Abstract
An atypical case of canine distemper (CD) was diagnosed in a vaccinated healthy adult dog. The patient was presented circling, seizuring, and blind. Postmortem examination resulted in a diagnosis of CD. Optic neuritis was diagnosed, a finding not previously described in the context of CD virus infection presenting solely with neurological signs.
Résumé
Névrite optique causée par le virus de la maladie de Carré chez un Jack Russell terrier. Un cas atypique de maladie de Carré (MC) a été diagnostiqué chez un chien adulte vacciné et en santé. Le patient a été présenté avec un comportement de tournoiements, des crises d’épilepsie et aveugle. Un examen post-mortem a produit un diagnostic de MC. Une névrite optique a été diagnostiquée, une constatation non décrite antérieurement dans le contexte d’une infection par le virus de MC présentant seulement des signes neurologiques.
(Traduit par Isabelle Vallières)
A 9-year-old spayed female Jack Russell terrier was referred to the Ontario Veterinary College Veterinary Teaching Hospital (OVC VTH) with a 1-week history of neurological deficits, including blindness. One week prior to presentation, episodes of lethargy were noted. No coughing, ocular or nasal discharge, or abnormal lung sounds were reported by the referring veterinarian. There was a brief mention in the medical record of an “abnormal breathing pattern” with a normal respiratory rate, but this observation was not clarified and subsequent clinical examinations failed to identify any such abnormality. There was also mention of a mild pyrexia of 39.6°C (reference range: 38.4°C to 39.4°C) (1). Pyrexia was not noted on any subsequent examinations. Empirical treatment with orbifloxacin (3 mg/kg q24h) and meloxicam (0.1 mg/kg q24h) had been initiated prior to referral. Despite therapy, the dog’s clinical condition deteriorated to include circling, falling, seizuring and, eventually, blindness. Prior to referral, phenobarbital therapy was initiated (8.5 mg/kg once as a loading dose, then 2 mg/kg q12h).
Case description
Upon presentation, temperature, pulse and respiration were within normal limits. Neurological examination revealed an obtunded mental status, circling and head tilt to the left, unilateral proprioceptive deficits involving the right front and hind limbs, blindness and a decreased response to right nasal septum stimulation. Neuro-ophthalmic examination revealed absence of a menace response bilaterally (OU), intact palpebral reflexes in addition to incomplete and sluggish direct and consensual pupillary light reflexes (PLR) OU. The lesion was characterized as a multifocal lesion involving the thalamocortex and cranial nerves II and VIII.
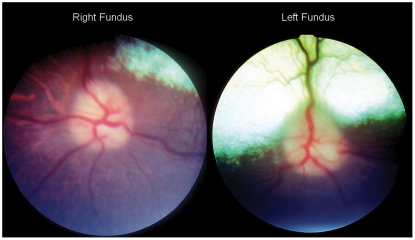
A photopic maze test was conducted. During this test, the patient compulsively circled to the left and repeatedly collided with objects within the maze. Bilateral resting mydriasis was noted on ocular examination. No ocular discharge was noted from either eye. Slit lamp biomicroscopic evaluation OU revealed a mild conjunctivitis as well as nuclear sclerosis. Indirect ophthalmoscopy revealed moderately swollen pink optic nerves with a loss of the physiologic cup as well as peripapillary edema bilaterally (Figure 1). Due to the critical condition of the patient, further ophthalmic testing was limited.
Figure 1.
Right and left fundus photographs. Notice the indistinct margins of the optic nerve, the peripapillary edema, and the swollen optic nerve heads.
Findings from a complete blood (cell) count (CBC) and urinalysis were within normal limits. The biochemistry panel revealed a mildly increased alanine transaminase (ALT) (386 U/L; reference range: 10 to 100 U/L), but was otherwise within normal limits.
A flash electroretinogram (ERG) was performed (Ephios Handheld ERG Unit Mjolner device; Linkoping, Sweden) after 30 min of dark adaptation. The results of the ERG were normal and indicated that retinal dysfunction did not account for the patient’s blindness.
Analysis of results from a cerebrospinal fluid (CSF) tap revealed a moderate mononuclear inflammation characterized by a nucleated cell count of 330 cells/μL (reference value: < 10 cells/μL) as well as an elevated protein of 0.65 g/L (reference value: < 0.4 g/L). A differential count revealed that 63% of the cells were lymphocytes and 37% were mononuclear cells. These findings are consistent with a mononuclear pleocytosis and elevated CSF protein. The remaining CSF sample volume was not sufficient to submit for culture or infectious titers.
