Summary
— The synthesis of 2,4-bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-1,3,5-triazine 6a and 2,4-bis[4-(1,4,5,6-tetrahydropyrimidin-2-yl)phenyl]-1,3,5-triazine 6b in 3 steps from either 4-bromobenzamidine or 4-(carbamoyl)benzamidine is reported. The synthesis of 4,6-bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-2-dimethylamino-1,3,5-triazine 9a and 4,6-bis[4-(1,4,5,6-tetrahydropyrimidin-2-yl)phenyl]-2-dimethylamino-1,3,5-triazine 9b in 2 steps from 1,4-dicyanobenzene is also described. The compounds 6b and 9b bind strongly to DNA model sequences and inhibit topoisomerase II from 2 microbial sources. Compounds 6a and 9a bind to both DNA and RNA model sequences whereas 6b and 9b essentially do not bind to the RNA model.
Keywords: triazine, diaryldiamidine, DNA, RNA, topoisomerase II
Introduction
Previous investigations in our laboratory have found that dicationic diaryldiamidines are active antiprotozoan agents [1] and exhibit strong binding to DNA [2–4]. Pentamidine and analogs have been found to be effective in treatment of Pneumocystic carinii pneumonia (PCP) and are useful in controlling AIDS-related PCP [5–7]. Relationships between DNA binding, inhibition of DNA-dependent enzymes, diamidine molecular shape and in vivo anti-PCP activity are beginning to emerge [8]. Typically, these dicationic molecules bind to DNA by selectively interacting with AT-rich regions of the minor groove. The complementarity of the curvature of the dicationic molecules with that of the minor groove of DNA has been considered an important determinant in the interaction of such groove binders with DNA [9–11]. Recently Cory et al have expanded upon the isohelicity paradigm of Cain, Dickerson, Lown and others by developing a method to estimate the radius of curvature of dicationic groove-binding molecules [8]. These workers found that pentamidine and analogs with radius of curvature values of between 10 and 100 Å strongly bind to DNA whereas analogs with smaller or larger values interact more weakly. However, it is important to note that the precise mode of binding of these dicationic molecules to DNA can be highly sensitive to structure [2–4]. Berenil and stilbamidine bind in the minor groove of DNA at sites which have 3 or more consecutive AT base pairs [12, 13]. DAPI, 2-[4′-guanyl-phenyl]-6-guanylindole, which has been known for some time to bind in the minor groove of DNA at AT sites, has recently been found to intercalate at GC sites [3]. The nucleic acid binding properties of a series of 2,5-diphenylfuran dications were found to range in interaction with DNA from AT selective minor groove binding, to classical intercalation at GC sites, to threading intercalation at GC sites [4]. The specific binding mode appears to depend upon the intrinsic nature of the cationic groups [4]. Although pentamidine and analogs and the 2,5-diphenylfuran dications have comparable radius of curvature values, the furan series can form a more extensive planar array and is thereby potentially capable of intercalation. Consequently, bis-benzamidines linked by planar rings (eg furans) appear prone to exhibit multiple binding modes to DNA.
We report here the synthesis of dicationic 2,4-diaryl-1,3,5-triazines, which have radius of curvature values in the range found by Cory et al and are favorable for strong binding to the DNA minor groove [14]. These molecules contain the triazine ring as the link between the bis-benzamidines, which is more hydrophilic than the furan link mentioned previously. Therefore, the dicationic triazines can be expected to exhibit different adsorption and distribution characteristics.
