Abstract
Background
Recent studies demonstrated that alcohol dependence and excessive alcohol consumption are associated with increased rates of obesity. In healthy light-drinkers, we and others have observed associations between elevated body mass index (BMI) and reductions in brain volumes, lower concentrations of N-acetyl-aspartate (NAA, marker of neuronal viability) and choline-containing compounds (Cho, involved in membrane turnover), and lower glucose utilization, particularly in frontal lobe – a brain region that is particularly vulnerable to the effects of alcohol dependence. Here, we evaluated whether BMI in alcohol dependent individuals was independently associated with regional measures of brain structure, metabolite concentrations, and neocortical blood flow.
Methods
As part of a study on the effects of alcohol dependence on neurobiology, we analyzed retrospectively data from 54 alcohol dependent males, abstinent from alcohol for about one month and with BMI between 20 and 37 kg/m2 by structural MRI, perfusion MRI (blood flow) and proton magnetic resonance spectroscopic imaging.
Results
After correction for age, smoking status, and various measures of alcohol consumption, higher BMI was associated with lower concentrations of NAA, Cho, creatine and phosphocreatine (Cr, involved in high energy metabolism), and myoinositol (m-Ino, a putative marker of astrocytes) primarily in the frontal lobe, in subcortical nuclei, and cerebellar vermis (p<0.004). Regional brain volumes and perfusion were not significantly related to BMI. Furthermore, co-morbid conditions, clinical laboratory measures, and nutritional assessments were not significant predictors of these MR-based measures.
Conclusion
The results suggest that BMI, independent of age, alcohol consumption and common comorbidities, is related to regional NAA, Cho, Cr, and m-Ino concentrations in this cohort of alcohol dependent individuals. Additionally, as some common co-morbid conditions in alcohol dependence such as cigarette smoking are associated with BMI, their associations with regional brain metabolite levels in alcohol-dependent individuals may also be influenced by BMI.
Keywords: alcohol dependence, body mass index, obesity, magnetic resonance spectroscopy, brain imaging
The adverse consequences of chronic and excessive alcohol consumption on brain biology and function have been described in preclinical and clinical research (Crews and Nixon, 2009; Durazzo and Meyerhoff, 2007; Sullivan, 2000). Neuroimaging studies of alcohol dependent individuals (ALC) report regional morphological and biochemical abnormalities in both white matter (WM) and gray matter (GM) and subcortical structures (for review see e.g., Sullivan, 2000). Some of these abnormalities cluster within cortico-striato-thalamo-cerebellar and ponto-cerebellar systems that support a wide range of neuropsychological processes that are compromised in ALC, such as executive skills, impulse control, regulation of mood and affect, visuospatial skills, and postural stability (Mega and Cummings, 1994; Sullivan, 2003).
Proton magnetic resonance spectroscopy (1H MRS) generally demonstrated lower concentrations of N-acetyl-aspartate (NAA) and choline-containing compounds (Cho), predominantly in the frontal lobes, medial temporal lobes and cerebellum (Bendszus et al., 2001; Gazdzinski et al., 2008a; Gazdzinski et al., 2008b; Parks et al., 2002). NAA is regarded as a marker of neuronal viability; low NAA may reflect neuronal loss, lower neuronal density, atrophied dendrites and axons, and/or deranged neuronal metabolism. Cho is involved in membrane turnover/synthesis (see Ross and Bluml, 2001). The findings regarding myo-inositol (m-Ino), which is an osmolyte and putative marker of astrocytes (Ross and Bluml, 2001), were not conclusive (Bartsch et al., 2007; Bendszus et al., 2001; Durazzo et al., 2004; Ende et al., 2005; Schweinsburg et al., 2001). Additionally, significantly lower cerebral blood flow, referred to also as perfusion, was detected among ALC (reviewed in e.g., Gazdzinski et al., 2006). Based on recent research, these alcohol-related abnormalities appear to be adversely modulated by co-morbid cigarette smoking (for review see Durazzo et al., 2007).