Magnetic resonance imaging (MRI) of the brain was pursued in light of the patient’s neurologic signs and blindness with a normal ERG. Apart from swollen optic nerves protruding into the posterior segment of the eye, no abnormalities were found on MRI.
Based on the patient’s clinical signs, signalment, and CSF tap findings, a tentative diagnosis of granulomatous meningoencephalitis (GME) was made. Supportive therapy including trimethoprim/sulfadiazine (TMS; Schering Canada, Pointe-Claire, Quebec), 30 mg/kg body weight (BW) q12h, famotidine (Omega, Montreal, Quebec), 0.5 mg/kg BW, q12h, dexamethasone (Vétoquinol, Lavaltrie, Quebec), 0.25 mg/kg, q24h, cytosine arabinoside (Mayne Pharma, Montreal, Quebec), 100 mg/m2 constant rate infusion, and Tear-Gel (Novartis, Dorval, Quebec), OU q4h was instituted. Cytosine arabinoside and dexamethasone were prescribed as the specific therapy for GME. Famotidine and TMS were prescribed according to hospital protocol as a gastroprotectant and a broad-spectrum antibiotic, respectively. While a bacterial etiology was less likely, it is common practice to place such patients on a broad-spectrum antibiotic capable of penetrating the blood brain barrier. With the patient’s seizure activity and altered mental status, her blinking frequency was decreased. Consequently, Tear-Gel was prescribed. Clinical response to this treatment protocol was poor, and the patient was humanely euthanized at the owner’s request 2 d after her initial presentation.
A postmortem examination was conducted and the patient was found to be in good body condition. Mild congestion was noted in the liver and lungs grossly. Gross examination of the remaining organ systems, including the central nervous system, revealed no lesions. Histopathology of the liver revealed hepatic glycogenosis. However, no histological lesions were noted in the lungs. Apart from lesions involving the central nervous system and the eyes, the histology of the remainder of the organ systems was unremarkable.
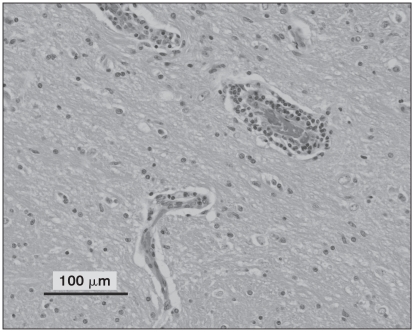
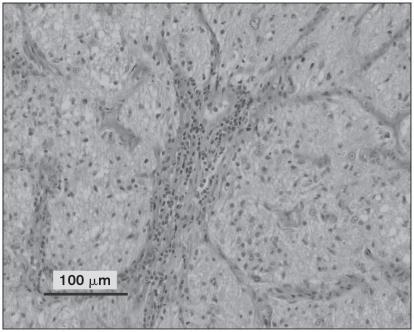
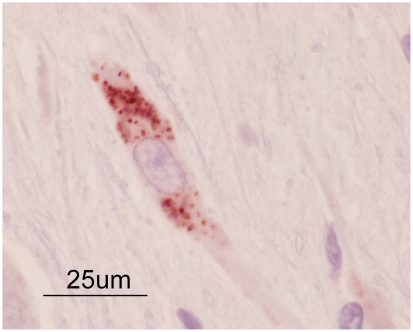
Multifocal perivascular aggregates of round cells throughout all levels of the brain as well as the spinal cord and right optic nerve were noted. Perivascular cuffing was noted in both the white and grey matter. The round cell population consisted of predominantly lymphocytes and macrophages with a lesser number of plasma cells, as shown in Figure 2. In addition, microgliosis and satellitosis were also noted in the brain and spinal cord sections. Apart from the optic nerve lesions, histological features of the globes, including the choroid and retinal tissue, were within normal limits. Perivascular cuffing and accumulation of round cells in the meninges were noted on examination of the right extraocular optic nerve as shown in Figure 3. Sufficient optic nerve was not available in the calottes from the left eye to adequately evaluate the extraocular nerve and meninges. While these findings could be consistent with GME, viral diseases remained on the differential list. Consequently, immunohistochemistry (IHC) for rabies (Canadian Food Inspection Agency, Nepean, Ontario), West Nile virus, and canine distemper (Animal Health Laboratory, University of Guelph, Ontario) was performed. Immunohistochemistry tests for rabies and West Nile virus were negative; however, the samples were positive for canine distemper. Strong cytoplasmic immunolabelling for canine distemper virus (CDV) was noted in neurons, astrocytes, and the occasional perivascular monocyte in sections from the brain and spinal cord. An example of this cytoplasmic CDV immunolabelling is depicted in Figure 4.
