate Δψ. Tim23 precursor was imported for 15 min at 25°C and samples were treated with 100 µg/ml trypsin when indicated. Tim23-f, trypsin-resistant N-terminal fragment of Tim23 that was partially translocated across the outer membrane. (D) Left panel, Tim23his12 was imported into mitochondria in the presence of Δψ. Central and right panels, mitochondria were preincubated with 50 µM carbonyl cyanide-m-chloro-phenylhydrazone (CCCP) to dissipate Δψ, and Tim23his12 was imported for 15 min at 25°C. Samples were treated with trypsin and mitochondria were reisolated in import buffer containing either 1 µM valinomycin (–Δψ, central panel) or 3 mM DTT to inactivate CCCP and 5 mM NADH (+Δψ, right panel). Where indicated mitochondria were converted to mitoplasts and treated with trypsin. Tim23Δ50his12, translation product starting at methionine 51 of Tim23his12; Tim23-f, trypsin-resistant N-terminal fragment of partially translocated Tim23his12; Tim23′his12 and Tim23′, trypsin-resistant portions of Tim23his12 that are inserted into the inner membrane. (E) Zn2+ dependence of translocation of Tim23 across the outer membrane. WT mitochondria were incubated with 10 mM EDTA and 2 mM o-phenanthroline (EDTA/o-phe) or left untreated (Control). An aliquot of the EDTA/o-phe-treated mitochondria was re-isolated in the presence of 0.1 mM ZnCl2 (Zn2+). Subsequently Δψ was dissipated with valinomycin/FCCP and then Tim23 precursor was imported for 15 min at 25°C. Samples were treated with 100 µg/ml trypsin (T) and subjected to SDS–PAGE and autoradiography (lower panel). Trypsin-resistant Tim23 and Tim23-f were quantified with a phosphoimaging system (upper panel).
In order to release soluble components of the intermembrane space, mitochondria from WT and Δ8/Δ13 cells were converted into mitoplasts. The mitoplasts were then incubated with Tim23 precursor (Figure 1B, right panels). Tim23 was efficiently imported into the mitoplasts generated from WT and Δ8/Δ13 mitochondria.
To investigate the possibility that the TIM8–13 complex plays a role in the translocation of Tim23 across the outer membrane, mitochondria were treated with valinomycin and carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) to dissipate Δψ and then Tim23 precursor was added (Figure 1C). In the absence of Δψ, a translocation intermediate of Tim23 accumulated in WT mitochondria. The translocation intermediate was clipped by trypsin to a specific fragment, Tim23-f (Figure 1C, left panel), but was completely degraded by PK (data not shown). This indicates that the precursor was partially translocated across the outer membrane. It was presumably still associated with the TOM complex in a similar manner to stage III translocation intermediates of the ADP/ATP carrier (Endres et al., 1999). When Tim23 precursor was incubated with Δ8/Δ13 mitochondria in the absence of Δψ no trypsin-resistant translocation intermediate was detected (Figure 1C, right panel). Therefore, the TIM8–13 complex is required for the partial translocation of Tim23 across the outer membrane in the absence of Δψ. In the presence of Δψ insertion of the C-terminal portion of Tim23 into the inner membrane is apparently sufficient to drive complete import of the N-terminal portion without assistance of the TIM8–13 complex.
For further characterization of the translocation intermediate we used Tim23his12, which carries a C-terminal tag of 12 histidine residues. When radiolabelled Tim23his12 was incubated with energized WT mitochondria the precursor was efficiently imported and inserted into the inner membrane (Figure 1D, left panel). Thus, when mitoplasts were generated after the import reaction and treated with trypsin, Tim23his12 was clipped and two fragments were generated. These fragments, Tim23′his12 and Tim23′, correspond to the membrane-inserted portion of Tim23 with and without his-tag, respectively. Tim23his12 was then incubated with mitochondria in the absence of Δψ. In order to test whether the precursor accumulates as a productive translocation intermediate the mitochondria were treated with trypsin to remove surface-bound precursors (Figure 1D, central and right panels). Like authentic Tim23, Tim23his12 was resistant to trypsin treatment. A fraction was clipped to a fragment of the same size as Tim23-f (i.e. without the his-tag), supporting the notion that the C-terminal portion of the translocation intermediate was exposed on the surface of the mitochondria (Káldi et al., 1998). The translocation intermediate was not inserted into the inner membrane and was therefore completely degraded when mitoplasts were generated and treated with trypsin (Figure 1D, central panel). However, when Δψ was restored, the translocation intermediate was imported and inserted into the inner membrane (Figure 1D, right panel).
Tim8 and Tim13 contain a putative Cys4 zinc-binding motif. To address whether metal ions were required for the accumulation of the translocation intermediate, WT mitochondria were pre-incubated with the chelating agents EDTA and o-phenanthroline (o-phe). Subsequently, Δψ was dissipated and Tim23 precursor was added. The formation of the trypsin-resistant translocation intermediate was significantly reduced when mitochondria were pretreated with EDTA/o-phe (Figure 1E). The translocation intermediate was efficiently formed again when the mitochondria were re-isolated in the presence of Zn2+ ions. This suggests that the formation of the translocation intermediate of Tim23 requires Zn2+ ions. When Δ8/Δ13 mitochondria were used the translocation intermediate of Tim23 was formed neither in the presence of EDTA/o-phe nor in the presence of Zn2+ ions (data not shown). In the presence of Δψ, import of Tim23 was not inhibited by EDTA/o-phe (data not shown), suggesting that other small Tim proteins such as Tim9 and Tim10 are not required.
Tim8 and Tim13 contact translocation intermediates of Tim23
To address whether the TIM8–13 complex is in close proximity to the translocation intermediate, Tim23 precursor was incubated with mitochondria in the absence of Δψ and chemical cross-linking was performed. Tim23 precursor was efficiently cross-linked to Tim8 and Tim13 in WT mitochondria while no cross-links were observed in Δ8/Δ13 mitochondria (Figure 2A). When WT mitochondria were pre-incubated with EDTA/o-phe, the efficiency of cross-linking was reduced (Figure 2B). Cross-linking was restored when Zn2+ ions were added back. Thus, the interaction of the TIM8–13 complex with the translocation intermediate of Tim23 was dependent on divalent metal ions.
