Abstract
An antemortem diagnosis of disseminated hemangiosarcoma with extensive hepatic and splenic involvement was made in a 9-year-old cow evaluated for lethargy, weight loss, regenerative anemia, thrombocytopenia, and elevated liver enzymes. This is the first report of hemangiosarcoma in a cow with a suspected primary location of the liver and/or spleen.
Résumé
Hémangiosarcome disséminé chez une vache. Un diagnostic ante-mortem d’hémangiosarcome disséminé avec une atteinte hépatique et splénique importante a été posé chez une vache âgée de 9 ans évaluée pour de la l’abattement, une perte de poids, de l’anémie régénérative, une thrombocytopénie et des enzymes hépatiques élevés. Il s’agit du premier rapport d’hémangiosarcome chez une vache avec l’emplacement primaire soupçonné du foie et/ou de la rate.
(Traduit par Isabelle Vallières)
A 9-year-old dry, open Brown Swiss cow was presented to the Field Service section of University of Pennsylvania’s New Bolton Center with a 2-week history of partial to complete anorexia, weakness, and weight loss. The cow was graded “Excellent” as a 2-year-old and scored 93 as a 5-year-old. In addition, the cow was a 3 time All-American as a fall calf, a fall yearling, and a 2-year-old. At the time of presentation, the owner suspected pneumonia and initiated treatment with oxytetracycline, 7.5 mg/kg BW, IV, q24h, sulfadimethoxine, 55 mg/kg BW, IV initially, then 27.5 mg/kg body weight (BW), IV, q24h and flunixin meglumine 1.1 mg/kg BW, IV, q24h during her initial illness. The owner elected to seek veterinary attention when no signs of improvement resulted from this course of therapy, and the cow remained depressed, weak, and inappetent.
Case description
Initial physical examination performed in the field revealed a decreased body condition score (2.5/5), mild tachycardia (80 beats/min), and decreased rumen motility (1 weak rumen contraction/min). The remaining physical examination was unremarkable.
Hematological analysis revealed a regenerative anemia, thrombocytopenia, and leukopenia with a lymphopenia. The packed cell volume was 0.21 L/L [reference interval (RI): 0.24 to 0.46 L/L], red blood cell count was 3.79 × 106/μL (RI: 7.4 to 11.6 × 106 μL). In addition, 1 nucleated red blood cell per high power field was observed. Platelets were 154 × 109/L (RI: 412 to 1003 × 109/L). The white blood cells were measured at 4810 × 106/L (RI: 6200 to 13 600 × 106/L) and the absolute lymphocyte count was 2200 × 106/L (RI: 4000 to 9000 × 106/L). Fibrinogen concentration was 3.85 g/L (RI: 3.00 to 7.75 g/L). The remaining hemogram values were unremarkable.
Serum biochemistry indicated the following abnormalities: γ-glutamyltransferase (GGT) concentration was 360 IU/L (RI: 22 to 64 IU/L), aspartate aminotransferase (AST) concentration was 275 IU/L (RI: 58 to 100 IU/L), and serum total protein concentration was 82 g/L (RI: 58 to 75 g/L). Other biochemistry values were within reference limits.
Given the significant hematological abnormalities observed, the cow was referred to the George D. Widener Hospital for Large Animals at the University of Pennsylvania’s New Bolton Center hospital for further diagnostic evaluation. Our differential diagnoses for a regenerative anemia with increased GGT in an aged cow were neoplasia (lymphosarcoma, hepatocellular carcinoma), abomasal ulcers, liver abscesses, reticuloperitonitis, and chronic abscessation.
On presentation to New Bolton Center, the cow was quiet but responsive with a kyphotic stance; however, she responded appropriately to a withers pinch. She was tachycardic (100 beats/min) with a normal pulse quality. Rectal examination revealed dark and tarry manure. In addition, a structure was transcutaneously palpated caudal to the costochondral junction in the right paralumbar fossa that was suspected to be liver. The results of the remaining physical examination were unchanged from those previously obtained in the field. Repeat analysis of liver enzyme values revealed an AST concentration of 319 IU/L and a GGT concentration of 410 IU/L. In addition, sorbitol dehydrogenase (SDH) concentration was determined to be 26.95 IU/L (RI: 6.10 to 18.40 IU/L). All other hemogram and serum biochemistry values were relatively unchanged from previous results.