A number of premorbid (e.g., genetic) and/or comorbid (e.g., medical, psychiatric) participant characteristics can promote substantial variability in the pattern and magnitude of neurobiological and neurocognitive abnormalities demonstrated in ALC (Durazzo and Meyerhoff, 2007; Meyerhoff and Durazzo, 2008). Prevalent comorbid medical conditions in alcohol use disorder include hypertension (daLuz and Coimbra, 2001; Klatsky, 1996; Parekh and Klag, 2001), coronary artery disease (Hennekens, 1996; Stinson et al., 2005; Tegos et al., 2001), and type-2 diabetes (Mansell et al., 2006), which are also associated with excess body weight (www.obesityresearch.nih.gov). Consumption of more than three alcohol drink equivalents per day per se is associated with increased rates of obesity, in particular abdominal obesity (Lukasiewicz et al., 2005; Schroder et al., 2007; Wannamethee and Shaper, 2003). Among ALC those with excessive body weight are at greater risk for metabolic syndrome (i.e., presence of type 2 diabetes, prediabetes, insulin resistance and two of the following: hypertension, dyslipidemia, abdominal obesity, or microalbuminuria) (Jarvis et al., 2007). Moreover, those with ALC may demonstrate an elevated waist-hip ratio (a measure of abdominal fat distribution) in the presence of normal body mass index (BMI, defined as body mass in kg divided by height in meters squared) (Addolorato et al., 1999), suggesting preference for abdominal fat deposition that is associated with insulin resistance and metabolic syndrome (Despres and Lemieux, 2006). A growing body of evidence suggests that excess body weight is associated with abnormalities in brain neurobiology. In older healthy adults, a higher BMI was associated with smaller global brain volumes (Ward et al., 2005). In young healthy individuals, regional reductions in gray matter (GM) volume and larger volumes of white matter (WM) were observed (e.g., Haltia et al., 2007; Pannacciulli et al., 2006). A positron emission tomography (PET) study found higher BMI associated with lower glucose metabolism, which in turn is related to cerebral blood flow and perfusion (e.g., Raichle et al., 1976), in prefrontal GM and cingulate gyrus (Volkow et al., 2009); this finding was related to lower striatal dopamine D2 receptor availability, suggesting overlap between neurobiological mechanisms associated with the development and/or maintenance of both obesity and alcohol dependence (Volkow et al., 2008b). Consistent with these findings, our proton magnetic resonance spectroscopy (1H MRS) studies of healthy middle-aged individuals (Gazdzinski et al., 2008c) showed that higher BMI was related to lower absolute concentrations of NAA throughout WM and in frontal GM, as well as lower frontal WM Cho. In an independent cohort of healthy elderly, we found that higher BMI was associated with lower NAA to Cho and NAA to Cr (creatine and phosphocreatine containing metabolites; involved in high energy metabolism) ratios in the anterior, but not posterior cingulate cortex (Gazdzinski et al., 2010). These finding were not explained by age or results of standard clinical labs, such as liver panel and complete blood count.
Taken together, high BMI is associated with abnormalities in brain morphology and metabolite levels in non-alcohol dependent cohorts. However, to date no study has specifically investigated the relationship of body weight to neurobiological measures in ALC. The goal of this retrospective analysis was to evaluate the relationship of BMI to regional brain volumes, metabolite concentrations, and perfusion in alcohol dependent individuals, while controlling for factors that may affect these MR-based measures. We hypothesized that higher BMI, cigarette smoking, age, and the magnitude of hazardous drinking levels are associated with smaller regional brain volumes, lower regional NAA levels, and lower cortical perfusion. Finally, as overeating and obesity were postulated to be protective against substance/ alcohol use disorders (Kleiner et al., 2004; Warren et al., 2005), we hypothesized an inverse relationship of measures of drinking severity to BMI.
MATERIALS AND METHODS
Participants
We studied 54 male treatment-seeking ALC, who were between 28 and 66 years and abstinent from alcohol for 32.2 ± 9.7 days. They were primarily recruited from the San Francisco VA Medical Center Substance Abuse Day Hospital and secondarily from the San Francisco Kaiser Permanente Chemical Dependence Recovery Program. The inclusion and exclusion criteria are fully described in (Durazzo et al., 2004). In short, all ALC met DSM-IV criteria for alcohol dependence with physiological dependence and consumed more than 150 standard alcoholic drinks per month for at least 8 years prior to enrollment into the study. Participants were free of general medical, neurologic, and psychiatric conditions, except for unipolar mood disorders, (medication controlled) hypertension, and hepatitis C due to their high prevalence among alcohol-dependent individuals (Hasin et al., 2007; Stinson et al., 2005). Alcohol consumption and smoking behavior over lifetime were assessed via the Lifetime Drinking History (LDH; Sobell and Sobell, 1992) and the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991), respectively. Clinical laboratory measures assessed for hepatocellular injury, red blood cell status, mean corpuscular volume, nutritional status (serum prealbumin) (Weinrebe et al., 2002). Participants were weighted within 1-3 days of the scan and their heights were generally obtained by self-report (when unsure, they were measured). BMI was calculated as body mass in kg divided by the height in meters squared. BMI ranged from 20.4 kg/m2 to 37.1 kg/m2.The Institutional Review Boards of the University of California San Francisco and the San Francisco VA Medical Center approved all procedures, and written informed consent was obtained from all participants prior to study.