Chemistry
The synthesis of the key precursor 3 of the desired dicationic 2,4-bis-diaryl-1,3,5-triazines was achieved by 2 similar approaches (see scheme 1). Fusion of 4-bromobenzamidine benzenesulfonate 1 and formamidine hydrochloride at 190°C for 16 h gave 2,4-bis-(4-bromophenyl)-1,3,5-triazine 2 in good yield [15]. Conversion of the bis-bromo compound 2 into the corresponding bis-nitrile 3 was also achieved in reasonable yield by the action of copper(I) cyanide in quinoline at reflux temperature [16]. The bis-nitrile 3 was also obtained from a 2-step process beginning with the fusion of 4-(carbamoyl)benzamidine 4 with formamidine hydrochloride to form 2,4-bis[(4-carbamoyl)phenyl]-1,3,5-triazine 5 [15]. The bis-amide 5 was readily dehydrated by the action of phosphorus oxychloride to give the bis-nitrile 3 in good yield [17]. Both the imidazoline 6a and the tetrahydropyrimidine 6b were obtained by reaction of the bis-nitrile 3 in the presence of hydrogen sulfide with the appropriate diamine [18]. In the case of the reaction of 3 with ethylenediamine a small amount of the mono-substitution product, 4-(4-cyanophenyl)-2-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-1,3,5-tri-azine 6c was also obtained. The synthesis of 4,6-(4-cyanophenyl)-2-dimethylamino-1,3,5-triazine 8 was accomplished in a 1-step process by the reaction of 1,4-dicyanobenzene 7 and 1,1-dimethylguanidine in the presence of sodium hydride (see scheme 2) [19]. The dicationic triazines 9a and 9b were obtained by fusion of the bis-nitrile 8 with ethylenediamine and 1,3-propanediamine hydrochloride, respectively [20].
Scheme 1.
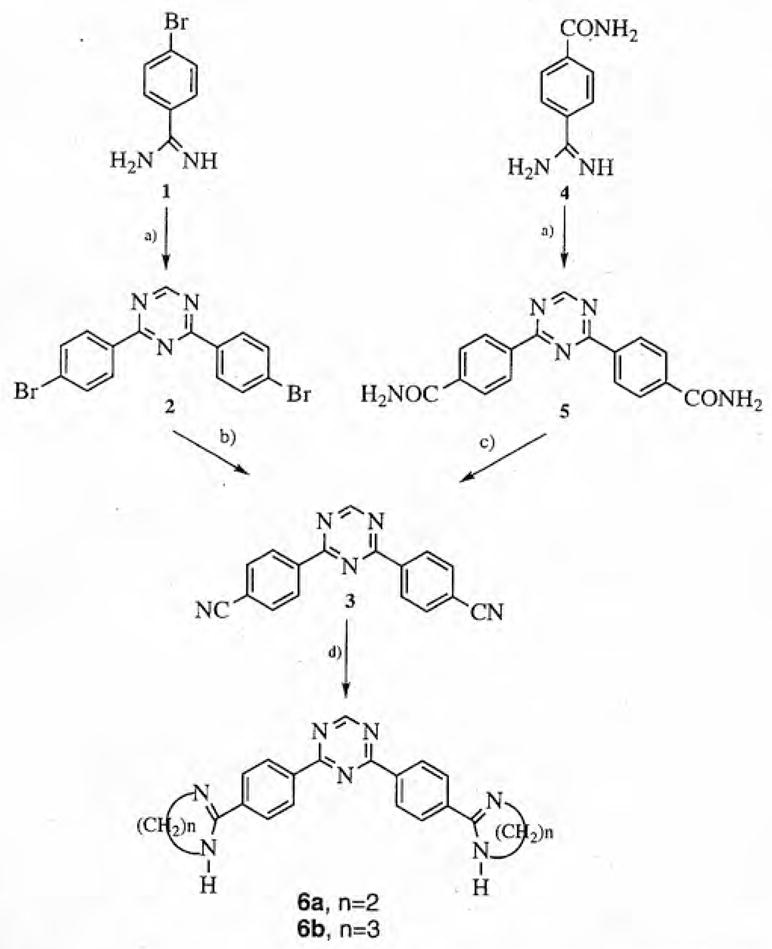
a) Formamidine hydrochloride, 190°C; b) CuCN, quinoline, reflux; c) POCl3, reflux; d) H2S, ethylenediamine or 1,3-diaminopropane, dimethoxyethane, reflux.
Scheme 2.