Sonographic evaluation of the abdomen revealed marked hepatomegaly with the liver extending caudal to the last rib into the right paralumbar fossa. Numerous anechoic to hypoechoic lesions were disseminated throughout the heteroechoic hepatic parenchyma (Figure 1). Some of the lesions contained echogenic strands while others were largely anechoic and cyst-like, appearing isoechoic to the lumens of the portal vessels. The masses ranged in size from a few millimeters up to 10 cm in diameter and created a “Swiss cheese”-like appearance in some areas. Blood flow was limited to the periphery of the lesions. The splenic architecture was markedly disrupted by similar lesions that were isoechoic to the lumens of the splenic vessels (Figure 2). The spleen appeared enlarged and measured 11 cm at its maximal thickness in the 9th intercostal space. Small hypoechoic masses with irregular margins were seen in the renal cortex and medulla.
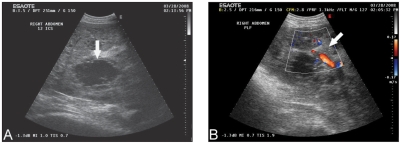
Figure 1.
Ultrasonograms with color Doppler (B) of the liver imaged at the 12th intercostal space (A) and paralumbar fossa (B) on the right side of the abdomen. The images depict an anechoic cystic lesion (arrow) within the liver parenchyma (A) with blood flow along the periphery of the lesion (arrow) (B).
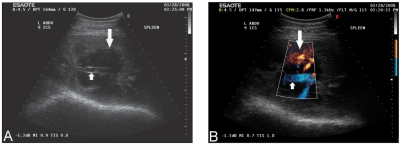
Figure 2.
Ultrasonograms with color Doppler (B) of the spleen at the 9th intercostal space on the left side of the abdomen. The images depict an anechoic cystic structure within the splenic parenchyma (large arrow) (A), with blood flow along the periphery of the lesion (large arrow) (B). The splenic vein is evident as it tracts through the center of this lesion (small arrow) (A) (B).
Following the sonogram, an ultrasound-guided tru-cut biopsy of the liver was performed. Histological evaluation revealed that most of the hepatic parenchyma was effaced by multifocal to coalescing, poorly demarcated and un-encapsulated proliferations of neoplastic endothelial cells (Figure 3; C, D, E). The cells were aligned end-to-end along collagen bundles, forming irregular blood-filled channels and sinuses. The nuclei were plump, ovoid, and large, with prominent discrete nucleoli. There was moderate anisokaryosis, but mitoses were not observed. Hepatocytes were atrophic and contained discrete small, clear vacuoles. The biopsy results confirmed a suspected diagnosis of visceral hemangiosarcoma with potential multiorgan involvement, or distant metastasis evidenced by the ultrasonographic findings.
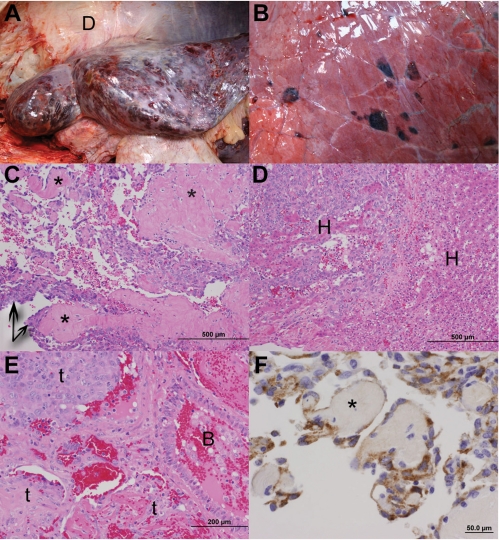
Figure 3.
Gross necropsy photographs depicting multifocal cavitated, blood filled nodules disseminated throughout all lobes of the liver (Figure 3A) and lung (Figure 3B). For reference, the peritoneal surface of the diaphragm is labeled (Figure 3A, D). Hematoxylin and eosin stained photomicrographs of formalin-fixed, paraffin embedded (FFPE) sections of the liver demonstrate a proliferation of neoplastic endothelial cells (Figure 3C, arrows) that align along collagen bundles (Figure 3C and Figure 3F, asterisks), and form blood-filled sinuses and clefts. Neoplastic cells efface the hepatic parenchyma and isolate small islands of hepatocytes (Figure 3D, H). Neoplastic cells of similar morphology are present within the lung (E, t); blood fills the lumen of an adjacent bronchiole (Figure 3E, B). An indirect immunohistochemical stain performed on FFPE sections of liver (Figure 3F) demonstrates strong expression of factor VIII antigen within neoplastic cells (DAKO, Factor VIII polyclonal-antibody, 1:1000 dilution).