Data Acquisition and Processing
All MR data were obtained on a standard 1.5T MR system (Siemens Vision, Iselin, NJ). 3D T1-weighted images were acquired for segmentation with a standard, coronal-oblique magnetization prepared rapid gradient echo sequence (TR/TE/TI = 10/7/300 ms, 15° flip angle, 1 × 1 × 1.5 mm3). Additionally, axial-oblique double spin-echo (TR/TE1/TE2 = 2500/20/80 ms, 1 × 1 × 3 mm3) yielded proton density and T2-weighted images. Metabolite spectra were acquired with proton multislice short-TE magnetic resonance spectroscopic imaging (1H MRSI; TR/TE/TI = 1800/25/165 ms) (Schuff et al., 1999) in three parallel, oblique-axial slices, each 15 mm thick, and 6 mm apart and covering centrum semiovale, nuclei of the basal ganglia, and cerebellum; an exemplary spectrum and its fit after subtraction of the fitted baseline are depicted in Figure 1. Finally, perfusion was acquired with pulsed arterial spin labeling using single shot EPI [TR/TE/TI2 (time between labeling pulse and the excitation pulse) = 2500/15/1500 ms] in five 8mm thick slices 2mm apart, above the Circle of Willis and oriented identical to the 1H MRSI slices (Jahng et al., 2003).
Figure 1.
Exemplary experimental spectrum and its fit after subtraction of the fitted baseline. This spectrum originates from parietal lobe. NAA, Cr, Cho, and m-Ino are observed at 2.0ppm, 3.0ppm, 3.2ppm, and 3.5ppm, respectively.
Probability maps of GM, WM, and cerebro-spinal fluid (CSF) in frontal, parietal, temporal, and occipital lobes were obtained by combining Expectation-Maximization Segmentation (EMS); (Van Leemput et al., 1999) with an atlas-based deformable registration method used to automatically identify regions of interest (ROIs) in the brain, as previously described; thalamus, caudate, lenticular nucleus, midbrain, and cerebellum were not divided into GM and WM, because their segmentation was not reliable (Cardenas, 2005). These maps were used to calculate tissue volumes. To account for individual variation in head size, absolute volumes of labeled structures were divided by intracranial volume defined as the sum of WM, GM, and CSF. Regional atrophy-corrected absolute metabolite concentrations of NAA, Cho, Cr, and m-Ino were calculated by combining SI and segmented MRI data (Meyerhoff et al., 2004). In particular, the MRSI dataset was water-suppressed with a finite impulse response deconvolution filter (Kuroda et al., 1989) and spectral noise was suppressed without affecting resonance line widths by using principal components analysis based on a deformation shape intensity model (Zhu et al., 2003). Integrals for the resonances corresponding to NAA, Cho, Cr, and m-Ino were estimated, including baseline correction (Soher et al., 1998) and multiplied with reference transmitter voltage to account for differences in coil loading due to different head sizes or positions of the head inside the RF coil, as described in (Michaelis et al., 1993). Concentrations of metabolites were then calibrated with intensity of water signal in the lateral ventricles as seen on proton density weighted MRI from the same imaging session that was appropriately corrected for coil loading and difference in receiver gains between sequences. To calculate metabolite concentrations for GM and WM in each ROI identified on MRI, the segmented MRI was spatially co-registered to the 1H MRSI dataset and reduced to MRSI resolution, taking into account the MRSI point-spread function, chemical-shift displacement, and slice profile (Schuff et al., 2001). Furthermore, metabolite peak quantities in each voxel were divided by the volume fraction of tissue, which was obtained from the co-registered tissue-segmented MRI (Schuff et al., 2001) (see below). Spectral data were subjected to automated quality-control measures described in detail in (Meyerhoff et al., 2004). For each tissue category in each ROI, average metabolite concentrations were calculated from all automatically selected voxels containing a sufficiently high tissue fraction: 80% or more for frontal WM and parietal WM, 50% or more for frontal GM, parietal GM, temporal GM, occipital GM, temporal WM , thalamus, midbrain and cerebellar vermis, and 30% or more for caudate and lenticular nucleus. Thalamus, midbrain, and cerebellar vermis were not divided into GM and WM, because their segmentation was not reliable (Cardenas, 2005). This method was utilized in our earlier report of relationships between higher BMI and lower regional metabolite concentrations in healthy middle-aged individuals (Gazdzinski et al., 2008c).
Average cerebral perfusion in frontal and parietal GM was calculated over voxels containing at least 80% GM tissue (for details of perfusion studies see Mon et al., 2009).
Statistical analyses
The main analyses used multiple linear regression to predict MR-based measures of regional brain volumes, metabolite concentrations, and perfusion with the following factors: age, smoking status, BMI, and average number of alcohol drinks per months over lifetime (proxy measure of drinking severity). These analyses were repeated with average number of alcohol drinks per month over the year preceding the study as a proxy measure for recent drinking severity. No significant interactions were observed; therefore, interaction terms were not included in the final models. In additional statistical analyses restricted to regions, where associations between BMI and metabolite concentrations were present, we used the following sets of selected factors as covariates at the same time (the sets were used one at a time): 1) prealbumin – a blood marker of nutrition, gamma-glutamyltransferase – a marker of liver function, red blood cell count, mean corpuscular volume, and hematocrit, as these factors were reported to affect brain recovery during abstinence from alcohol (Pfefferbaum et al., 2004) or 2) presence of common co-morbidities in ALC (hypertension, hepatitis C, chronic obstructive pulmonary disease), unipolar mood disorders (recurrent major depression, major depression in partial or sustained full remission), history of substance abuse/dependence (e.g., Durazzo et al., 2008).