Figure 2.
All levels of brain examined demonstrated mild to moderate perivascular aggregates of round cells within both the grey and white matter as shown in this image. Hematoxylin and eosin.
Figure 3.
Right optic nerve section demonstrating gliosis and lymphocytic perivascular cuffing. Hematoxylin and eosin.
Figure 4.
Positive immunohistochemistry staining for canine distemper virus in a neuron present in cerebral tissue.
Discussion
Sudden blindness can be caused by intraocular lesions, post-retinal lesions (optic nerve, chiasm, and tracts) or lesions within the cerebrum or brain stem. Intraocular lesions include those involving the clear ocular media (cataracts), the retina, and the intraocular portion of the optic nerve. Retinal causes of sudden blindness include retinal detachment, toxin exposure (Ivermectin), and sudden acquired retinal degeneration syndrome (SARDS) (2–4). Glaucoma and optic neuritis are common conditions affecting the intraocular portion of the optic nerve. Based on the ophthalmic examination, bilaterally swollen optic nerves, and the normal ERG, this patient’s sudden blindness was attributable to optic neuritis.
The inflammation associated with optic neuritis may affect various sections of the optic nerve including the optic nerve head and the retrobulbar optic nerve including the optic chiasm. Should only the retrobulbar nerve be affected, the fundus will appear normal. When the optic nerve head is affected, it appears raised (swollen) with indistinct margins, the physiologic cup may be absent, and the nerve will appear pink. Peripapillary edema and retinitis as well as focal hemorrhage within the optic nerve head may be present with optic neuritis (5).
Differential diagnoses for swelling of the optic nerve head include papilledema, pseudopapilledema, neoplasia of the optic nerve, and optic neuritis (5,6). Papilledema, in its early stages, is not typically associated with blindness (7). Pseudopapilledema describes excessive myelination of the optic nerve head which does not affect vision. Optic nerve neoplasia, such as gliomas and meningiomas, is uncommon (6) and would not likely account for the other systemic signs noted in this patient.
In both humans and animals the cause of the optic neuritis is often difficult to determine (8). Etiologies include infectious, inflammatory, neoplastic, nutritional, traumatic, toxic, idiopathic causes, and spread of systemic disease or meningitis. Infectious causes of optic nerve inflammation include viral, bacterial, protozoal, fungal, and rickettsial disease (9,10). Non-infectious etiologies of optic neuritis include inflammation (GME) (11), neoplasia (optic nerve or orbital) (9), and toxin exposure (Closantel) (12).
Prior to presentation in November 2007, the patient had received routine veterinary care consisting of yearly wellness examinations and a rotating vaccination schedule as per the American Animal Hospital Association canine vaccination guidelines. The geographical location (Ontario, Canada), the lack of travel history, and current vaccine status decreased the likelihood of infectious disease. In addition, the patient lived in an urban setting, was fed a commercial diet and had no known exposure to toxins.
A tentative diagnosis of GME was made based on signalment, clinical signs, and CSF findings. While GME can affect any age or breed of dog, a predisposition has been proposed in middle-aged small breed dogs with terriers and poodles being overrepresented (13,14). This patient was a middle-aged, terrier, consistent with signalment often noted in cases of GME. Cerebrospinal fluid findings in dogs with GME are variable; however, an increase in total protein and a mononuclear pleocytosis, as seen in this patient, can be typical of GME (15,16). These CSF characteristics are often observed with other etiologies such as infectious meningoencephalitis (CDV, toxoplasmosis, Rocky Mountain spotted fever) and neoplasia (malignant histiocytosis, lymphoma) (17–20).
The histopathology of this case did not completely coincide with a diagnosis of GME. In GME, multifocal perivascular aggregates of round cells (lymphocytes, macrophages, and plasma cells) are typically present in the white matter of the brain and spinal cord (17,21). In the present case, perivascular cuffing was observed throughout both grey and white matter. In addition, microgliosis and satellitosis were noted and are not typically seen in cases of GME. These findings are more often observed in demyelinating disease. Microgliosis and satellitosis are often found in cases of CDV; however, they are not specific for the disease (22). This combination of atypical histopathology findings prompted the additional diagnostic testing that led to identification of CDV.