The cow remained under the care of New Bolton Center for 4 d. Flunixin meglumine (Banamine, Schering-Plough Animal Health, Summit, New Jersey, USA), 1.1 mg/kg BW, IV, q24h was initiated upon admission to the tertiary care facility and continued daily. Over the duration of her hospital care, the cow quickly declined in health and comfort. The heart rate was persistently tachycardic (80 to 100 beats/min). Dyspnea, shown by increased respiratory effort with an abdominal component despite a normal respiratory rate (24 to 32 breaths/min), was often observed. In addition, she continued to have a kyphotic stance. After the first day, she became consistently weaker and she would only stand with assistance. On day 4, the cow was unable to stand and was inappetent. Due to her decreased comfort level, recumbency, and grave prognosis of disseminated hemangiosarcoma, the owner consented to humane euthanasia.
At necropsy, disseminated masses consistent with hemangiosarcoma were observed. There was extensive involvement of the liver and spleen with suspected metastasis to the lungs, kidney, nasal turbinates, right corneal limbus, and right perirenal lymph nodes. Severe hepatomegaly was observed with multifocal to coalescing, slightly raised, dark red-purple to tan, soft, friable nodules, 0.5 to 5 cm in diameter, disseminated throughout the surface and parenchyma (Figure 3; A, B). These nodules were cavitated and contained liquid to clotted blood admixed with friable tan material. There was a large 20 cm × 10 cm blood-filled cavity at the caudal pole of the spleen, and an additional 5-cm diameter, mottled tan-white to dark red nodule that expanded and effaced the parenchyma of the cranial end. Scattered throughout the parenchyma of both kidneys were pinpoint, red, nodular foci that were 1 mm in diameter. The right perirenal lymph node was enlarged and effaced by cavitated, blood-filled, red friable nodules. The lungs contained disseminated dark red foci that were 2 to 3 cm in diameter. In addition, chronic adhesions were present in the abdomen and multiple ulcers were observed in the abomasum. Mild melena was present within the lumen of the intestines.
Histomorphological evaluation of tissues harvested at necropsy, and immunohistochemical analysis of the liver, which demonstrated abundant Factor VIII-related antigen expression by neoplastic endothelial cells, confirmed the diagnosis of hemangiosarcoma that was made on biopsy (Figure 3; F). Given the extensive lesions involving both the liver and spleen, it is suspected that this tumor arose within either or both of these organs with metastases to other organs. However, we cannot rule out a multicentric origin of this neoplasm. CVJ
Discussion
Hemangiosarcoma is a very rare disease in adult cattle. Of confirmed neoplasms detected at Canadian slaughterhouses from 1974 to 1980, hemangiosarcoma represented 0.3% (4/1370) (1). Few case reports have been published in the literature and only 1 of these recorded the presence of hemangiosarcoma in visceral organs other than the lungs (2–9). That case report described a congenital multifocal hemangiosarcoma in a stillborn Hereford calf with skin, skeletal muscle, bone, kidney, spleen, mesentery, liver, lung, and heart involvement (2). Other sites suspected as primary include cutaneous (3), cutaneous external nares (4), long bones (5), skeletal muscle (6), vertebrae (7), extradural spinal cord and brain (8), and mandible (9). Among these 7 case reports, suspected metastasis to the lungs was observed in 4 but the spleen, liver, and kidneys were devoid of hemangiosarcoma lesions (3,5–9). One case with an antemortem diagnosis of non-visceral hemangiosarcoma was lost to follow-up before further investigation of metastasis could be determined (4). Clinical signs reported are non-specific and depend on the site of the lesions. These signs include weakness, tachypnea, decreased milk production, a solitary mass with rapid regrowth following excision and central and peripheral neuropathies (3–9).
Several aspects of clinical presentation and diagnostic results in this case were similar to canine splenic hemangiosarcoma with metastasis. The non-specific clinical signs initially observed on physical examination (inappetence, weakness, weight loss, and tachycardia) are consistent with those clinical signs commonly observed in the dog (10). In addition, dyspnea, as observed in this cow while in the hospital, is often seen in dogs with metastasis to the lungs (11). Also, metastasis can occur to the liver, omentum, and mesentery via hematogenous routes or transabdominal transplantation in the dog (10).