Finally, to account for variability in tissues contributing to ROIs, in additional analyses we simultaneously corrected for mean WM and GM contributions to these regions, as well as for interaction between BMI and tissue contributions to these ROIs.
For BMI, the results were stringently corrected for multiple comparisons (13 regions: GM and WM of the frontal, temporal, parietal, occipital lobes, thalamus, caudate, lenticular nucleus, midbrain, and cerebellar vermis) and p = 0.05/13 = 0.0038 was considered significant. We did not correct for the number of MR outcome measures, as their relationships with demographic, clinical, and drinking variables were treated as separate scientific questions. Relationships between BMI and measures of drinking and smoking severity were evaluated with Spearman correlations. All statistical tests were conducted with SPSS-16.0 for Windows (SPSS; Chicago, IL).
RESULTS
Participant demographics
ALC were male, US Armed Services Veterans, 50.8 ± 8.9 years old, had 13.7 ± 1.9 years of education, and their BMI ranged from 20.4 kg/m2 to 37.1 kg/m2. Of the 54 participants, 41 were Caucasian (76%), six African American (11%), four Latino (7%), and three Native Americans (6%). Thirty two ALC were active chronic smokers and 22 ALC were non-smokers. Eleven ALC had a history of a unipolar mood disorder, five met criteria for substance abuse/dependence in early full remission or in sustained full remission, 13 ALC had medically controlled hypertension, and only one had type-2 diabetes. Among the smoking ALC, Fagerstrom score was 6.0±1.9, indicating medium to high levels of nicotine dependence, and they smoked on average 23±12 cigarettes per day for 28.5±12.1 years, resulting in 25±23 pack-years.
Associations of BMI, metabolite concentrations MR outcome measures, alcohol consumption and smoking variables
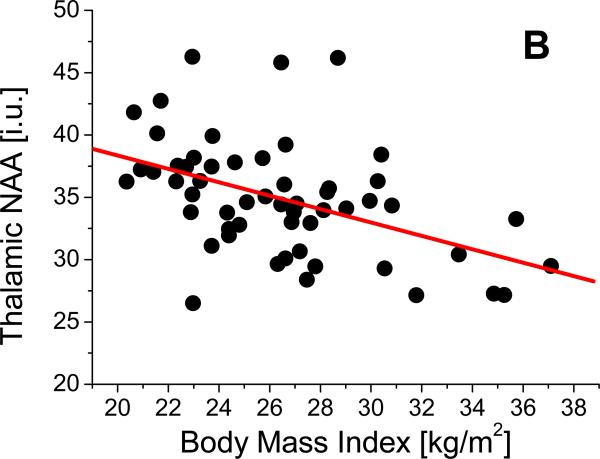
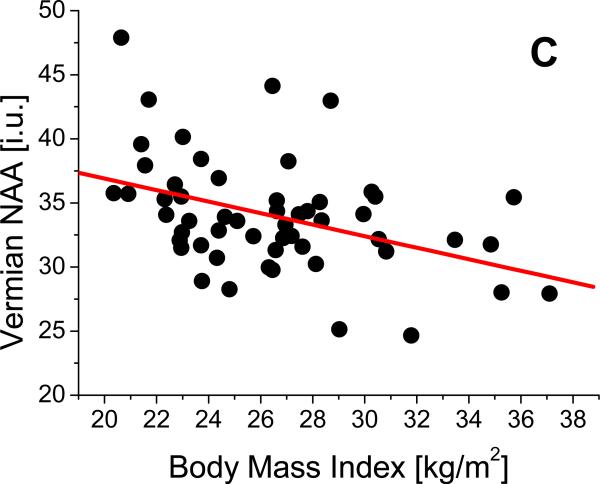
Linear regression analyses revealed that after adjustment for age, average number of drinks per month over lifetime, and smoking status, higher BMI was independently associated with lower NAA in frontal GM (β= -0.52, p=0.001, Figure 2a), frontal WM (β= -0.47, p = 0.002), thalamus (β= -0.51, p= 0.001, Figure 2b), midbrain (β= -0.44, p=0.003), and cerebellar vermis (β= -0.46, p= 0.003, Figure 2c). Higher BMI was also associated with lower Cho in the following regions: frontal GM (β= -0.52, p =0.001, Figure 2d), thalamus (β= -0.48, p=0.001), and cerebellar vermis (β= -0.47, p= 0.002). Higher BMI was also independently related to lower Cr in frontal GM (β= -0.53, p=0.001), frontal WM (β= -0.47, p=0.001), thalamus (β= -0.45, p=0.001), and lenticular nucleus (β= -0.46, p=0.002) and tended to be associated with lower Cr in caudate (β=-0.48, p=0.004). Also independent of age, smoking status, and drinking severity, higher BMI was associated with lower m-Ino in cerebellar vermis (β= -0.47, p=0.002). BMI was not significantly related to brain volume or perfusion in any region (see Table 1). None of these significant correlations were affected by additionally including one of the following sets of factors in the linear regression model (all factors within each set were used at the same time): 1) prealbumin, gamma-glutamyl-transferase, red blood cell count, mean corpuscular volume, and hematocrit, or 2) co-morbid medical conditions, unipolar mood disorders, or history of substance abuse/dependence . Similar patterns of relationships between BMI and metabolite concentrations were obtained when average number of alcoholic drinks per month over lifetime was substituted for average number of drinks per month over the year preceding the study.