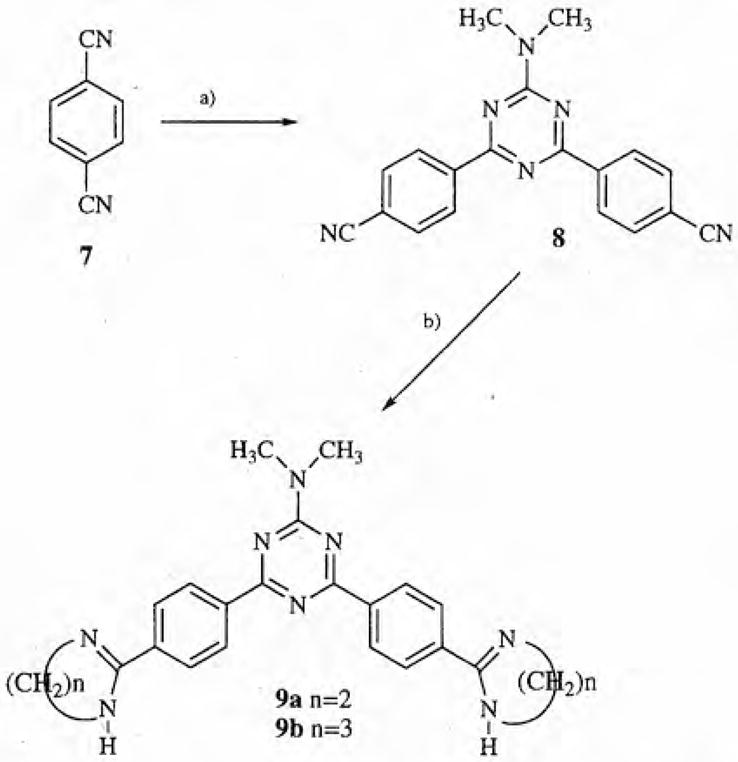
a) 1,1-Dimethylguanidine sulfate, NaH, DMF; b) ethylenediamine or 1,3-diaminopropane hydrochloride.
Biological results and discussion
The results for the biological evaluation of dicationic diaryl triazines 6a, 6b, 9a and 9b are found in table I. The interaction of the dicationic triazines with DNA was assessed by measuring the increase in thermal melting temperature (ΔTm) on complex formation with poly dA·dT [21]. As can be seen from table I, the 2-dimethylaminotriazines 9a and 9b give larger ΔTm values than the unsubstituted triazines 6a and 6b. In both sets of triazines the tetrahydropyrimidine compounds 6b and 9b exhibit larger ΔTm values than their imidazoline counterparts 6a and 9a. The greater ΔTm values for both sets being compared may be attributable to the theoretically greater van-der-Waals interactions of the respective triazines within the DNA minor groove. As expected, the mono cation 6c interacts only very weakly with DNA. The imidazoline derivatives 6a and 9a exhibit significant binding to the RNA model poly A·U, whereas 6b and 9b only weakly interact. The stronger binding of the dicationic imidazolines relative to the tetrahydropyrimidines with RNA is consistent with the previously reported study which suggested that such molecules bind to RNA by intercalation [21]. The dicationic triazines were evaluated against HIV-1 in a previously described cell culture screen [22], since 6a and 9a showed significant binding to our RNA model. Significant anti-HIV activity was noted for the stronger RNA binding molecules 6a and 9a, but significant levels of cell toxicity were also noted suggesting that these compounds may not be selective inhibitors against HIV-1 in culture. Since topoisomerase inhibition has been implicated in the mode of antimicrobial action of other diamidines the triazines were also evaluated against topoisomerase II isolated from 2 organisms [23, 24]. The 2 dicationic compounds 6b and 9b, which exhibit the significantly stronger binding to DNA, also show the greater inhibition of Giardia lamblia topoisomerase II, a similar trend is noted for inhibition of topoisomerase II isolated from P carinii. Three of the dicationic triazines 6a, 9a and 9b were evaluated in vivo against P carinii pneumonia using the immunosuppressed rat model [25]. Unlike many other dicationic diaryldiamidines, the triazine compounds exhibited severe toxicity to the animals and further synthetic investigation of the diaryltriazine system was discontinued.