Similar hematological changes observed in this cow can be seen in dogs with splenic hemangiosarcoma. Common clinicopathologic findings include anemia, thrombocytopenia, and neutrophilic leukocytosis (10,12). Elevated liver enzymes are observed when the liver is affected (10,12). Interestingly, this cow was leukopenic with a lymphopenia and absolute neutrophil count within normal reference range.
The fibrinogen concentration in this cow was in the lower limits of the reference range. Fibrinogen is produced exclusively by the liver and, given the extensive infiltration of neoplastic cells within the liver parenchyma, the decreased concentration may have been due to decreased production or increased utilization as in disseminated intravascular coagulation (DIC). Fibrinogen levels are not routinely measured in dogs but hypofibrinogenemia has been reported in cases of hemangiosarcoma in young horses (< 3 y old), a species in which fibrinogen levels are routinely measured (13). However, this finding contrasts with fibrinogen levels measured in mature horses with disseminated hemangiosarcoma (14).
Diagnostic modalities such as ultrasound and liver biopsy were extremely useful for narrowing the differential diagnoses list. Sonographic evaluation of the abdomen revealed numerous masses whose echogenicity and blood flow pattern were most consistent with fluid filled cavitations, specifically hematomas at various stages of maturity (15). Given the involvement of multiple organs and marked disruption of the hepatic and splenic architecture, a neoplastic process that would result in areas of hemorrhage and necrosis seemed most likely and elevated the suspicion of hemangiosarcoma over other neoplastic processes such as lymphosarcoma or carcinoma. Furthermore, the sonographic appearance and distribution of the lesions is comparable to lesions described in dogs with splenic hemangiosarcoma (16). An ultrasound-guided biopsy was important for obtaining a diagnostic liver sample thus leading to an antemortem diagnosis of hemangiosarcoma.
Information regarding treatment and survival time of hemangiosarcoma in cattle is limited. In the dog, it has been reported that survival times are longer with a lower meta-static potential in cutaneous hemangiosarcoma compared with visceral hemangiosarcoma where there is rapid and widespread metastasis and the tendency to bleed within body cavities (hemoabdomen, hemoperitoneum, hemothorax) (17). In this case of disseminated hemangiosarcoma, the survival time following initial examination and prior to euthanasia was 6 d. Although chemotherapeutic agents have been used in treatment of hemangiosarcoma in other species, there is no Food and Drug Administration approved chemotherapeutic agent for use in treatment of neoplastic conditions in cattle. Off-label treatments can be performed with the understanding that the animal’s milk or meat would not be available for human consumption.
Several significant factors could have contributed to development of hemangiosarcoma in this cow. She was an older cow and the incidence of neoplasia is thought to increase with age. Interestingly, hemangiosarcoma has been reported in approximately 0.3% to 2% of recorded canine necropsies, which is similar to the incidence of hemangiosarcoma in cattle generated from the Canadian slaughterhouse (1,11). It is possible that hemangiosarcoma is not observed frequently in dairy cattle since most cows are culled before they reach an older age. The average age of cows reported in Wisconsin Holstein herds is 5 y (18). However, this suggestion is in contrast to the previous case reports of bovine hemangiosarcoma which indicated an age of 4 y or younger (2–9).
Purebred animals have an increased frequency of cancer (11). The German shepherd, golden retriever, Labrador retriever, and standard poodle are over-represented with incidence of hemangiosarcoma (10,11). In horses, a true breed predilection has not been established (13). Although reported cases of hemangiosarcoma in cattle have been of different breeds, a previous case of disseminated hemangiosarcoma in an aged Brown Swiss cow was diagnosed at New Bolton Center in April of 2007. Therefore, it is unknown if disseminated hemangiosarcoma has an over-representation of 1 breed or is a purely sporadic disease, more similar to that in the horse. More reports involving cattle would be necessary for validation.
There is still debate regarding whether disseminated visceral canine hemangiosarcoma represents metastatic spread from a primary tumor, or whether multicentric neoplastic transformation of endothelial cells occurs (19). In this case, the primary location within the spleen and/or liver is speculative and based on the extent of the lesions within these locations. Thus, the diagnosis of disseminated versus a primary splenic and/or hepatic visceral hemangiosarcoma was reached.
Given the malignant properties of this neoplasm with evidence of multiorgan involvement, and the clinical deterioration of the cow, a grave prognosis was warranted. In-hospital treatment was palliative, and the cow was appropriately humanely euthanized. To the authors’ knowledge, this is the first account of an antemortem diagnosis of disseminated hemangiosarcoma with a suspected primary location of the liver and/or spleen in a cow. This case report suggests the inclusion of disseminated hemangiosarcoma as a differential diagnosis of cattle with non-specific clinical signs (lethargy, weight loss, anorexia), anemia, thrombocytopenia, and elevated liver enzymes.