Figure 2.
Relationships between body mass index (BMI) and concentrations of (A) frontal gray matter (GM) N-acetylaspartate (NAA), (B), thalamic NAA, (C), vermian NAA, and (D) frontal GM choline containing compounds (Cho).
Table 1.
Standardized regression coefficients β for associations between BMI and regional metabolite concentrations and (selected) amounts of variance explained by BMI.
| ROI | β / % Variance explained by BMI | |||
|---|---|---|---|---|
| NAA | Cho | Cr | m-Ino | |
| Frontal GM | -.052**/22.6 | -0.52**/21.9 | -0.53**/25.3 | -0.33 |
| Frontal WM | -0.47*/18.6 | -0.36 | -0.47**/19.0 | 0.03 |
| Thalamus | -0.51**/21.5 | -0.48**/19.0 | -0.45**/18.8 | -0.35 |
| Lenticular nucleus | -0.35 | -0.37 | -0.46*/17.0 | -0.15 |
| Midbrain | -0.44*/18.0 | -0.30 | -0.32 | -0.12 |
| Vermis | -0.46*/16.8 | -0.47*/18.0 | -0.37 | -0.47*/17.8 |
p<0.0038
p<0.0001.
When WM and GM tissue contributions, as well as the interactions between BMI and these tissue contributions were simultaneously added to the main model, a significant interaction between BMI and frontal WM contribution to frontal GM voxels emerged and it was associated with lower frontal GM NAA (β= -0.61, p=0.03, not corrected for multiple comparisons), but BMI was no longer associated with frontal GM NAA (p=0.72). The GM or WM content of thalami and cerebellar vermis were significant predictors of NAA, Cho, and Cr concentrations. However, tissue segmentation in subcortical brain is less reliable than in the neocortex. Nevertheless, when correcting for these tissue contributions, the reported relationships between BMI and subcortical metabolite concentrations were not appreciably altered. We have not observed similar associations involving tissue contributions in our previous study of healthy, middle-aged controls that used the same methodology (Gazdzinski et al., 2008c).
Cigarette smoking status, after adjustment for age, drinking severity, and BMI, was independently associated with lower NAA in frontal (β= -0.40, p = 0.01) and temporal WM (β= -0.37, p = 0.02). Additionally, after simultaneously correcting for GM and WM content in cerebellar vermis voxels, as well as their interactions with BMI, smoking status was associated with lower NAA (β= -0.29, p = 0.04). Larger average number of drinks over lifetime, after correction for age, smoking status, and BMI, was associated with higher m-Ino in frontal (β= 0.35, p = 0.02) and parietal WM (β= 0.36, p = 0.02), thalamus (β=0.29, p=0.04) and lenticular nucleus (β= 0.34, p = 0.02), as well as with smaller volumes of frontal (β=-0.31, p=0.02) and temporal GM (β=-0.31, p=0.05). .
BMI and alcohol and cigarette consumption
Finally, in this cohort, BMI was not significantly associated with measures of alcohol consumption (|rho|<0.18, p>0.10). However, among sALC, lower BMI was associated with higher pack-years (rho=-0.45, p=0.008) and higher number of cigarettes per day (rho=-0.38, p=0.04).
DISCUSSION
In this study of 1-month-abstinent, treatment-seeking alcohol dependent individuals, we observed that higher BMI was associated with lower concentrations of NAA, Cho and Cr in certain brain regions, but not with regional brain volumes or perfusion. Furthermore, while lower metabolite concentrations were generally not related to measures of drinking severity, more severe alcohol consumption was related to elevated m-Ino in multiple brain regions and to smaller volumes of frontal and temporal GM. Smoking status, after adjusting for age, BMI, and drinking severity, was associated with lower NAA in frontal and temporal lobes, consistent with our previous results in a smaller sample (Durazzo et al., 2006). Finally, whereas drinking severity was not related to BMI in this study, greater cigarette smoking severity was associated with lower BMI.