Table I.
Biological evaluation of substituted diaryltriazines.
| Compound no | ΔTm(DNA)a | ΔTm (RNA)b | IC50(μM) 50% topoisomerase II inhibition (μM) |
Anti-HIV activity (EC50)e | In vivo activity against Pneumocystic carniii |
|||
|---|---|---|---|---|---|---|---|---|
| G lambliac | P cariniid | (mg/kg per day) | Toxicity | cyst/g Lung (% of control) | ||||
| 6a | 15.6 | 10.8 | 100 | 150 | 0.3h | 5.0 | 2+ | 96 |
| 6b | 23.9 | 1.2 | 5 | 60 | 133 | – | – | – |
| 6c | 1.0 | 1.2 | – | – | – | – | – | – |
| 9a | 20 | 11.0 | 5 | 14 | 0.12f | 5.0 | 2+ | 65 |
| 9b | >24 | 3.2 | 3 | 10 | 60g | 2.5 | 4+ | 26 |
| Pentamidine | 12.8 | 0 | – | >100 | – | 10.0 | 2+ | – |
| Saline | – | – | – | – | – | – | 0 | 100 |
Increase in thermal melting of poly dA·dT see ref [21);
increase in thermal melting of poly A·U, see ref [21];
inhibition of topoisomerase II isolated from G lamblia, see ref [24];
inhibition of topoisomerase II isolated from P carinii, see ref [23];
evaluation of the dicationic triazines against HIV-1 (strain LAV) in human PBM cells as described in ref [22];EC50 is the median effective concentration (μM);
toxic to PBM cells at ca 10 μM;
toxic to PBM cells at ca 100 μM;
toxic to PBM cells at ca 1.0 μM;
evaluation of iv dosage of the triazines against P carinii in rats as described in ref [25].
Experimental protocols
2,4-Bis(4-bromophenyl)-1,3,5-triazine 2
4-Bromobenzamidine benzenesulfonate [26] (7.32 g, 0.02 mol) and formamidine hydrochloride (2.15 g, 0.03 mol) were mixed and heated for 16 h in an oil-bath at 190°C After cooling, the mixture was poured into water, washed with water, dried and crystallized from acetone to give the product. Yield, 3.37 g (84%), mp 199°C. 1H-NMR (CDCl3, TMS), δ 7.68 (d, 4H, J = 8.3 Hz), 8.48 (d, 4H, J = 8.3 Hz), 9.23 (s, 1H). 13C-NMR (CDCl3, TMS), δ 128.1, 130.4, 132.1, 134.3, 166.9, 170.7. IR (KBr): ν 1589, 1576, 1543, 1512, 1415, 1071, 1011, 801 cm−1. MS, m/z: 389, 391, 393 (peaks in the molecular ion region).
2,4-Bis(4-cyanophenyl)-1,3,5-triazine 3
A mixture of 2,4-bis(4-bromophenyl)-1,3,5-triazine (3.17 g, 0.008 mol) and copper(I) cyanide (1.8 g, 0.02 mol) in freshly distilled quinoline (30 mL) was refluxed for 2 h. After cooling the reaction mixture was poured into ether (100 mL), washed with ether and water, and then extracted in a Soxhlet apparatus using acetone as a solvent. The crude product was crystallized from pyridine, washed with ether and recrystallized from acetone. Yield 1.49 g (65%), mp 263°C. Anal calc C17H9N5: C, 72.07; H, 3.20; N, 24.73; found: C, 71.79; H, 3.28; N, 24.64. 1H-NMR (DMSO-d6), δ 8.06 (d, 4H, J = 8.3 Hz), 8.71 (d, 4H, J = 8.3 Hz), 9,54 (s, 1H). 13C-NMR (DMSO-d6), δ 115.1, 117.9, 129.0, 132.7, 138.6, 167.4, 169.3. IR (KBr): ν 2224, 1544, 1515, 1422, 1315, 1016, 812 cm−1. MS, m/z: 283 (M+).