Acknowledgement
The authors thank Dr. JoAnn Slack for providing the ultrasound images and assistance in interpreting the ultrasound findings. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Dukes TW, Bundza A, Corner AH. Bovine neoplasms encountered in Canadian slaughterhouses: A summary. Can Vet J. 1982;23:28–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Badylak SF. Congenital multifocal hemangiosarcoma in a stillborn calf. Vet Pathol. 1983;20:245–247. doi: 10.1177/030098588302000213. [DOI] [PubMed] [Google Scholar]
- 3.Urdaz JH, Tyler JW, Henry CJ, Gautz P, Turk JR, Turnquist SE. Primary cutaneous hemangiosarcoma in a cow. Vet Rec. 2001;149:306–307. doi: 10.1136/vr.149.10.306. [DOI] [PubMed] [Google Scholar]
- 4.Queen WG, Masterson MA, Weisbrode SE. Hemangiosarcoma of the external nares in a cow. J Am Vet Med Assoc. 1992;201:1411–1412. [PubMed] [Google Scholar]
- 5.Guard C, Wilkinson JE. Hemangiosarcoma in a cow. J Am Vet Med Assoc. 1984;185:789–790. [PubMed] [Google Scholar]
- 6.Vermunt JJ, Thompson KG. Hemangiosarcoma in a 4-month-old calf. New Zealand Vet J. 2001;49:120–121. doi: 10.1080/00480169.2001.36216. [DOI] [PubMed] [Google Scholar]
- 7.Zachary JF, Jones MG, Wolff WA. Multicentric osseous hemangiosarcoma in a Chianina-Angus steer. Vet Path. 1981;18:266–270. doi: 10.1177/030098588101800215. [DOI] [PubMed] [Google Scholar]
- 8.Sutton RH, McLennan MW. Hemangiosarcoma in a cow. Vet Path. 1982;19:456–458. doi: 10.1177/030098588201900413. [DOI] [PubMed] [Google Scholar]
- 9.Poulsen KP, McSloy AC, Perrier M, Prichard MA, Steinberg H, Semrad SD. Primary mandibular hemangiosarcoma in a bull. Can Vet J. 2008;49:901–903. [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond TN, Pesillo-Crosby SA. Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003–2005) J Am Vet Med Assoc. 2008;4:553–558. doi: 10.2460/javma.232.4.553. [DOI] [PubMed] [Google Scholar]
- 11.MacEwen EG. Miscellaneous tumors. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. 3rd ed. New York: WB Saunders; 2001. pp. 639–646. [Google Scholar]
- 12.Hammer AS, Couto CG, Swardson C, Getzy D. Hemostatic abnormalities in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:11–14. doi: 10.1111/j.1939-1676.1991.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 13.Johns I, Stephen JO, Del Piero F, Richardson DW, Wilkins PA. Hemangiosarcoma in 11 young horses. J Vet Intern Med. 2005;19:564–570. doi: 10.1892/0891-6640(2005)19[564:hiyh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Southwood LL, Schott HC, II, Henry CJ, et al. Disseminated hemangiosarcoma in the horse: 35 cases. J Vet Intern Med. 2000;14:105–109. doi: 10.1892/0891-6640(2000)014<0105:dhithc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Wicks JD, Silver TM, Bree RL. Gray scale features of hematomas: An ultrasonic spectrum. Am J Roentgenol. 1978;131:977–980. doi: 10.2214/ajr.131.6.977. [DOI] [PubMed] [Google Scholar]
- 16.Wrigley RH, Park RD, Konde LJ, Lebel JL. Ultrasonographic features of splenic hemangiosarcoma in dogs: 18 cases (1980–1986) J Am Vet Med Assoc. 1988;192:1113–1117. [PubMed] [Google Scholar]
- 17.Schultheiss PC. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J Vet Diagn Invest. 2004;16:522–526. doi: 10.1177/104063870401600606. [DOI] [PubMed] [Google Scholar]
- 18.Sattler CG, Detine MR. Trends in herd age structure and the relationships with management characteristics in Wisconsin Holstein herds. J Dairy Sci. 1989;72:1027–1034. [Google Scholar]
- 19.Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors of Domestic Animals. 4th ed. Ames, Iowa: Blackwell Publ; 2002. pp. 99–100. [Google Scholar]