In ALC, lower NAA (Bartsch et al., 2007; Bendszus et al., 2001; Ende et al., 2005; Schweinsburg et al., 2001) and abnormal Cho concentrations (Bartsch et al., 2007; Bendszus et al., 2001; Ende et al., 2005; Schweinsburg et al., 2001) are generally considered to reflect alcohol induced brain injury. However, drinking severity has not been consistently related to lower metabolite concentrations, suggesting that other factors account for metabolite abnormalities in ALC. Here, we report inverse relationships between BMI and regional metabolite concentrations in the frontal lobe, thalamus and cerebellar vermis - regions that are components of the ponto-cerebellar and cerebello-thalamo-cortical systems (Sullivan, 2003) as well as major constituents of the brain reward pathway (Volkow et al., 2008a). These networks demonstrate neurobiological abnormalities in alcohol and substance use disorders (Baler and Volkow, 2006; Sullivan and Pfefferbaum, 2005) as well as in obesity (Volkow, 2008a).
The reported relationships between BMI and metabolite concentrations do not appear to be secondary to systematic error. For example, if coil loading were a factor influencing the observed relationships, all metabolite signal intensities in all brain regions would be uniformly decreased with higher BMI. This is not observed in this study (see Table 1), nor was it observed in our previous studies in healthy controls (one performed on the same scanner with the same methodology), in which correlations of BMI with metabolite concentrations were largely limited to NAA (Gazdzinski et al., 2008c; Gazdzinski et al., 2010). Additionally, the latter study utilized NAA concentrations scaled to Cr or Cho. However, the variability in contributions from various tissues to our spectroscopic regions in this study may potentially introduce some bias into the reported results; in our previous study that used the same methodology, the correlations between NAA and BMI were not affected by tissue contributions to spectroscopic ROIs, nor by interactions between BMI and other factors (Gazdzinski et al., 2008c). Finally, our previously reported associations between BMI and metabolite levels in healthy controls are consistent with correlations between BMI and frontal glucose metabolism obtained by FDG-PET (Volkow et al., 2009).
The associations between BMI and NAA in ALC, in the absence of correlations between BMI and volumes, are consistent with our findings in healthy light-drinking controls (Gazdzinski et al., 2008c; Gazdzinski et al., 2010). The mechanisms behind these relationships are not entirely clear; they may possibly reflect some level of insulin dysregulation that is common among obese individuals (about 20% of our sample) and is reported to be associated with reduced insulin transport into the brain and impaired glucose utilization (Craft, 2007), consistent with lower NAA (e.g., Baslow, 2003). However, the associations of BMI with Cho, Cr, and m-Ino concentrations were largely specific to this ALC cohort and not observed in our studies of healthy controls; this suggests dysfunction beyond simply brain energy metabolism in ALC. In fact, chronic and excessive ethanol consumption per se is associated with development of insulin resistance (Kang et al., 2007; Virmani et al., 2006) via inhibition of insulin and insulin-like-growth factor that can lead to neurodegeneration and gliosis (Cohen et al., 2007; de la Monte et al., 2008). However, it needs to be noted that we have no direct indications of insulin resistance in our sample. Taken together, this suggests that brain injury in alcohol dependence is not solely mediated by the effects of ethanol, but rather by a complex interplay among hazardous levels of drinking and comorbid factors such as elevated BMI and chronic cigarette smoking. A small postmortem study of six alcohol dependent individuals came recently to a similar conclusion (de la Monte et al., 2008).
BMI was not associated with brain volumetric changes, suggesting that structural changes in ALC may be preceded by metabolite abnormalities, or that the associations with BMI and morphology may be more localized to subregions within the large lobar regions we examined in this study. BMI was also not related to lobar gray matter perfusion in this cohort for similar reasons or because of a potential bias from variability in blood transit times (Gazdzinski et al., 2006).
Finally, our cohort did not demonstrate significant associations between higher alcohol consumption and lower BMI, which does not support the hypothesis that excessive food consumption protects against excessive alcohol use (Kleiner et al., 2004; Warren et al., 2005).
Our study has several limitations that may limit the generalizability of the findings. Among males consuming alcohol in excess, BMI is strongly related to measures of abdominal obesity (Addolorato et al., 1999), which in turn is strongly related to insulin resistance and metabolic syndrome (Despres and Lemieux, 2006). However, such obesity and insulin resistance measures were not obtained in this study. Moreover, ALC were examined after one month of abstinence from alcohol, so that the effects of alcohol consumption on metabolite concentrations per se might have been underestimated (assuming metabolic recovery as described in similar cohorts (Durazzo et al., 2006; Ende, 2005). Additionally, as alcohol dependence and obesity seem to share at least some neurobiological underpinnings (Volkow et al, 2008a), we cannot rule out that our relationships between BMI and metabolite abnormalities in ALC reflect some premorbid (e.g., genetic) factors common to both obesity and alcohol dependence. Finally, potential unrecorded group differences in nutrition, stress, exercise and general fitness, overall physical health, and genetic predispositions may mediate the results described in this study.