2,4-Bis[(4-carbamoyl)phenyl]-1,3,5-triazine 5
The title compound was prepared from commercially available 4-amidinobenzamide hydrochloride (24 g, 0.120 mol) and formamidine hydrochloride (12.6 g, 0.156 mol) in essentially the same way as described for 2,4-bis(4-bromophenyl)-1,3,5-triazine. The crude product (18.7 g, 98%) was extracted overnight with chloroform in a Soxhlet apparatus. The colorless crystals were filtered (2 g, mp 285°C). The insoluble product remaining in the thimble (16.5 g) was used in the next step. 1H-NMR (DMSO-d6), δ 7.48 (br s, 2H), 8.05 (d, 4H, J = 8.3 Hz), 8.10 (br s, 2H), 8.49 (d, 4H, J = 8.3 Hz), 9.43 (s, 1H). 13C-NMR (DMSO-d6), δ 128.0, 128.2, 136.9, 138.3, 166.6,167.1, 169.3. IR (KBr): ν 3408, 3194,1660, 1574, 1514, 1440, 1397, 1313,818 cm−1.
Formation of 3 by dehydration of 2,4-bis[(4-carbamoyl)phenyl]-1,3,5-triazine
2,4-Bis[(4-carbamoyl)phenyl]-1,3,5-triazine (16.5 g, 0.05 mol) was placed in phosphorus oxychloride (90 mL) and refluxed for 3 h. The excess phosphorus oxychloride was removed under diminished pressure and the residue carefully decomposed with ice. The solid was filtered off, washed with water, dried and extracted with acetone in a Soxhlet device to afford 2,4-bis(4-cyanophenyl)-1,3,5-triazine (12.11 g, 83%). Spectral and analytical data were given above.
2,4-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-1,3,5-triazine dihydrochloride dihydrate 6a
A solution of 2,4-bis[(4-cyanophenyl)]-1,3,5-triazine (0.5 g, 0.002 mol) and 1,2-diaminoethane (5 mL, 0.08 mol) in 1,2-dimethoxyethane (25 mL) was saturated with hydrogen sulfide and then refluxed overnight. Progress of the reaction was monitored by TLC (CHCl3/CH3OH/NH4OH, 11:4:1, v/v/v). After cooling, the precipitate was collected and extracted overnight with acetone in a Soxhlet apparatus. The acetone solution was concentrated and crystals of 4-(4-cyanophenyl)-2-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-1,3,5-triazine 6c separated (109 mg, 19%), mp 271°C. Anal calc C19H14N6·0.25H2O: C, 68.97; H, 4.41; N, 25.40; found: C, 68.75; H, 4.39; N, 25.25. 1H-NMR (DMSO-d6, TMS), δ 3.73 (s, 4H), 8.06 (d, 2H, J = 8.3 Hz), 8.07 (d, 2H, J = 8.3 Hz), 8.63 (d, 2H, J = 8.3 Hz), 8.73 (d, 2H, J = 8.3 Hz), 9.49 (s, 1H). 13C-NMR (DMSO-d6, TMS),δ 48.8, 114.9, 117.9, 127.6, 128.3, 128.9, 132.6, 133.5, 136.4, 138.8, 162.9, 167.1, 169.1, 170.0. IR (KBr): ν 3197, 2924, 2230, 1605, 1573, 1525, 1410, 818 cm−1. MS, m/z: 326 (M+).
The residue from the thimble was refluxed with dimethylformamide and filtered hot, washed with water and acetone (mp > 300°C). The hydrochloride had mp > 300°C (0.44 g, 52%). Anal calc C21H19N7·2HCl·2H2O: C, 52.39; H, 5.24; N, 20.37; found: C, 52.65; H, 5.29; N, 20.50. 1H-NMR (D2O, (CH3)3SiCH2CH2COONa), δ 4.14 (s, 8H), 7.97 (d, 4H, J = 8.3 Hz), 8.61 (d, 4H, J = 8.3 Hz), 9.38 (s, 1H). 13C-NMR (D2O, (CH3)3SiCH2CH2COONa), δ 47.6, 128.9, 131.5, 132.4, 141.6, 168.5, 169.9, 173.2. IR (KBr): ν 3344, 1616, 1567, 1540, 1510, 1422, 1289, 820 cm−1. MS (free base) m/z: 369 (M+).