Canine distemper virus is capable of infecting mammals other than the Canidae. The Ontario government Ministry of Natural resources lists raccoons as a reservoir of CDV, rabies, and mange (23). It is not uncommon for raccoons to come in contact with domestic pets. Furthermore, outbreaks of CD in urban raccoon populations occur periodically. The most recently reported outbreak occurred in Toronto, Ontario, approximately 55 km from this patient’s home (24). We postulate that this patient contracted CDV from direct or indirect contact with an infected raccoon. The reasons behind the ineffectiveness of CDV vaccination in this case are not clear. No history of underlying disease or therapy that might suppress an appropriate immune response is present. Exposure to a highly virulent strain or to very high levels of CDV may have occurred. Alternatively, problems with the manufacture, storage, or administration of any vaccine have the potential to impact protection.
In the present case, canine distemper virus, and indeed other infectious causes of optic neuritis, were considered to be low on the list of differential diagnoses due to the patient’s current vaccination history, lack of travel to areas where various pathogens listed earlier are endemic, the patient’s signalment and presentation. The disease is more typically reported in young puppies (3 to 6 mo of age) or unvaccinated or immunocompromized adults (25). This patient was a healthy, fully vaccinated middle-aged terrier. With the exception of a brief mention of an “abnormal breathing pattern” and a very slight fever in the medical records prior to her presentation to OVC, which were not confirmed in any subsequent testing, her presentation was exclusively neurologic. In addition, adults presenting with a neurologic form of CD typically present with a progressive tetraparesis rather than the seizures, altered mental status, and blindness as seen in this patient (26,27).
A recent report examining atypical presentations of CD described 8 cases that presented solely with neurological signs. Half of these dogs (4/8) had been previously vaccinated with 3 or more vaccines and the average age of presentation was 59.4 mo (26). Another report examining the prevalence of CD in Denmark also reported cases of CD in vaccinated animals (28). Our case was similar to these published cases in that the patient demonstrated neurologic signs exclusively and had been vaccinated for CDV. However, this case was unusual in that the presenting neurological signs were atypical for an adult dog. More importantly, this case presented with optic neuritis, a condition that has not previously been reported in canine distemper cases presenting solely with neurologic signs.
An additional unusual feature of this case is the presence of optic neuritis in the absence of histological or clinical evidence of chorioretinitis. Chorioretinitis is commonly reported to occur in cases of CDV encephalomyelitis (27); the literature examining cases of neurological CD mention ophthalmic clinical signs infrequently. Importantly, optic neuritis is not mentioned in the literature pertaining to solely neurological presentation of CD. One retrospective study of 38 cases of CDV encephalomyelitis reported that 9 out of the 22 cases that received an ophthalmic examination had lesions of chorioretinitis (27). However, that study did not report any cases of blindness or optic neuritis. A similar study examining 19 cases of CDV encephalomyelitis, 4 of which presented solely with neurological signs, described 2 cases of chorioretinitis. Again, no cases of optic neuritis were diagnosed and no visual deficits noted (22). The remaining literature on cases of CDV presenting solely with neurological signs also report no cases of optic neuritis (26,29).
The mild conjunctival hyperemia noted in this case could have been attributable to CDV; however, conjunctivitis is a non-specific finding associated with many ocular and systemic conditions (30). Other factors, such as recent general anesthesia, which causes a decrease in tear production (31), as well as a significant decrease in blinking frequency could also have accounted for the mild conjunctivitis noted on examination. In a recent study examining CD and keratoconjunctivitis sicca (KCS), conjunctival hyperemia was noted in only 45% of the cases, while 70% of the cases of KCS had mucopurulent ocular discharge (32). The animal in the current case did have mild conjunctivitis; however, she did not have mucopurulent ocular discharge at any point in her history.
A complete ophthalmic examination is an important but often overlooked component of the diagnostic workup of any patient presenting with neurological signs. In the case presented here, mydriasis and visual deficits were noted by the referring veterinarian on the same days as the first suspected seizure. An ophthalmic examination can add to the patient’s problem list and thereby help to direct the diagnostic plan and modify the differential diagnoses.