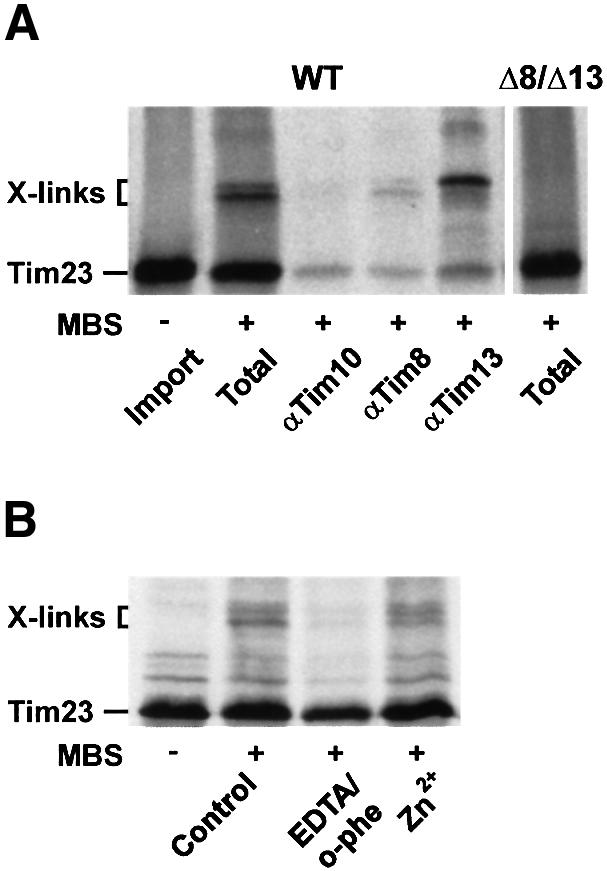
Fig. 2. Interaction of Tim8 and Tim13 with translocation intermediates of Tim23. (A) WT mitochondria were incubated with Tim23 precursor for 15 min at 25°C in the absence of Δψ (Import). Then 100 µM MBS was added and samples were incubated on ice for 30 min (Total). Aliquots were removed, mitochondria were lysed with 1% SDS and the samples were then diluted 20-fold with Tris-buffered saline containing 0.5% Triton X-100. After a clarifying spin the supernatant fractions were subjected to immunoprecipitation with antibodies against Tim10, Tim8 and Tim13. Right panel, cross-linking of Tim23 in Δ8/Δ13 mitochondria. X-links, Tim23-specific adducts. (B) Zn2+-dependent interaction of Tim8 and Tim13 with Tim23 precursor. WT mitochondria (Control) were incubated with EDTA/o-phe, and, where indicated, re-isolated in the presence of 0.1 mM ZnCl2 (see above). Subsequently Tim23 precursor was imported in the absence of Δψ and the samples were cross-linked with MBS.
Fully imported Tim23 was not cross-linked to the TIM8–13 complex (data not shown). Thus, the adducts with Tim8 and Tim13 were specifically formed with translocation intermediates of Tim23. Similarly, endogenous Tim23 was not cross-linked (data not shown). The translocation intermediate of Tim23 was cross-linked with low efficiency to Tim10 in WT mitochondria (Figure 2A) but not in Δ8/Δ13 mitochondria. Yet, cross-links with Tim23 precursor were also observed when mitochondria were used that were depleted of Tim10 (data not shown), indicating that the interaction of the TIM8–13 complex with the precursor was independent of the TIM9–10 complex. The low efficiency of cross-linking with Tim10 could indicate an interaction of the Tim23 translocation intermediate with the membrane-bound TIM9–10–12 complex that is associated with the TIM22 complex (Sirrenberg et al., 1998; Adam et al., 1999).
Tim8 and Tim13 interact with the N-terminal half of the Tim23 precursor
To identify the region in Tim23 that interacts with Tim8 and Tim13, we constructed a number of truncated Tim23 precursors (Figure 3A, left). In the presence of Δψ radiolabelled precursors of Tim23Δ20, Tim23Δ30, Tim23Δ50 and Tim23Δ55–91 were imported into WT and Δ8/Δ13 mitochondria and inserted into the inner membrane, while Tim23(1–180) and Tim23(1–144), which lack the Δψ-dependent insertion signal in the C-terminal portion of Tim23 (Káldi et al., 1998), were not inserted (data not shown). As expected, none of the precursors was imported into mitochondria from Δ8/Δ13 cells in the absence of Δψ (data not shown). In WT mitochondria, the N-terminally truncated precursors Tim23Δ20 and Tim23Δ30 and the C-terminally truncated precursors Tim23(1–180) and Tim23(1–144) accumulated as intermediates, which were partially translocated across the outer membrane. Thus, they were either partially or completely resistant to trypsin treatment (Figure 3A, right) but degraded by PK (data not shown). Tim23Δ50 and Tim23Δ55–91 were, however, not imported (Figure 3A, right).