2,4-Bis[4-(1,4,5,6-tetrahydropyrimidin-2-yl)phenyl]-1,3,5-triazine dihydrochloride dihydrate 6b
This compound was prepared from the dinitrile (0.5 g) in essentially the same way as described above for the imidazolinyl derivative, but traces of a monocyano intermediate were not isolated. (TLC, CHCl3/CH3OH/NH4OH, 7:5:2, v/v/v). The hydrochloride has mp > 300°C (0.60 g, 67%). Anal calc C23H23N7·2HCl·2H2O: C, 54.55; H, 5.77; N, 19.36; found: C, 54.63; H, 5.85; N, 19.29. 1H-NMR (DMSO-d6, TMS), δ 2.04 (t, 4H, J = 4.88 Hz), 3.55 (t, 8H, J = 5.38 Hz), 8.04 (d, 4H, J = 8.30 Hz), 8.78 (d, 4H, J = 8.30 Hz), 9.56 (s, 1H), 10.27 (s, 4H). 13C-NMR (DMSO-d6), δ 17.4, 38.6, 128.3, 128.7, 132.2, 138.7, 158.5, 167.4, 169.6. IR (KBr): ν 3446, 3160, 3022, 1647, 1541, 1422, 1373, 1314, 817, 740 cm−1. MS (free base), m/z: 397 (M+). Caution: an excess of 1,2-diaminoethane causes decomposition of the triazine ring and 1,4-bis[2-(4,5-dihydro-1H-imidazol-2-yl]benzene is a major product (mp 301–303°C). Application of the fusion reaction (below) with ethylenediamine hydrochloride for disubstituted triazines leads to the same undesired product [27]. 1H-NMR (DMSO-d6, TMS) δ 3.65 (s, 8H), 6.71 (br s, 2H), 7.84 (s, 4H). 13C-NMR (CD3COOD, TMS), δ 46.1, 128.4, 130.5, 166.2. IR (KBr), ν 3175, 2927, 2862, 1603, 1524, 1485, 1461, 1342, 1307, 1271 cm−1. MS, m/z: 214 (M+).
4,6-Bis(4-cyanophenyl)-2-dimethylamino-1,3,5-triazine 8
A mixture of 1,4-dicyanobenzene (25.6 g, 0.2 mol), 1,1-dimethylguanidine sulfate (13.6 g, 0.05 mol), sodium hydride (8.0 g of 60% oil dispersion, 0.2 mol), and N,N-dimethylformamide (200 mL) was stirred first at 0°C and then at room temperature for 3 h. After heating at 75°C for 3 h (TLC (CHCl3) showed the absence of 1,4-dicyanobenzene), the solvent was evaporated under vacuum and water (100 mL) was added. The solid was filtered, washed with water and methanol, subjected to silica-gel column chromatography, and eluted with chloroform. The eluent on concentration and addition of ethanol gave analytically pure bis-nitrile. The product can be recrystallized from pyridine. Yield, 8.7 g (27%), mp 264°C. Anal calc C19H14N6: C, 69.92; H, 4.32; N, 25.76; found: C, 69.85; H, 4.31; N, 25.78. 1H-NMR (DMSO-d6, TMS), δ 3.34 (s, 6H), 8.01 (d, 4H, J = 8.3 Hz), 8.65 (d, 4H. J = 8.3 Hz). 13C-NMR (DMSO-d6, TMS), δ 36.1, 114.3, 118.3, 128.8, 132.5, 140.2, 164.8. 168.7. IR (KBr): ν 2228, 1593, 1507, 1382, 1003, 810, 549 cm−1. MS, m/z: 326.