Additional diagnostic tests may have proven informative with respect to this case but were not performed for several reasons. A Schirmer tear test (STT) would have been useful as some dogs with CD have KCS. In this case, CD was not among our initial differential diagnoses and our access to the patient was limited due to her critical medical condition. Consequently, the testing done on the patient was limited to those tests deemed absolutely necessary. Titers for viral (CDV, rabies, West Nile), rickettsial, fungal (cryptococcus, blastomycosis, histoplasmosis), and protozoal diseases (toxoplasmosis) could have been performed. Antibody titers on CSF and serum may have allowed the clinician to determine significance of the titer levels, especially in a patient not exhibiting clinical signs typical in published reports of the infection. Visual evoked potentials would have enhanced the ophthalmic examination of the patient’s blindness and helped to more precisely localize the cause of the blindness. Despite these limitations, a conclusive diagnosis of CD was achieved, through immunohistochemistry, in a case that presented atypically with respect to the clinical signs. This case should prove useful in increasing the awareness of CD as an important differential diagnosis when veterinary clinicians are confronted with acute blindness and neurologic signs, even in fully vaccinated canids. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Aiello SE, Mays A, editors. The Merck Veterinary Manual. 8th ed. Whitehouse Station, New Jersey: Merck & Co; 1997. The Merck Veterinary Manual Reference guides; p. 2188. [Google Scholar]
- 2.Shelah M, Weinberger D, Ofri R. Acute blindness in a dog caused by an explosive blast. Vet Ophthalmol. 2007;10:196–198. doi: 10.1111/j.1463-5224.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Kenny PJ, Vernau KM, Puschner B, Maggs DJ. Retinopathy associated with ivermectin toxicosis in two dogs. J Am Vet Med Assoc. 2008;233:279–284. doi: 10.2460/javma.233.2.279. [DOI] [PubMed] [Google Scholar]
- 4.Venter IJ, Petrick SW. Acute blindness in a dog caused by sudden acquired retinal degeneration. J S Afr Vet Assoc. 1995;66:32–34. [PubMed] [Google Scholar]
- 5.Brooks DE. Diseases of the canine optic nerve. In: Gelatt KN, editor. Veterinary Ophthalmology. 4th ed. Ames, Iowa: Blackwell Publ; 2007. pp. 1059–1086. [Google Scholar]
- 6.Caswell J, Curtis C, Gibbs B. Astrocytoma arising at the optic disc in a dog. Can Vet J. 1999;40:427–428. [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer AC, Malinowski W, Barnett KC. Clinical signs including papilloedema associated with brain tumours in twenty-one dogs. J Small Anim Pract. 1974;15:359–386. doi: 10.1111/j.1748-5827.1974.tb06512.x. [DOI] [PubMed] [Google Scholar]
- 8.Nell B. Optic neuritis in dogs and cats. Vet Clin North Am Small Anim Pract. 2008;38:403–15. viii. doi: 10.1016/j.cvsm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Shamir MH, Ofri R. In: Veterinary Ophthalmology. 4th ed. Gelatt KN, editor. Ames, Iowa: Blackwell Publ; 2007. p. 1442. [Google Scholar]
- 10.Leiva M, Naranjo C, Pena MT. Ocular signs of canine monocytic ehrlichiosis: A retrospective study in dogs from Barcelona, Spain. Vet Ophthalmol. 2005;8:387–393. doi: 10.1111/j.1463-5224.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa M, Okada M, Watari T, Sato T, Kanayama K, Sakai T. Ocular granulomatous meningoencephalomyelitis in a dog: Magnetic resonance images and clinical findings. J Vet Med Sci. 2009;71:233–237. doi: 10.1292/jvms.71.233. [DOI] [PubMed] [Google Scholar]
- 12.McEntee K, Grauwels M, Clercx C, Henroteaux M. Closantel intoxication in a dog. Vet Hum Toxicol. 1995;37:234–236. [PubMed] [Google Scholar]
- 13.Thomas WB. Inflammatory diseases of the central nervous system in dogs. Clin Tech Small Anim Pract. 1998;13:167–178. doi: 10.1016/S1096-2867(98)80038-8. [DOI] [PubMed] [Google Scholar]
- 14.Munana KR, Luttgen PJ. Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases (1982–1996) J Am Vet Med Assoc. 1998;212:1902–1906. [PubMed] [Google Scholar]