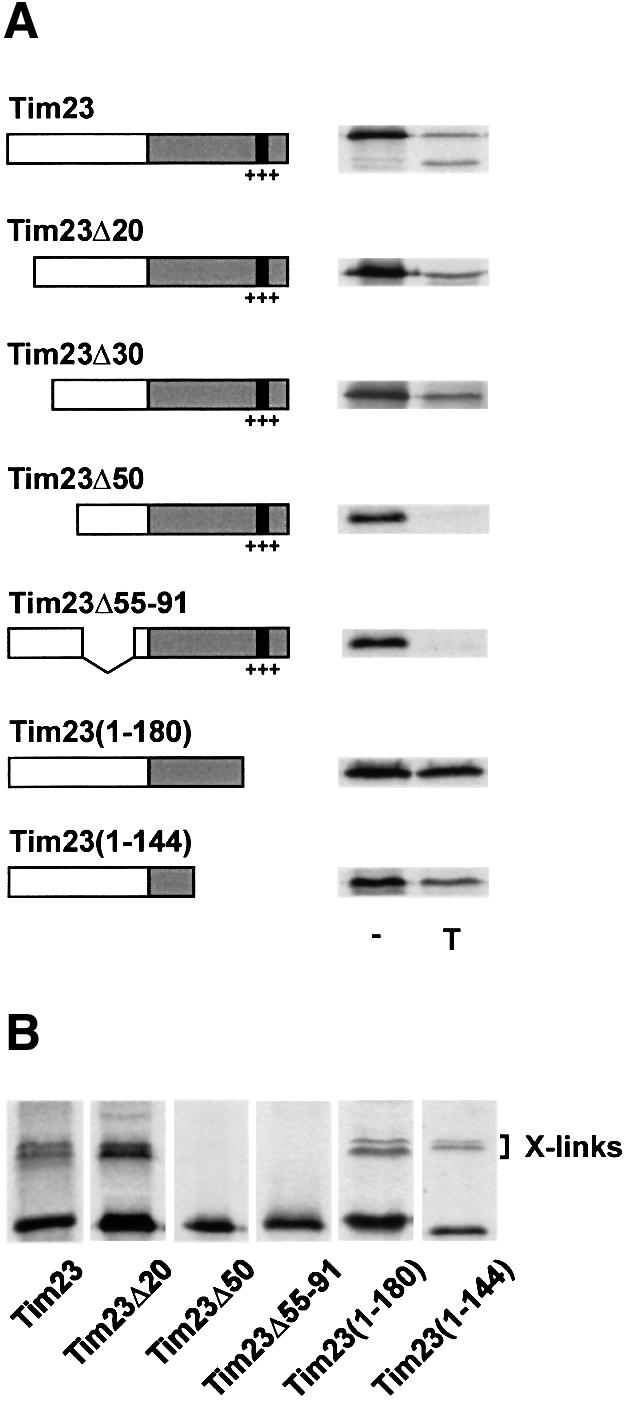
Fig. 3. Interaction of the TIM8–13 complex with the N-terminal portion of Tim23. (A) Translocation of Tim23-derived precursors across the outer membrane. Tim23-derived precursor constructs are schematically outlined. The grey box indicates the C-terminal portion, which is integrated into the inner membrane, the black box and the +++ refer to the Δψ-dependent import signal (Káldi et al., 1998). Numbers refer to amino acid residues of Tim23, deletions are indicated by Δ. The precursors were incubated with WT mitochondria in the absence of Δψ for 15 min at 25°C. Aliquots were treated with100 µg/ml trypsin (T). (B) Cross-linking of Tim8 and Tim13 with Tim23-derived precursor constructs. Precursors were imported into WT mitochondria in the absence of Δψ and then cross-linked with 100 µM MBS.
In order to detect an interaction of the Tim23-derived precursors with the TIM8–13 complex cross-linking was performed (Figure 3B). Tim23Δ20, Tim23(1–180) and Tim23(1–144) were cross-linked to the TIM8–13 complex, while no adducts with Tim23Δ50 and Tim23Δ55–91 were detected (Figure 3B). Together, these observations suggest that the TIM8–13 complex interacts with the N-terminal portion of Tim23, presumably between amino acid residues 30 and 90.
For confirmation, we fused the N-terminal half of Tim23 (amino acid residues 1–101) to the ADP/ATP carrier, AAC (Figure 4A). In the presence of Δψ, the hybrid precursor, Tim23(1–101)–AAC, was efficiently imported into mitochondria from WT and Δ8/Δ13 cells (Figure 4B). To analyse the interaction of Tim23 (1–101)–AAC with small Tim proteins of the intermembrane space, the chimeric precursor was incubated with mitochondria in the absence of Δψ and cross-linking was performed (Figure 4C). Cross-linked adducts were subsequently identified by immunoprecipitation with antibodies against Tim10 and Tim13. As expected, the translocation intermediate was cross-linked with Tim10, which is strictly required for the import of carrier proteins (Koehler et al., 1998a; Sirrenberg et al., 1998; Endres et al., 1999). However, the arrested Tim23(1–101)–AAC precursor was also cross-linked to Tim13 (Figure 4C). Since the AAC does not interact with Tim13 (Koehler et al., 1999), these observations indicate that the portion corresponding to Tim23(1–101) interacts with the TIM8–13 complex. Similarly, Tim23(1–101)–Tim17, a fusion of the N-terminal half of Tim23 with Tim17, was cross-linked to the TIM8–13 complex while Tim17 was not (data not shown).
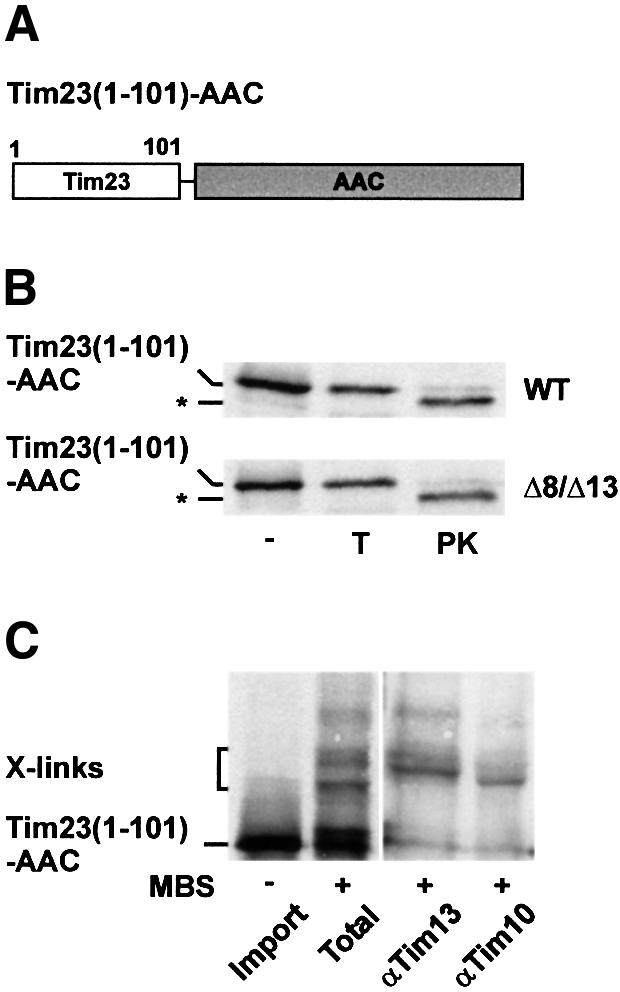
Fig. 4. Interaction of Tim23(1–101)–AAC with small Tim proteins of the intermembrane space. (A) Schematic outline of Tim23(1–101)–AAC. (B) Radiolabelled Tim23(1–101)–AAC precursor was imported for 10 min at 25°C into WT and Δ8/Δ13 mitochondria, which were energized with NADH. Samples were treated with100 µg/ml trypsin (T) or 200 µg/ml PK. *, fragment generated by cleavage of Tim23 by PK. (C) Cross-linking of Tim23(1–101)–AAC with small Tim proteins. Tim23(1–101)–AAC was imported into WT mitochondria in the absence of Δψ (Import) and then cross-linked with 100 µM MBS (Total). Aliquots were subjected to immunoprecipitation with antibodies against Tim13 and Tim10 as described in Figure 2. X-links, Tim23(1–101)–AAC-specific adducts.
