Abstract
Although the rectal mucosa remains the traditional site for measuring body temperature in dogs, an increasing number of clinicians have been using auricular temperature to estimate core body temperature. In this study, 88 mature healthy dogs had body temperatures measured with auricular and rectal thermometers. The mean temperature and confidence intervals were similar for each method, but Bland-Altman plots showed high biases and limits of agreement unacceptable for clinical purposes. The results indicate that auricular and rectal temperatures should not be interpreted interchangeably.
Résumé
Comparaison entre les thermomètres auriculaires et rectaux standard pour la mesure de la température corporelle chez les chiens. Même si les muqueuses rectales demeurent le site traditionnel pour la mesure de la température corporelle chez les chiens, un nombre grandissant de cliniciens utilisent la température auriculaire pour estimer la température corporelle centrale. Dans cette étude, la température de 88 chiens adultes en santé a été mesurée à l’aide de thermomètres auriculaires et rectaux. La température moyenne et les intervalles de confiance étaient semblables pour chaque méthode, mais les représentations graphiques Bland-Altman ont montré des biais élevés et des seuils de concordance inacceptables à des fins cliniques. Les résultats indiquent que les températures auriculaires et rectales ne devraient pas être interprétées de manière interchangeable.
(Traduit par Isabelle Vallières)
Introduction
Measuring body temperature allows the identification of variations of core temperature associated with medical conditions (1). Body temperature in dogs has traditionally been obtained by using rectal thermometers. Besides being stressful for many dogs, use of rectal thermometers is time-consuming and can be a potential source of cross-contamination and injury to the patient and the veterinarian (2–4). Moreover, many conditions, including digestion, peristaltic movements, fecal masses, muscle tone, and physical activity may affect temperatures acquired by rectal thermometry (5).
Several types of clinical thermometers are available, including non-contact non-invasive, mildly-invasive contact, and invasive contact devices. The latter are used more frequently in anesthetized and critical care patients. Most often, dogs have their body temperature obtained by placing a mildly invasive contact thermometer, such as a glass thermometer or a digital thermometer, against the rectal mucosa for varying lengths of time (1,3,6).
Recently there has been an increase in the use of non-contact non-invasive thermometers, such as the infrared auricular thermometer, presumably because of the shorter time needed to obtain body temperature, the supposed accuracy, and better patient compliance in dogs and cats (1,4). These thermometers utilize pyroelectric sensors to detect the temperature of the tympanic membrane, which theoretically provides a more accurate measurement of core body temperature (7,8).
Studies have shown varying results when comparing auricular with rectal temperatures. In many studies, however, only a small number of animals was assessed (1,4,9). In this study, therefore, we hypothesized that a correlation exists between auricular and rectal temperatures in a large population of clinically healthy dogs.
Materials and methods
Animals
Eighty-eight adult dogs [mean weight 13.2 kg ± 11.8 kg (standard deviation, s)] of either sex were used. Several breeds were represented. All animals enrolled in the study were brought to a Veterinary Teaching Hospital for various purposes, including routine vaccinations and neutering. Written consent was obtained from the owners and all animals were determined to be healthy based on physical examination. Once the temperatures had been obtained, the animal was returned to its owner. The study was conducted in accordance with guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Temperature measurements
Body temperatures were obtained in a room that had a mean temperature of 26.2°C ± 0.1°C and relative air humidity of 67.0% ± 17.2%. Each animal was acclimatized to the temperature in the room for 30 min before measurements were taken.
Commercially available thermometers were used, including an auricular infrared device (Thermoscan IRT 4520; Braun, Kronberg, Germany), a glass-mercury thermometer (Accumed; G-Tech, São Paulo, Brazil), and a digital equilibrium thermometer (Digital Soft Tip; CVS, Woonsocket, Rhode Island, USA). After being positioned in the ear canal descending to the eardrum, the activation push-button was pressed and the auricular infrared thermometer provided readings within seconds. The rectal glass-mercury thermometer was inserted a minimum of 2 cm into the rectum, and kept in contact with the rectal mucosa either until an apparently steady temperature was observed (stabilization of the mercury column) or for 3 min. The digital thermometer was also inserted a minimum of 2 cm into the rectum, where it remained until an endpoint reading audible beep was heard. Prior to the study, accuracy of both rectal thermometers was validated in a temperature controlled water bath against a reference thermometer. A temperature controlled thermal plate was used to validate the auricular thermometer.