In conclusion, although the mechanisms have yet to be identified, higher BMI in this cohort of ALC was related to regionally lower metabolite markers of neuronal viability and membrane turnover in regions encompassing the reward pathway and cortico-ponto-cerebellar and cerebello-thalamo-cortical circuits. These networks support a wide range of neuropsychological processes that may be adversely affected by alcohol, substance use disorders, and potentially obesity. This data reinforces the observation that a number of common comorbid conditions can promote substantial variability in the pattern and magnitude of neurobiological and neurocognitive abnormalities observed in individuals with alcohol use disorders.
Acknowledgements
This project was supported by AA10788 (DJM), grants that PARTLY supported the project JU-WRBW-8/IF/2010 (SG), and partly by DA025202 (DJM) and DA24136 (TCD). This material is the result of work supported with resources and the use of facilities at the Radiology Research Service of the Veterans Administration Medical Center in San Francisco. We thank Mr J. O'Hara, and Drs G. Matson, and A. Ebel for technical support. We also extend our gratitude to Mary Rebecca Young and Bill Clift of the Veterans Administration Substance Abuse Day Hospital and Dr. David Pating, Karen Moise and their colleagues at the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco for their valuable assistance in recruiting participants. Finally, we thank our participants who made this research possible.
Contributor Information
Stefan Gazdzinski, Center for Imaging of Neurodegenerative Diseases, San Francisco Veterans Administration Medical Center, San Francisco, CA, USA. Marian Smoluchowski Institute of Physics, Jagiellonian University, Krakow, Poland..
Timothy C. Durazzo, Department of Radiology and Biomedical Imaging, University of California San Francisco, and Center for Imaging of Neurodegenerative Diseases, San Francisco Veterans Administration Medical Center, San Francisco, CA, USA..
Anderson Mon, Department of Radiology and Biomedical Imaging, University of California San Francisco, and Center for Imaging of Neurodegenerative Diseases, San Francisco Veterans Administration Medical Center, San Francisco, CA, USA..
Dieter J. Meyerhoff, Department of Radiology and Biomedical Imaging, University of California San Francisco, Center for Imaging of Neurodegenerative Diseases, and San Francisco Veterans Administration Medical Center, San Francisco, CA, USA.
REFERENCES
- Addolorato G, Capristo E, Caputo F, Greco AV, Ceccanti M, Stefanini GF, Gasbarrini G. Nutritional status and body fluid distribution in chronic alcoholics compared with controls. Alcohol Clin Exp Res. 1999;23:1232–1237. doi: 10.1111/j.1530-0277.1999.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Boning J, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. AJNR Am J Neuroradiol. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31:1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daLuz PL, Coimbra SR. Alcohol and atherosclerosis. An Acad Bras Cienc. 2001;73:51–55. doi: 10.1590/s0001-37652001000100006. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Cohen AC, Sheedy D, Harper C, Wands JR. Insulin and insulin-like growth factor resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res. 2008;32:1630–1644. doi: 10.1111/j.1530-0277.2008.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol Alcohol. 2007;42:174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Meyerhoff DJ. The relationships of sociodemographic factors, medical, psychiatric, and substance-misuse comorbidities to neurocognition in short-term abstinent alcohol-dependent individuals. Alcohol. 2008;42:439–449. doi: 10.1016/j.alcohol.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the Effects of Chronic Alcohol Consumption and Abstinence on Brain Metabolism: A Longitudinal Proton Magnetic Resonance Spectroscopy Study. Biol Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo T, Jahng GH, Ezekiel F, Banys P, Meyerhoff D. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol Clin Exp Res. 2006;30:947–958. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Weiner MW, Meyerhoff DJ. Are treated alcoholics representative of the entire population with alcohol use disorders? A magnetic resonance study of brain injury. Alcohol. 2008a;42:67–76. doi: 10.1016/j.alcohol.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008b;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008c;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Millin R, Kaiser LG, Durazzo TC, Mueller SG, Weiner MW, Meyerhoff DJ. Body mass index and neuronal integrity in healthy, cognitively normal elderly: a proton magnetic resonance spectroscopy study. Obesity. 2010;18:743–748. doi: 10.1038/oby.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia LT, Viljanen A, Parkkola R, Kemppainen N, Rinne JO, Nuutila P, Kaasinen V. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hennekens CH. Alcohol and Risk of Coronary Events. In: Zahkari S, Wassef M, editors. Alcohol and the Cardiovascular System. National Institutes of Health: National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1996. p. 15. [Google Scholar]