In summary, the TIM8–13 complex interacts with the N-terminal portion of Tim23 and facilitates its translocation across the outer membrane. In the absence of Δψ, the TIM8–13 complex is strictly required for the partial translocation of Tim23 across the outer membrane. In the presence of Δψ the insertion signal in the C-terminal portion of Tim23 is sufficient to drive efficient import and insertion of Tim23 even in the absence of the TIM8–13 complex.
Tim8 and Tim13 facilitate import of Tim23 at low membrane potential
Tim8 and Tim13 are not essential for the viability of yeast. To assess the function of Tim8 and Tim13, we analysed growth of Δ8/Δ13 cells under varying conditions. Δ8/Δ13 cells grew at 30 and 15°C in media containing the non-fermentable carbon source, glycerol (Figure 5A). In media containing glucose as a carbon source Δ8/Δ13 cells grew at 30 but not at 15°C.
Fig. 5. The TIM8–13 complex facilitates import of Tim23 at low Δψ. (A) Cold-sensitive growth phenotype of Δ8/Δ13 cells in the presence of glucose. Δ8/Δ13 cells and the parental WT cells were grown at 30°C in YPD medium to an OD578 of 1. The cultures were subjected to serial 10-fold dilutions and 2 µl aliquots were spotted onto YPD and YPG agar plates. The plates were incubated for 2 days at 30°C and for 3 days at 15°C. (B) Assessment of Δψ. Mitochondria from WT cells (100 µg/ml) were incubated for 10 min in import buffer with 2 mM 3,3′-dipropylthiadicarbocyanide iodide. Where indicated the samples contained 8 µM antimycin, 20 µM oligomycin and 2.5 mM ATP to lower Δψ or 1 µM valinomycin (val) and 80 mM KCl to dissipateΔψ. Subsequently the mitochondria were removed by centrifugation and the fluorescence (excitation at 622 nm, emission at 670 nm) ofthe supernatant fractions was determined at 25°C (Rassow, 1999). (C) Import of Tim23 at low Δψ. WT and Δ8/Δ13 mitochondria were incubated with antimycin, oligomycin and ATP to generate a low Δψ. Tim23 precursor was imported for 15 min at 25 and 15°C and samples were treated with 200 µg of PK as indicated. Samples were analysed by SDS–PAGE (upper panel) and quantified with a phosphoimager (lower panel). (D) Import of precursors into mitoplasts at high and low Δψ. WT mitochondria were converted into mitoplasts by swelling in 20 mM HEPES–KOH pH 7.2. The mitoplasts were incubated with NADH or anti/oli. pSu9(1–94)-DHFR (upper panel) and Tim23 precursor (lower panel) were imported into the mitoplasts for 15 min at 25°C. When indicated the mitoplasts were treated with 200 µg/ml PK. p, precursor; m, mature form of Su9(1–94)-DHFR; Tim23′′, PK-resistant fragment of inserted Tim23.
When cells are grown in the presence of glucose Δψ is low, generated mainly by the ADP/ATP carrier, which then shuttles ATP from the cytosol into the matrix space in exchange for ADP from the matrix. We therefore examined the possibility that the TIM8–13 complex is required for Tim23 import at low Δψ. In order to generate a low Δψ, isolated mitochondria were pre-incubated with antimycin, oligomycin and ATP (anti/oli). To assess the strength of Δψ, the mitochondria were incubated with 3,3′- dipropylthiadicarbocyanide iodide [DiSC3(5)], a fluorescent dye that interacts with mitochondrial membranes in a Δψ-dependent manner (Sims et al., 1974). Subsequently the mitochondria were removed by centrifugation and the fluorescence of the supernatant was measured (Figure 5B). DiSC3(5) was efficiently taken up by mitochondria with high Δψ and the fluorescence in the supernatant was low. In contrast, the fluorescence in the supernatant was high when Δψ was dissipated with valinomycin (Figure 5B). In the presence of anti/oli the fluorescence was also high, but significantly lower than with valinomycin, indicating that a low Δψ was generated under these conditions (Figure 5B).