Four temperatures were obtained by the same experienced observer in the following order: 1) rectal glass-mercury thermometer for 3 min, 2) auricular thermometer, 3) rectal digital thermometer, and 4) rectal glass-mercury thermometer until stabilization of the column (steady temperature). A second experienced observer independently performed a second measurement of auricular temperature at the end of the 4 initial readings.
Statistical analyses
The results are expressed as the mean, standard deviation (s), and 95% confidence intervals (CI). The agreement between auricular and rectal temperatures was assessed using Bland-Altman, in which the difference between 2 techniques (auricular versus rectal) is plotted against their mean and the limits of agreement calculated (10). The same method was used to assess how the several rectal temperatures compare with each other. Pearson’s correlation coefficient was calculated between all rectal temperatures and auricular measurements, as well as between the results of auricular temperatures obtained by the 2 observers.
Results
Table 1 gives the results of mean, s, and 95% CI of both auricular and rectal temperatures. Measurements were obtained by the 4 protocols in every animal. Auricular measurements were well-tolerated in 89.7% of the dogs, whereas rectal measurements were well-tolerated in only 68.2% of the dogs. The maximal temperatures documented for auricular and rectal thermometers were 40.3°C (auricular), 39.9°C (glass-mercury for 3 min), 39.5°C [glass-mercury thermometer (steady state)], and 39.5°C (digital). Signs attributable to fever were not documented in any dog.
Table 1.
Temperatures (°C) measured by auricular and rectal thermometers in 88 clinically healthy dogs
| Type of measurement | Mean | s | 95% CI |
|---|---|---|---|
| Mercury thermometer — column stabilization | 38.6 | 0.5 | 38.5, 38.7 |
| Mercury thermometer — 3 minutes | 38.8 | 0.4 | 38.7, 38.9 |
| Digital thermometer | 38.7 | 0.4 | 38.6, 38.8 |
| Auricular thermometer (1st observer) | 39.0 | 0.5 | 38.9, 39.1 |
| Auricular thermometer (2nd observer) | 39.0 | 0.6 | 38.9, 39.2 |
s — standard deviation; CI — confidence interval.
Bland-Altman plots revealed a better agreement between auricular temperatures and rectal measurements using the glass-mercury thermometer for 3 min (Table 2). The bias, or average difference between the 2 methods, was −0.1716°C, with limits of agreement showing that the discrepancy between auricular and rectal (glass-mercury for 3 min) temperatures for an individual animal ranged from −1.335°C to 0.532°C. If we arbitrarily consider any error > ± 0.50°C to be clinically unacceptable, then there is a lack of agreement between the 2 types of body temperature measurements. The other 2 types of rectal temperatures showed even greater biases, also failing to agree with auricular readings. Bland-Altman plots showed that the differences in temperature (auricular minus rectal) are not close to zero, thereby indicating that the tested methods do lack agreement. Also, Bland-Altman was used to assess how the several rectal temperatures compare with each other (Table 3), showing much narrower ranges for the limits of agreement.
Table 2.
Bias and limits of agreement (°C) between temperatures measured by auricular and rectal thermometers in 88 clinically healthy dogs
| Type of measurement | Bias | s | 95% limits of agreement |
|---|---|---|---|
| Mercury thermometer — column stabilization | −0.4011 | 0.4762 | −1.335, 0.5322 |
| Mercury thermometer — 3 minutes | −0.1716 | 0.4604 | −1.074, 0.7307 |
| Digital thermometer | −0.3182 | 0.4409 | −1.182, 0.5460 |
s — standard deviation.
Table 3.