4,6-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-2-dimethylamino-1,3,5-triazine dihydrochloride dihydrate 9a
A mixture of the above bis-nitrile (2 g, 6 mmol), ethylenediamine dihydrochloride (8.2 g, 0.06 mol), and ethylenediamine (4.1 mL, 0.06 mol) was maintained in a sand-bath at 300–310°C for 10 min, during which time ammonia was evolved. The crude product was dissolved in hot water and a brown solid was filtered and discarded; crystals separated on cooling the filtrate. After recrystallization from boiling water chromatographically pure product was collected, washed with cold water, and dried overnight in a vacuum oven at 70°C. The product (0.95 g, 30%) did not melt at temperatures up to 360°C. Anal calc C23H24N8·2HCl·2H2O; C, 52.98; H, 5.80; N, 21.49; found: C, 53.00; H, 5.82; N, 21.39. 1H-NMR (DMSO-d6, TMS), δ 3.38 (s, 6H), 4.06 (s, 8H), 8.17 (d, 4H, J = 8.3 Hz), 8.76 (d, 4H, J = 8.3 Hz), 10.75 (brs, 4H). 13C-NMR (D2O, (CH3)3SiCH2CH2COONa), δ 37.1, 46.0, 125.0, 129.1, 130.2, 142.4, 165.3, 166.0, 169.4. IR (KBr): ν 3418, 3086, 2954, 1581, 1506, 1384, 1286, 1001, 816 cm−1. MS, (free base) m/z: 412 (M+).
2-Dimethylamino-4,6-bis[4-(1,4,5,6-tetrahydropyrimidin-2-yl)phenyl]-1,3,5-triazine dihydrochloride dihydrate 9b
This compound was prepared from the above bis-nitrile (2 g, 6 mmol) 1,3-diaminopropane dihydrochloride (9.0 g, 0.06 mol) and 1,3-diaminopropane (5.1 mL, 0.06 mol) as described for the imidazoline derivative. The hydrochloride was sparingly soluble in water and it was preferable to liberate the base from the reaction mixture by means of 1 N sodium hydroxide solution (50 mL). The crude base (1.54 g, 57%) was filtered, washed with water and crystallized from ethanol/acetone/water solution. The purified base (mp 303–305°C) was converted into the hydrochloride by means of hydrogen chloride in ethanol and then precipitated with ethyl ether. Yield, 1.1 g (32%). The product did not melt at temperatures up to 360°C. Anal calc C25H28N8·2HCl·2H2O: C, 54.64; H, 6.24; N, 20.40; found: C, 54.74; H, 6.23; N, 20.33. 1H-NMR (DMSO-d6, TMS), δ 2.02 (brt, 4H), 3.42 (s, 6H), 3.54 (brt, 8H), 8.04 (d, 4H, J = 8.3 Hz), 8.61 (d, 4H, J = 8.3 Hz), 10.54 (brs, 4H). 13C-NMR (DMSO-d6, TMS), δ 17.5, 36.0, 38.6, 128.0, 128.4, 131.1, 140.0, 158.3, 164.6, 168.7. IR (KBr): ν 3416, 3146, 2995, 1644, 1588, 1534, 1507, 1374, 1316, 1202, 1002, 815 cm−1. MS (free base), m/z: 440 (M+).
Acknowledgments
This work was supported by NIH Grants NIAID AI-27196, AI-33363, and by the Georgia VA Research Center for AIDS and HIV Infections. An award from the Chemical Instrumental Program of NSF (CHE 8409599) provided partial support for acquisition of the Varian VXR400 spectrometer. We appreciate the technical assistance of W Brake and B Bender with the animal model for P carinii.