Mitochondria from WT and Δ8/Δ13 cells were incubated at 25 and 15°C with anti/oli to establish a low Δψ. Tim23 precursor was added and import was analysed. Tim23 was imported at both temperatures into WT mitochondria but not into mitochondria from Δ8/Δ13 cells (Figure 5C). Thus, the TIM8–13 complex apparently facilitates import of Tim23 under conditions of low Δψ. For further analysis, mitochondria from WT cells were incubated in hypotonic buffer solution to generate mitoplasts in order to release the TIM8–13 complex from the intermembrane space. Subsequently, the mitoplasts were supplemented with either NADH or anti/oli to strengthen or weaken Δψ, respectively. Subsequently the mitoplasts were incubated with Tim23 precursor and, for control, with the matrix-targeted precursor pSu9(1–94)-DHFR. pSu9(1–94)-DHFR was efficiently imported into mitoplasts energized with either NADH or anti/oli (Figure 5D, upper panel), indicating that the levels of Δψ were sufficiently high to promote import into the matrix. Tim23 was efficiently imported into mitoplasts energized with NADH but not into mitoplasts in the presence of anti/oli (Figure 5D, lower panel). Thus, at low Δψ the TIM8–13 complex is required to present the Tim23 precursor to the TIM22 complex in the inner membrane.
Import of human Tim23 requires a high membrane potential
MTS is associated with mutations in the DDP1 gene, which encodes the human Tim8a protein (Jin et al., 1996; Bauer et al., 1999b). We analysed the import of human Tim23 (Bauer et al., 1999a). Radiolabelled precursor of human Tim23 was synthesized in vitro and incubated with energized mitochondria from WT and Δ8/Δ13 yeast cells (Figure 6A). Import of human Tim23 into mitochondria from Δ8/Δ13 cells was slightly reduced (∼25%) compared with import into WT mitochondria. Thus, like yeast Tim23, import of human Tim23 was not strongly dependent on the TIM8–13 complex at high levels of Δψ. Mitochondria were then incubated with anti/oli to generate a low Δψ, and for control with NADH, and human and yeast Tim23 precursors were added (Figure 6B). Both Tim23 precursors were efficiently imported at high Δψ. At low Δψ, import of yeast Tim23 was reduced 2-fold, while import of human Tim23 was reduced at least 8-fold (Figure 6B). The same results were obtained when rat liver mitochondria were used. At low levels of Δψ, import of yeast Tim23 was reduced ∼2-fold while import of human Tim23 was inefficient and close to background levels (Figure 6B). This would suggest that the import of human Tim23 requires higher Δψ levels than import of yeast Tim23.
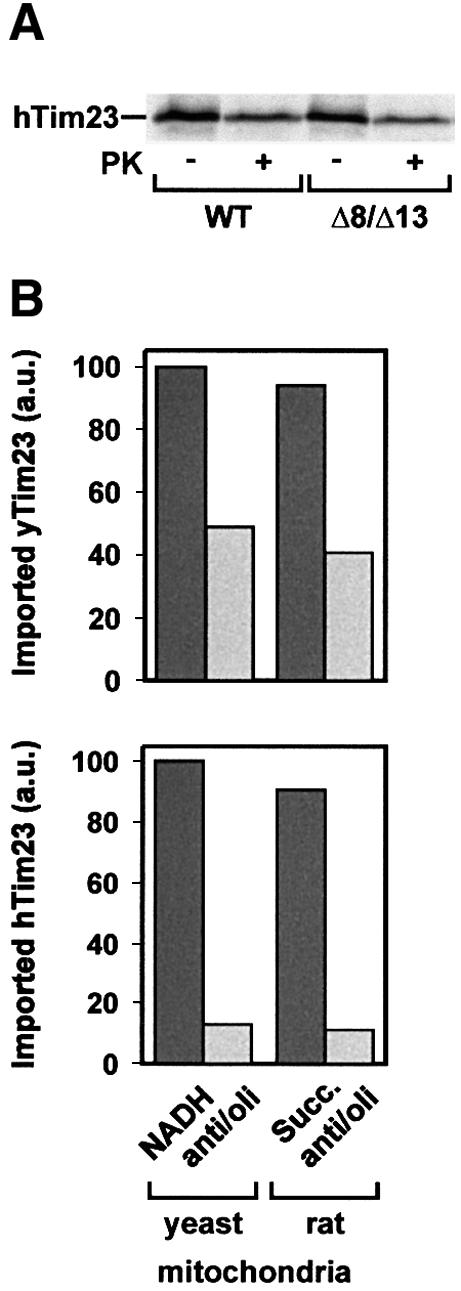
Fig. 6. Import of human Tim23. (A) Radiolabelled human Tim23 (hTim23) precursor was synthesized in reticulocyte lysate and imported for 15 min at 25°C into WT and Δ8/Δ13 mitochondria from yeast that were energized with NADH. Samples were treated with 100 µg/ml PK. (B) Import of human Tim23 requires high Δψ. The precursors of yeast Tim23 (yTim23) (upper panel) and human Tim23 (lower panel) were imported into yeast mitochondria and mitochondria from rat liver that were energized with NADH or succinate (Succ.), respectively. Samples were treated with 100 µg/ml PK, subjected to SDS–PAGE and imported Tim23 was quantified with a phosphoimaging system.
Discussion
Biogenesis of nuclear-encoded mitochondrial inner membrane proteins is a multi-step process of considerable complexity. When the precursors are delivered to the receptors of the TOM complex cytosolic chaperones are released to allow translocation across the outer membrane. In the absence of chaperones, however, hydrophobic precursors are susceptible to misfolding and loss of translocation competence. The TIM22-dependent import pathway mediates the translocation of subsets of hydrophobic inner membrane proteins (Sirrenberg et al., 1996). Mitochondrial carrier proteins constitute the major class of precursors that are imported via this pathway (Sirrenberg et al., 1996, 1998; Koehler et al., 1998a,b; Endres et al., 1999). Import of carrier proteins is strictly dependent on the TIM9–10 and TIM9–10–12 complexes, which are located in the intermembrane space and mediate the transfer of the precursors from the TOM complex to the TIM22 complex in the inner membrane (Koehler et al., 1998a,b; Sirrenberg et al., 1998; Adam et al., 1999; Endres et al., 1999).
Tim23, a polytopic component of the inner membrane translocase for matrix-targeted precursors, is also imported via the TIM22 complex but does not require the assistance of the TIM9–10 complex (Káldi et al., 1998; Leuenberger et al., 1999). Rather, Tim23 precursor interacts with the structurally related TIM8–13 complex (Leuenberger et al., 1999).