Bias and limits of agreement (°C) between temperatures measured by several rectal thermometers in 88 clinically healthy dogs
| Temperatures compared | Bias | s | 95% limits of agreement |
|---|---|---|---|
| Mercury thermometer (3 minutes) versus Mercury thermometer (column stabilization) | −0.2295 | 0.2203 | −0.2022, 0.6613 |
| Mercury thermometer (3 minutes) versus Digital thermometer | 0.1465 | 0.1999 | −0.2453, 0.5385 |
| Digital thermometer versus Mercury thermometer (column stabilization) | 0.0829 | 0.2492 | −0.4055, 0.5714 |
s — standard deviation.
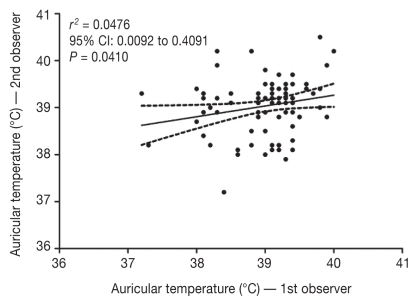
Pearson’s correlation coefficient (r2) indicated a weak correlation between auricular and rectal temperatures; r2 varied between 0.343 and 0.372 for the 3 methods of measuring rectal temperatures. The comparison between near-simultaneous duplicate auricular temperatures performed by 2 different observers resulted in a weak correlation (r2 = 0.048), as measurements are considered more repeatable when the coefficient approaches 1.
Discussion
Rectal measurement has been the gold standard for the measurement of body temperature in veterinary practice, possibly because of the good agreement that exists between this technique and core body temperature (3,11). In children, although rectal measurement was an established method of measuring temperature, infrared thermometers are now frequently used (12,13).
The measurement of temperature at the ear has advantages over measurement at the rectum, such as the more practical anatomical location of the ear and the much faster result. However, to be clinically acceptable, both methods should agree sufficiently well to permit interchangeable use of either thermometer (4,12).
In a large systematic review of several investigations comparing ear and rectal temperatures in children (12), wide limits of agreement were found between the 2 methods, although the mean differences between temperature measurements were small. Therefore, ear thermometry was concluded to be an unreliable approximation of rectal temperature. On the contrary, auricular temperature was documented to be an accurate estimation of core temperature in dogs, although lower than temperature measured at the rectal mucosa (11). In the present investigation, Bland-Altman plots showed that the agreements between auricular and rectal temperature were greatest with glass-mercury thermometry for 3 min and least with the stabilization of the mercury column. However, the lack of a randomized sequence for obtaining temperature readings might have played a role in the results because the last temperatures obtained were more likely to be influenced by the multiple manipulations the dogs had undergone.
In hypothermic anesthetized dogs, auricular thermometry was correlated with rectal temperature (1). Nonetheless, lower correlation was obtained as animals recovered from anesthesia and body temperature increased. When Pearson’s correlation was considered, our results also documented a correlation between auricular and rectal measurements. However, this analysis is not appropriate for comparing 2 methods of measurement. In this case, for example, biases indicated that auricular thermometry might unreliably overestimate rectal measurements. This poor concurrence has implications for clinical management when temperature needs to be measured accurately. Also, in cats with varying temperatures the documented limits of agreement between auricular and rectal measurements were believed to be unacceptable for clinical purposes (4). A study in dogs (5), however, documented a good agreement between these measurements sites, as supported by very small biases.
There was a weak correlation between duplicate measurements performed by 2 observers (r = 0.218), contrasting with previously published results (1), which indicated that measurements were significantly repeatable (r = 0.999). The inadequate repeatability of near-simultaneous measurements in this study could be ascribed to several factors, including multiple manipulations of the animals, excitement, activity, as well as inadequate positioning of the thermometer probe in the ear canal (4).
Although none of the dogs in the study was diagnosed with otitis externa, inflammation of the ear canal does not represent a concern when measuring auricular temperature (6).