References
- 1.Das BP, Boykin DW. J Med Chem. 1977;20:531–536. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 2.Wilson WD, Tanious FA, Buczak H, Ratmeyer LS, Venkatramanan MK, Kumar K, Boykin DW, Munson BR. In: Structure and Function, Vol 1 Nucleic Acids. Sarma RH, Sarma MH, editors. Adenine Press; New York: 1992. pp. 83–105. [Google Scholar]
- 3.Wilson WD, Tanious FA, Barton HJ, Strekowski L, Boykin DW, Jones RL. J Am Chem Soc. 1989;111:5008–5010. [Google Scholar]
- 4.Wilson WD, Tanious FA, Buczak H, Venkatramanan MK, Das BP, Boykin DW. In: Jerusalem Symposia on Quantum Chemistry and Biochemistry. Pullman B, Jortner J, editors. Vol. 23. Kluwer Academic Publishers; The Netherlands: 1990. pp. 331–353. [Google Scholar]
- 5.Montgomery AB, Luce JM, Turner J, Lin ET, Debs RJ, Corkery KJ, Brunette EN, Hopewell PC. Lancet. 1987:480–482. doi: 10.1016/s0140-6736(87)91794-6. [DOI] [PubMed] [Google Scholar]
- 6.Debs RJ, Blumenfeld W, Brunette EN, Straubinger RM, Montgomery AB, Lin E, Agabian N, Papahadjopoulos D. Antimicrob Agents Chemother. 1987;31:37–41. doi: 10.1128/aac.31.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tidwell RR, Jones SK, Geratz JD, Ohemeng KA, Cory M, Hall JE. J Med Chem. 1990;33:1252–1257. doi: 10.1021/jm00166a026. [DOI] [PubMed] [Google Scholar]
- 8.Cory M, Tidwell RR, Fairley TA. J Med Chem. 1992;35:431–438. doi: 10.1021/jm00081a003. [DOI] [PubMed] [Google Scholar]
- 9.Luck G, Zimmer G, Schweizer D. Stud Biophys. 1988;125:107–119. [Google Scholar]
- 10.Cain BF, Atwell GJ, Seelye RN. J Med Chem. 1969;12:199–206. doi: 10.1021/jm00302a001. [DOI] [PubMed] [Google Scholar]
- 11.Goodsell D, Dickerson RE. J Med Chem. 1986;29:727–733. doi: 10.1021/jm00155a023. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer C, Wahnert U. Prog Biophys Mol Biol. 1986;47:31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]
- 13.Neidle S, Pearl LH, Shelly JV. Biochem J. 1987;243:1–13. doi: 10.1042/bj2430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairley TA, Tidwell RR, Donkor I, Nainen NA, Ohemeng KA, Lombardy RJ, Bently JA, Cory M. J Med Chem. 1993;36:1746–1753. doi: 10.1021/jm00064a008. [DOI] [PubMed] [Google Scholar]
- 15.Fisher H, Summers LA. J Heterocycl Chem. 1980;17:333–336. [Google Scholar]
- 16.Ellis GP, Romney-Alexander TM. Chem Rev. 1987;87:779–794. [Google Scholar]
- 17.Weiss CD. J Org Chem. 1962;27:3514–3520. [Google Scholar]
- 18.Marxer A. J Am Chem Soc. 1957;79:467–172. [Google Scholar]
- 19.Alsofrom D, Grossberg H, Sheffer H. J Heterocycl Chem. 1976;13:917–919. [Google Scholar]
- 20.Oxley P, Short WF. J Chem Soc. 1947:497–505. [PubMed] [Google Scholar]
- 21.Wilson WD, Ratmeyer L, Zhao M, Strekowski L, Boykin DW. Biochemistry. 1993;32:4098–4104. doi: 10.1021/bi00066a035. [DOI] [PubMed] [Google Scholar]
- 22.Dixon DW, Kim MS, Kumar V, Obara G, Marzilli W, Schinazi RF. Antiviral Chem Chemother. 1992;3:279–283. [Google Scholar]
- 23.Dykstra CC, Tidwell RR. J Protozool. 1991;38:78S–81S. [PubMed] [Google Scholar]
- 24.Bell CA, Dykstra CC, Naimen NA, Cory M, Fairley TA, Tidwell RR. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tidwell RR, Jones SK, Naimen NA, Berger LC, Brake WB, Dykstra CC, Hall JE. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oxley P, Short WF. J Chem Soc. 1946:147–152. [Google Scholar]
- 27.Smolin EM, Rapoport L. s-Triazines and Derivatives. Interscience; New York: 1959. [Google Scholar]