The data show that the TIM8–13 complex interacts specifically with translocation intermediates of Tim23 that are partially translocated across the outer membrane. It does not, however, bind to fully imported or assembled Tim23. Thus, binding sites are either inaccessible in assembled Tim23 or the TIM8–13 complex recognizes translocation intermediates specifically in association with the TOM complex. The interaction of the TIM8–13 complex with Tim23 precursor is dependent on divalent metal ions, suggesting that Tim8 and Tim13 are Zn2+-finger proteins like the structurally related Tim10 (Sirrenberg et al., 1998). Tim8 and Tim13 bind to the N-terminal/intermediate domain of Tim23, presumably between amino acid residues 30 and 90. A sequence motif in this region, LHPLA, is strictly conserved in Tim23 proteins of fungi and is similar to corresponding segments of Tim23 proteins from other eukaryotes (data not shown). Whether this motif is recognized by the TIM8–13 complex is presently under investigation.
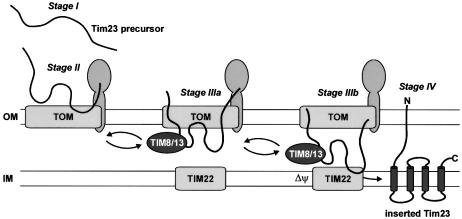
The TIM8–13 complex is not required for import of Tim23 under all conditions. The Δψ-responsive import signal in the C-terminal portion of Tim23 (Káldi et al., 1998) is sufficient and necessary to drive import and membrane insertion of Tim23 even without the assistance of the TIM8–13 complex. What then is the role of the TIM8–13 complex in the biogenesis of Tim23? In the absence of Δψ, when membrane insertion is blocked, the TIM8–13 complex is strictly required to accumulate precursor, which is partially translocated across the TOM complex. Thus, the TIM8–13 complex binds to the incoming Tim23 precursor and probably prevents retrograde translocation through the TOM complex (Figure 7). As a consequence the TOM-associated precursor accumulates at the inner side of outer membrane (stage IIIa) where it is readily accessible to the TIM22 complex (stage IIIb) to allow membrane insertion (stage IV) at high Δψ as well as under sub-optimal levels of Δψ. In the absence of the TIM8–13 complex the Tim23 precursor cannot be trapped in the intermembrane space and accumulates predominantly on the surface of the mitochondria (stage II). Yet, when Δψ is high, the TIM22 complex interacts efficiently with the small fraction of the precursor that is exposed to the intermembrane space and this shifts the equilibrium towards insertion of Tim23 into the inner membrane (stage IV). Therefore, the requirement of the TIM8–13 complex can be circumvented at high Δψ. At low Δψ, however, a direct interaction of the Tim23 precursor with the TIM22 complex would be inefficient, i.e. membrane insertion might require multiple rounds of interaction of the TOM-bound precursor with the TIM22 complex. In the absence of the TIM8–13 complex and at low Δψ the equilibrium is therefore shifted towards retrograde translocation. When the hydrophobic precursor is exposed on the surface of the mitochondria, reactions like misfolding or aggregation may ultimately result in the loss of translocation competence.
Fig. 7. Proposed role of the TIM8–13 complex in the import pathway of Tim23. Cytosolic Tim23 precursor (stage I) binds to receptors on the surface of the outer membrane (stage II). It is then translocated across the TOM complex and trapped by the TIM8–13 complex in the intermembrane space (stage IIIa). The TIM22 complex interacts with the accumulated precursor (stage IIIb) and mediates in a Δψ-dependent manner its release from the TOM complex and insertion into the inner membrane (stage IV). IM, inner membrane; OM, outer membrane.
The structurally related TIM9–10 complex may fulfil a corresponding function in the import of mitochondrial carrier proteins. Import of carriers was found to be completely abolished when Δψ was depleted by addition of antimycin and oligomycin (Pfanner and Neupert, 1985), which is in contrast to what is being reported here for import of Tim23. Import of carriers requires a higher Δψ (Pfanner and Neupert, 1985) than import of Tim23. In addition, carriers are considerably more hydrophobic than the Tim23 precursor. These might be reasons why import of carriers is strictly dependent on the TIM9–10 complex even under optimal conditions of Δψ.
Import of human Tim23 into yeast mitochondria and into mammalian mitochondria requires a higher Δψ than import of yeast Tim23. The biogenesis of human Tim23 may thus be more dependent on the TIM8–13 complex. Accordingly, mutations in DDP1, which encodes the human homologue of Tim8, may significantly affect the biogenesis of Tim23 in humans, particularly under conditions when the Δψ is low. Impaired import of Tim23, which is an essential component of the import machinery for matrix-targeted proteins, should lead to a significant reduction of many mitochondrial functions. MTS, which is due to mutations in DDP1, may thus result from impaired import of Tim23. The pleiotropic symptoms of MTS patients are characteristic of mitochondrial diseases that are often present as neuromuscular disorders and neurodegenerative syndromes (Wallace, 1999).
In summary, the TIM8–13 complex and the related TIM9–10 complex are components of the intermembrane space that act along the TIM22-dependent import pathway. They specifically assist the import of subsets of hydrophobic precursors, Tim23 and carrier proteins. The two complexes do not have the same substrate specificity but function in a comparable manner. They trap incoming precursors in the intermembrane space and prevent retrograde movement. Thereby the precursors are maintained in a translocation competent conformation, which ensures their Δψ-dependent insertion into the inner membrane mediated by the TIM22 complex.