The contrasting results of this investigation with previous studies may be partially ascribed to differences in the model of auricular thermometers. We chose an infrared auricular thermometer designed for use in humans because it is more widely available than veterinary infrared thermometers and is more common in clinical practice. Nevertheless, differences in the anatomy of the ears of dogs and humans might lead to an inadequate positioning of the thermometer probe in the dog’s ear canal descending to the eardrum, thereby having the thermometer read the temperature of other parts of the external acoustic meatus instead of the tympanic membrane.
Even though the statistical analysis disclosed unacceptable limits of agreement for clinical purposes, auricular thermometry may be used with discretion to avoid misinterpretation of the animal’s clinical status. The results of this investigation are especially indicative that auricular and rectal temperatures readings should not be interpreted interchangeably, but rather, compared against a reference range of temperatures for that particular site. Although a few studies exist, agreement between auricular and rectal measurements is yet to be studied in a large population of hypothermic and hyperthermic dogs to better clarify the accuracy of this novel method of temperature acquisition. CVJ
Figure 1.
Correlation of auricular temperature obtained in healthy dogs (n = 88) by 2 independent observers. Dotted lines represent the 95% confidence intervals.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Southward ES, Mann FA, Dodam J, et al. A comparison of auricular, recta and pulmonary artery thermometry in dogs with anesthesia-induced hypothermia. J Vet Emerg Crit Care. 2006;16:172–175. [Google Scholar]
- 2.Fraden J. The development of thermoscan instant thermometer. Clin Pediatr. 1991;30:11–12. doi: 10.1177/0009922891030004S04. [DOI] [PubMed] [Google Scholar]
- 3.Michaud A. Comparison of an infrared ear thermometer to rectal thermometers in cats. Fel Pract. 1996;24:25–30. [Google Scholar]
- 4.Kunkle G, Nicklin C, Sullivan-Tamboe D. Comparison of body temperature in cats using a veterinary infrared thermometer and a digital rectal thermometer. J Am Anim Hosp Assoc. 2004;40:42–46. doi: 10.5326/0400042. [DOI] [PubMed] [Google Scholar]
- 5.Rexroat J, Benish K, Fraden J. Clinical accuracy of Vet-TempTM instant ear thermometer: Comparative study with dogs and cats. [Last accessed February 7, 2011];Advances Monitor Corporation. 1999:1–4. Available from www.mimi12.com/img/Clinical_Accuracy.pdf. [Google Scholar]
- 6.González A, Mann F, Preziosi D, et al. Measurement of body temperature by use of auricular thermometers versus rectal thermometers in dogs with otitis externa. J Am Vet Med Assoc. 2002;221:378–380. doi: 10.2460/javma.2002.221.378. [DOI] [PubMed] [Google Scholar]
- 7.Brinnel H, Cabanac M. Tympanic temperature is a core temperature in humans. J Therm Biol. 1988;14:47–53. [Google Scholar]
- 8.Huang H, Shih H. Use of infrared thermometry and effect of otitis externa on external canal temperature in dogs. J Am Anim Hosp Assoc. 1998;213:26–79. [PubMed] [Google Scholar]
- 9.Wiedemann G, Scalon M, Paludo G, et al. Comparison between tympanic and anal temperature with a clinical infrared ray thermometer in dogs. Arq Bras Med Vet Zootec. 2006;58:503–505. [Google Scholar]
- 10.Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 11.Greer R, Cohn L, Dodam J, et al. Comparison of three methods of temperature measurement in hypothermic, euthermic, and hyperthermic dogs. J Am Vet Med Assoc. 2007;230:1841–1848. doi: 10.2460/javma.230.12.1841. [DOI] [PubMed] [Google Scholar]
- 12.Craig J, Lancaster G, Taylor S, et al. Infrared ear thermometry compared with rectal thermometry in children: a systematic review. Lancet. 2002;360:603–609. doi: 10.1016/S0140-6736(02)09783-0. [DOI] [PubMed] [Google Scholar]
- 13.Sermet-Gaudelus I, Chadelat I, Lenoir G. La mesure de la température en pratique pédiatrique quotidienne. Arch Pediatr. 2005;12:1292–1300. doi: 10.1016/j.arcped.2005.01.034. [DOI] [PubMed] [Google Scholar]