- 15.Bailey CS, Higgins RJ. Characteristics of cerebrospinal fluid associated with canine granulomatous meningoencephalomyelitis: A retrospective study. J Am Vet Med Assoc. 1986;188:418–421. [PubMed] [Google Scholar]
- 16.Thomas JB, Eger C. Granulomatous meningoencephalomyelitis in 21 dogs. J Small Anim Pract. 1989;30:287–293. [Google Scholar]
- 17.Suzuki M, Uchida K, Morozumi M, et al. A comparative pathological study on granulomatous meningoencephalomyelitis and central malignant histiocytosis in dogs. J Vet Med Sci. 2003;65:1319–1324. doi: 10.1292/jvms.65.1319. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman K, Almy F, Carter L, et al. Cerebrospinal fluid from a 10-year-old dog with a single seizure episode. Vet Clin Pathol. 2006;35:127–131. doi: 10.1111/j.1939-165x.2006.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu JM, Georgy MF, Burroughs FH, Weir EG, Rosenthal DL, Ali SZ. Lymphoma, leukemia, and pleiocytosis in cerebrospinal fluid: Is accurate cytopathologic diagnosis possible based on morphology alone? Diagn Cytopathol. 2009;37:820–824. doi: 10.1002/dc.21110. [DOI] [PubMed] [Google Scholar]
- 20.Bush WW, Throop JL, McManus PM, Kapatkin AS, Vite CH, Van Winkle TJ. Intravascular lymphoma involving the central and peripheral nervous systems in a dog. J Am Anim Hosp Assoc. 2003;39:90–96. doi: 10.5326/0390090. [DOI] [PubMed] [Google Scholar]
- 21.Braund KG. Granulomatous meningoencephalomyelitis. J Am Vet Med Assoc. 1985;186:138–141. [PubMed] [Google Scholar]
- 22.Koutinas AF, Polizopoulou ZS, Baumgaertner W, Lekkas S, Kontos V. Relation of clinical signs to pathological changes in 19 cases of canine distemper encephalomyelitis. J Comp Pathol. 2002;126:47–56. doi: 10.1053/jcpa.2001.0521. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Natural Resources. [Last accessed January 25, 2011]. [homepage on the Internet]. [updated 2009] Available from http://www.mnr.gov.on.ca/MNR_E002208.pdf.
- 24.Canadian Broadcasting Corporation. [Last accessed January 25, 2011]. [homepage on the Internet]. [updated 2010] Available from http://www.cbc.ca/canada/toronto/story/2010/02/18/toronto-disptemper.html.
- 25.Greene C, Appel M. Chapter 3: Canine distemper. In: Greene C, editor. Infectious Diseases of the Dog and Cat. 3rd ed. St Louis, Missouri: Saunders/Elsevier; 2006. pp. 25–41. [Google Scholar]
- 26.Amude AM, Alfieri AA, Alfieri AF. Clinicopathological findings in dogs with distemper encephalomyelitis presented without characteristic signs of the disease. Res Vet Sci. 2007;82:416–422. doi: 10.1016/j.rvsc.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Thomas WB, Sorgonen DC, Steiss JE. A retrospective evaluation of 38 cases of canine distemper encephalomyelitis. J Am Anim Hosp Assoc. 1993;29:129. [Google Scholar]
- 28.Blixenkrone-Moller M, Svansson V, Have P, et al. Studies on manifestations of canine distemper virus infection in an urban dog population. Vet Microbiol. 1993;37:163–173. doi: 10.1016/0378-1135(93)90190-i. [DOI] [PubMed] [Google Scholar]
- 29.Amude AM, Alfieri AA, Alfieri AF. Antemortem diagnosis of CDV infection by RT-PCR in distemper dogs with neurological deficits without the typical clinical presentation. Vet Res Commun. 2006;30:679–687. doi: 10.1007/s11259-006-3308-2. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix DVH. Canine conjunctiva and nictitating membrane. In: Gelatt KN, editor. Veterinary Ophthalmology. 4th ed. Ames, Iowa: Blackwell Publ; 2007. pp. 662–689. [Google Scholar]
- 31.Herring IP, Pickett JP, Champagne ES, Marini M. Evaluation of aqueous tear production in dogs following general anesthesia. J Am Anim Hosp Assoc. 2000;36:427–430. doi: 10.5326/15473317-36-5-427. [DOI] [PubMed] [Google Scholar]
- 32.de Almeida DE, Roveratti C, Brito FL, et al. Conjunctival effects of canine distemper virus-induced keratoconjunctivitis sicca. Vet Ophthalmol. 2009;12:211–215. doi: 10.1111/j.1463-5224.2009.00699.x. [DOI] [PubMed] [Google Scholar]