Materials and methods
Disruption of the TIM8 and TIM13 genes
For disruption of TIM8, a fragment that carries the HIS3 gene of Schizosaccharomyces pombe and flanking regions of the TIM8 gene was amplified by PCR from pFA6a-HIS3MX6 (Wach et al., 1997) using the oligonucleotide primer GCGTCTTGTTTGTTGTTACGCTGCATATCAAAGCGAACGGTTAGTTATTAACTAACGTAACGTACGCTGCAGGTCGAC and the reverse primer TTACAGATATATTAAGGTAAAGAGAATAATGACTCGGAGAGATAAATCGGTTTCATACATCGATGAATTCGAGCTC. For disruption of TIM13 a PCR fragment was amplified from pFA6a-URA3MX6 (Wach et al., 1994), which carries the URA3 gene of S.pombe using the oligonucleotide primers CCATACCCTTGACGATTTGACAGGTCGCTACAGATTCAGAAAGAGATCATATCAATACAATTCAATTCATCA and AACGTAATAGCATTTTCTTGGTATCCTTTGAATCTTATATACAAGTACGAGTACATACTGAGTTTAGTATAC. Sequences in italic correspond to regions flanking the TIM8 and the TIM13 open reading frames, respectively; bold sequences correspond to HIS3 and URA3, respectively. The diploid Saccharomyces cerevisiae strain MB2 was transfected with both PCR fragments and transformants were selected on minimal medium without histidine and uracil (Maarse et al., 1992).
Preparation of mitochondria
Mitochondria were isolated as described (Herrmann et al., 1994). For preparation of rat mitochondria an adult male albino rat was starved for 15 h prior to sacrifice. Liver mitochondria were prepared in a medium containing 0.25 M sucrose, 2 mM EDTA and 20 mM HEPES–KOH pH 7.4 (Werner and Neupert, 1972).
Construction of Tim23-derived precursors
Tim23Δ20, Tim23Δ30 and Tim23(1–144) DNA were amplified from pGEM4–Tim23 (Berthold et al., 1995) with specific oligonucleotide primers by PCR. The PCR products were digested with BamHI and HindIII and subcloned into pGEM4. For the construction of pGEM4–Tim23Δ55–91, Tim23(92–222) was first amplified by PCR with the upstream primer CCTAAGGTCGACGGGTGGACCGATGACCTA and the downstream primer AAGCTTTCATTTTTCAAG using pGEM4–Tim23 as a template. The PCR fragment was subcloned into pGEM4–Tim23 vector, which was digested with SalI and HindIII to delete a fragment encoding amino acid residues 55–222. For construction of Tim23(1–101)–AAC, pGEM4–Tim23 was digested with Asp718 and HindIII to remove the region encoding amino acid residues 102–222, and a PCR fragment encoding the AAC2 gene of Neurospora crassa was subcloned into the resulting vector.
In vitro synthesis of precursor proteins and import into mitochondria
Precursor proteins were synthesized by coupled transcription/translation in reticulocyte lysate (Promega) in the presence of [35S]methionine (Pelham and Jackson, 1976). Import reactions into isolated yeast mitochondria were carried out at 25°C in 180 µl of import buffer [0.6 M sorbitol, 0.1 mg/ml bovine serum albumin (BSA), 80 mM KCl, 10 mM Mg(OAc)2, 2.5 mM EDTA, 2 mM KH2PO4, 50 mM HEPES–KOH pH 7.2] in the presence of 5 mM NADH and 2.5 mM ATP. Import reactions into rat mitochondria were carried out at 30°C in 100 µl of import buffer [0.25 M sucrose, 0.1 mg/ml BSA, 80 mM KCl, 5 mM Mg(OAc)2, 10 mM MOPS–KOH pH 7.2] in the presence of 10 mM succinate and 2.5 mM ATP. The reaction contained 50 µg (yeast) or 100 µg (rat) of mitochondrial protein and 3% reticulocyte lysate with the radiolabelled precursor protein. Membrane potential was dissipated by omission of NADH and pre-incubation for 2 min at 25°C with 1 µM valinomycin and 25 µM FCCP. For generation of a low Δψ, mitochondria were pre-incubated for 2 min at 25°C with 8 µM antimycin, 20 µM oligomycin and 2.5 mM ATP. To generate mitoplasts mitochondria were diluted 10-fold with 20 mM HEPES–KOH pH 7.2. Protease treatment of mitochondria and mitoplasts was carried out on ice for 20 min.
Cross-linking
Import reactions were performed in import buffer using 5% reticulocyte lysate. One hundred micrograms of mitochondria were used for total cross-links, 200 µg for cross-linking and subsequent immunoprecipitation. The cross-linking reaction was carried out on ice for 30 min with 100 µM m-maleimidobenzoyl-N-hydroxysuccinimide-ester (MBS) in sorbitol–HEPES buffer (0.6 M sorbitol, 20 mM HEPES–KOH pH 7.2). The cross-linking reaction was quenched with 80 mM glycine pH 8.0. For immunoprecipitation, mitochondria were lysed on ice for 15 min in 1% SDS, 0.5% Triton X-100, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 mM Tris–HCl pH 7.4. After a clarifying spin the supernatant was subjected to immunoprecipitation.
Acknowledgments
Acknowledgements
We thank Melanie Honcia and Christiane Kotthoff for expert technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 184 (Teilprojekt B2 and B12 and Ba1438/2), the Human Frontiers of Science Program, the Fonds der Chemischen Industrie, the Wilhelm–Sander Stiftung (97.061.1), the Münchener Medizinische Wochenschrift (to M.F.B. and to M.B.) and by the Bundesministerium für Bildung und Forschung (01KW9605/4) (to M.B. and M.F.B.).