- Jahng GH, Zhu XP, Matson GB, Weiner MW, Schuff N. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49:307–314. doi: 10.1002/mrm.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis CM, Hayman LL, Braun LT, Schwertz DW, Ferrans CE, Piano MR. Cardiovascular risk factors and metabolic syndrome in alcohol- and nicotine-dependent men and women. J Cardiovasc Nurs. 2007;22:429–435. doi: 10.1097/01.JCN.0000297387.21626.88. [DOI] [PubMed] [Google Scholar]
- Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcohol Clin Exp Res. 2007;31:1581–1588. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Blood Pressure and Alcohol Intake: Clinical Aspects. In: Zahkari S, Wassef M, editors. Alcohol and the Cardiovascular System. National Institutes of Health: National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1996. p. 173. [Google Scholar]
- Kleiner KD, Gold MS, Frost-Pineda K, Lenz-Brunsman B, Perri MG, Jacobs WS. Body mass index and alcohol use. J Addict Dis. 2004;23:105–118. doi: 10.1300/J069v23n03_08. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Wada A, Yamazaki T, Nagayama K. Postacquisition data processing method for suppression of the solvent signal. Journal of Magnetic Resonance. 1989;84:604–610. [Google Scholar]
- Lukasiewicz E, Mennen LI, Bertrais S, Arnault N, Preziosi P, Galan P, Hercberg S. Alcohol intake in relation to body mass index and waist-to-hip ratio: the importance of type of alcoholic beverage. Public Health Nutr. 2005;8:315–320. doi: 10.1079/phn2004680. [DOI] [PubMed] [Google Scholar]
- Mansell D, Penk W, Hankin CS, Lee A, Spiro A, 3rd, Skinner KM, Hsieh J, Kazis LE. The illness burden of alcohol-related disorders among VA patients: the veterans health study. J Ambul Care Manage. 2006;29:61–70. doi: 10.1097/00004479-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner H. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype? Alcohol Clin Exp Res. 2008;32:1146–1158. doi: 10.1111/j.1530-0277.2008.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis T, Merboldt KD, Bruhn H, H,,nicke W, Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: Quantification of localized proton MR spectra. Radiology. 1993;187:219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ. The Impact of Chronic Cigarette Smoking on Recovery From Cortical Gray Matter Perfusion Deficits in Alcohol Dependence: Longitudinal Arterial Spin Labeling MRI. Alcohol Clin Exp Res. 2009;33:1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr Opin Nephrol Hypertens. 2001;10:385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Serventi KL, Sullivan EV. Brain volumes, RBC status, and hepatic function in alcoholics after 1 and 4 weeks of sobriety: predictors of outcome. Am J Psychiatry. 2004;161:1190–1196. doi: 10.1176/appi.ajp.161.7.1190. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Jr., Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol. 1976;33:523–526. doi: 10.1001/archneur.1976.00500080001001. [DOI] [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Schroder H, Morales-Molina JA, Bermejo S, Barral D, Mandoli ES, Grau M, Guxens M, de Jaime Gil E, Alvarez MD, Marrugat J. Relationship of abdominal obesity with alcohol consumption at population scale. Eur J Nutr. 2007;46:369–376. doi: 10.1007/s00394-007-0674-7. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend D, Knowlton R, Tanabe J, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in hippocampus by proton MR spectroscopic imaging and MRI neurobiology of aging. Neurobiology of Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res. 2001;25:924–934. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc.; 1992. pp. 41–72. [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. NIAAA Research Monograph No. 34: Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tegos TJ, Kalodiki E, Sabetai MM, Nicolaides AN. The genesis of atherosclerosis and risk factors: a review. Angiology. 2001;52:89–98. doi: 10.1177/000331970105200201. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- Virmani A, Binienda Z, Ali S, Gaetani F. Links between nutrition, drug abuse, and the metabolic syndrome. Ann N Y Acad Sci. 2006;1074:303–314. doi: 10.1196/annals.1369.027. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008a;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008b;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y, Pradhan K. Inverse Association Between BMI and Prefrontal Metabolic Activity in Healthy Adults. Obesity (Silver Spring) 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr. 2003;77:1312–1317. doi: 10.1093/ajcn/77.5.1312. [DOI] [PubMed] [Google Scholar]
- Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis. 2005;24:95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]
- Weinrebe W, Graf-Gruss R, Schwabe R, Stippler D, Fusgen I. The two-factor method--a new approach to categorizing the clinical stages of malnutrition in geriatric patients. J Am Geriatr Soc. 2002;50:2105–2107. doi: 10.1046/j.1532-5415.2002.50637.x. [DOI] [PubMed] [Google Scholar]
- Zhu X-P, Du A-T, Jahng G, Maudsley AA, Weiner MW, Schuff N. Improved Proton MRSI using a Deformation Shape Intensity Model. Magn Reson Med. 2003;50:474–482. doi: 10.1002/mrm.10572. [DOI] [PMC free article] [PubMed] [Google Scholar]