References
- Adam A., Endres,M., Sirrenberg,C., Lottspeich,F., Neupert,W. and Brunner,M. (1999) Tim9, a new component of the TIM22.54 translocase in mitochondria. EMBO J., 18, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M.F., Sirrenberg,C., Neupert,W. and Brunner,M. (1996) Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell, 87, 33–41. [DOI] [PubMed] [Google Scholar]
- Bauer M.F., Gempel,K., Reichert,A.S., Rappold,G.A., Lichtner,P., Gerbitz,K.-D., Neupert,W., Brunner,M. and Hofmann,S. (1999a) Genetic and structural characterization of the human mitochondrial inner membrane translocase. J. Mol. Biol., 289, 69–82. [DOI] [PubMed] [Google Scholar]
- Bauer M.F., Rothbauer,U., Mühlenbein,N., Smith,R.J.H., Gerbitz,K.-D., Neupert,W., Brunner,M. and Hofmann,S. (1999b) The mitochondrial TIM22 preprotein translocase is highly conserved throughout the eukaryotic kingdom. FEBS Lett., 464, 41–47. [DOI] [PubMed] [Google Scholar]
- Bauer M.F., Hofmann,S., Neupert,W. and Brunner,M. (2000) Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol., 10, 25–31. [DOI] [PubMed] [Google Scholar]
- Berthold J., Bauer,M.F., Schneider,H.-C., Klaus,C., Dietmeier,K., Neupert,W. and Brunner,M. (1995) The MIM complex mediates preprotein translocation across the mitochondrial inner membrane and couples it to the mt-Hsp70/ATP driving system. Cell, 81, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Donzeau M., Káldi,K., Adam,A., Paschen,S., Wanner,G., Guiard,B., Bauer,M.F., Neupert,W. and Brunner,M. (2000) Tim23 links the inner and outer mitochondrial membranes. Cell, 101, 401–412. [DOI] [PubMed] [Google Scholar]
- Endres M., Neupert,W. and Brunner,M. (1999) Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22.54 complex. EMBO J., 18, 3214–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J.M., Fölsch,H., Neupert,W. and Stuart R.A. (1994) Isolation of yeast mitochondria and study of mitochondrial protein translation. In Celis,J.E. (ed.), Cell Biology: A Laboratory Handbook. Vol. 1. Academic Press, San Diego, CA, pp. 538–544. [Google Scholar]
- Jin H. et al. (1996) A novel X-linked gene, DDP, shows mutations in families with deafness (DFN-1), dystonia, mental deficiency and blindness. Nature Genet., 14, 177–180. [DOI] [PubMed] [Google Scholar]
- Jin H., Kendall,E., Freeman,T.C., Roberts,R.G. and Vetrie,D.L.P. (1999) The human family of deafness/dystonia peptide (DDP) related mitochondrial import proteins. Genomics, 61, 259–267. [DOI] [PubMed] [Google Scholar]
- Káldi K., Bauer,M.F., Sirrenberg,C., Neupert,W. and Brunner,M. (1998) Biogenesis of Tim23 and Tim17, integral components of the TIM machinery for matrix-targeted preproteins. EMBO J., 17, 1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O., Holder,J., Srinivasan,M., Leung,R.S. and Jensen,R.E. (1997) The Tim54p–Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J. Cell Biol., 139, 1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler C.M., Jarosch,E., Tokatlidis,K., Schmid,K., Schweyen,R.J. and Schatz,G. (1998a) Import of mitochondrial carrier proteins mediated by essential proteins of the intermembrane space. Science, 279, 369–373. [DOI] [PubMed] [Google Scholar]
- Koehler C.M., Merchant,S., Oppliger,W., Schmid,K., Jarosch,E., Dolfini,L., Junne,T., Schatz,G. and Tokatlidis,K. (1998b) Tim9p, an essential partner subunit of Tim10p for the import of mitochondrial carrier proteins. EMBO J., 17, 6477–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler C.M., Leuenberger,D., Merchant,S., Renold,A., Junne,T. and Schatz,G. (1999) Human deafness dystonia syndrome is a mitochondrial disease. Proc. Natl Acad. Sci. USA, 96, 2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger D., Bally,N.A., Schatz,G. and Koehler,C.M. (1999) Different import pathways through the mitochondrial intermembrane space for inner membrane proteins. EMBO J., 18, 4816–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarse A.C., Blom,J., Grivell,L.A. and Meijer,M. (1992) MPI1, an essential gene encoding a mitochondrial membrane protein, is possibly involved in protein import into yeast mitochondria. EMBO J., 11, 3619–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R.B. and Jackson,R.J. (1976) An efficient mRNA dependent translation system from reticulocyte lysates. Eur. J. Biochem., 67, 247–256. [DOI] [PubMed] [Google Scholar]
- Pfanner N. (1998) Mitochondrial import: crossing the aqueous intermembrane space. Curr. Biol., 8, R262–R265. [DOI] [PubMed] [Google Scholar]
- Pfanner N. and Meijer,M. (1997) The Tom and Tim machines. Curr. Biol., 7, R100–R103. [DOI] [PubMed] [Google Scholar]
- Pfanner N. and Neupert,W. (1985) Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J., 4, 2819–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J. (1999) Protein folding and import into organelles. In Higgins,S.J. and Hames,B.D. (eds), Post-translational Processing: A Practical Approach. Oxford University Press, Oxford, UK, pp. 43–94. [Google Scholar]
- Ryan K.R. and Jensen,R.E. (1995) Protein translocation across mitochondrial membranes: what a long strange trip it is. Cell, 83, 517–519. [DOI] [PubMed] [Google Scholar]
- Schatz G. (1996) The protein import system of mitochondria. J. Biol. Chem., 271, 31763–31766. [DOI] [PubMed] [Google Scholar]
- Sims P.J., Waggoner,A.S., Wang,C.-H. and Hoffmann,J.F. (1974) Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry, 13, 3315–3330. [DOI] [PubMed] [Google Scholar]
- Sirrenberg C., Bauer,M.F., Guiard,B., Neupert,W. and Brunner,M. (1996) Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature, 384, 582–585. [DOI] [PubMed] [Google Scholar]
- Sirrenberg C., Endres,M., Fölsch,H., Stuart,R.A., Neupert,W. and Brunner,M. (1998) Carrier protein import into mitochondria mediated by the intermembrane proteins Tim10/Mrs11p and Tim12/Mrs5p. Nature, 391, 912–915. [DOI] [PubMed] [Google Scholar]
- Wach A., Brachat,A., Pohlmann,R. and Philippsen,P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast, 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- Wach A., Brachat,A. and Philipsen,P. (1997) Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast, 13, 1065–1075. [DOI] [PubMed] [Google Scholar]
- Wallace D.C. (1999) Mitochondrial diseases in man and mouse. Science, 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Werner S. and Neupert,W. (1972) Functional and biogenetical heterogeneity of the inner membrane of rat-liver mitochondria. Eur. J. Biochem., 25, 379–396. [DOI] [PubMed] [Google Scholar]