Abstract
Baseline neurodynamics are believed to play an important role in normal brain function. A potentially intrinsic property of the brain is the weak coupling between networks at rest, which enables it to be flexible, adapt, process novel stimuli, and learn. Brain regions become differentially coordinated in response to cognitive task and behavior demands and external stimuli. However, abnormally synchronized resting brain networks may also be associated with different pathologies. We investigated baseline network dynamics in the epileptic brain using information theoretic parameters to quantify coupling and directionality of information flow between different cortical regions. We estimated relative entropy, conditional mutual information and a related measure of directional coupling, from EEGs of patients with epilepsy and healthy subjects. At rest, the healthy brain appears to be characterized by low and non-directional network coupling, whereas the epileptic brain appears to be transiently and directionally synchronized.
I. INTRODUCTION
Epilepsy is a common neurological disorder affecting almost 1% of the US population. It is characterized by transient episodes of abnormal electrical activity in the brain and hyper-synchronization of distant neuronal networks. In addition to the ictal interval, seizure-related activity occurs prior to and following clinical onset/offset, as well as between seizures (inter-ictal activity). Despite this activity, the baseline (resting) epileptic brain is considered to be otherwise neurophysiologically normal. In the absence of other pathologies, brain function of patients with epilepsy is usually normal and may be disrupted by pre-ictal precursors and actual seizures. Resting brain dynamics are believed to play an important role in normal brain function, including our ability to adapt and learn [16]. At rest, the healthy brain is believed to be in a dynamic state of synchronized very low-frequency (≤ 0.1 Hz) oscillations but weakly-coupled networks, particularly at higher frequencies [5][11][3]. Low baseline network coupling may be an intrinsic property of the brain that enables it to be flexible. Brain regions become differentially activated and coordinated in response to cognitive and behavior demands and external stimuli. Coordination measures, e.g., frequency band-specific coherence, phase and generalized synchronization, and entropy measures have been proposed to describe neuronal synchrony in the healthy brain [8][4][19] and in pre-ictal electroencephalograms (EEG) of patients with focal and multi-focal seizures [15]. This paper focuses on differences in baseline neurodynamics of the healthy and epileptic brains.
In the context of epilepsy, network analysis has been previously applied to detect patterns of activity in EEG either in the pre-ictal interval or at seizure onset, and to localize the epileptic source and estimate the directionality of seizure propagation [8][6]. In addition, inter-region correlations of functional magnetic resonance imaging (fMRI) time series and EEG at rest have also been investigated, and there is increasing evidence that there is altered functional connectivity in the resting epileptic brain, including increased connectivity in medial temporal lobes but decreased connectivity in frontal and parietal lobes, at least in patients with mesial temporal epilepsy [10][20][1]. Brain connectivity can be studied at different spatio-temporal scales. This study investigates baseline network dynamics and large-scale inter-region connectivity using information theoretic measures, including relative entropy, conditional mutual information and a related measure of directional coupling, to quantify the information flow between cortical regions. All parameters are estimated from baseline scalp EEG data of patients with focal and multi-focal seizures and are compared to corresponding parameters from healthy control EEGs.
II. METHODS
All EEG data were recorded using a standard international 10-20 EEG system and a referential montage, and were collected at Beth Israel Deaconess Medical Center, Boston MA, in the Clinical Neurophysiology Laboratory of the Comprehensive Epilepsy Center. Baseline (at rest) scalp EEGs from 6 patients with diagnosed epilepsy and heterogeneous focal and/or multi-focal (or broad-focus) seizures were analyzed. These were classified clinically as non-ictal segments (20-30s long), unrelated to any pre-ictal or ictal activity, and with no visually identifiable inter-ictal waveforms, e.g., spikes. Baseline EEGs from 5 healthy adults (60-300s long) were analyzed for comparison. All data were sampled at 500 Hz.
A. EEG Data
Power-line noise was attenuated with a stop-band filter-bank centered at the noise 60 Hz harmonics, in the range 60-250 Hz, with a 2 Hz bandwidth for center frequencies ≤ 150 and a 4 Hz bandwidth for center frequencies > 150 Hz. Second order elliptical filters (20 dB attenuation in the stop-band, 0.5 dB ripple in the passband) were used. Signals were filtered in both forward and reverse directions to eliminate potential phase distortions due to the non-linear phase of the filter. Other artifacts related to eye blinking were eliminated using a matched-filtering approach, as described in [14].
B. Information theoretic measures
Inter-channel coupling was quantified using relative entropy (Kullback divergence D(p ∥ q)), which measures the distance between the distributions p(x) and q(y) of two random variables x and y, and mutual information I(x; y):
| (1) |
| (2) |
The statistics of baseline EEG signals are typically dynamic. Thus, to estimate the potentially time-varying probability density (pdf) of the EEG data we applied a 2s sliding window to each EEG signal of epilepsy patients, and a 5s window to EEGs of healthy controls. We estimated the pdf of each channel non-parametrically using an exponential kernel. From simple histograms of the data, we determined that given the asymmetry of the empirical distribution this was more appropriate than a Gaussian kernel. The optimum bandwidth was selected using cross-validation [2][13]. Similarly, the joint pdf p(x, y) between channels, was estimated using a two-dimensional kernel. Although relative entropy and mutual information quantify the inter-dependence between two channels, they do not describe the directionality of this dependence. Instead, conditional entropy measures may be estimated for this purpose. Directional relationships between brain regions are often established using Granger causality which assesses whether knowledge of a measurement from one region, helps to predict a corresponding measurement from another region [7]. Although this concept is applicable to a wide range or coupled linear processes, for non-linear processes methods based on information theory may be more appropriate. We applied a methodology adapted from [17]. Specifically, we defined the capacity of process x to predict process y as a conditional mutual information given by:
| (3) |
| (4) |
where nΔy is a time lag of n points and H(·) denotes entropy. Note that the expression in Equation 3 does not include the history of process y on itself, as we are interested only in the common information between the two processes [17]. We can derive a similar expression for I(y; x + Δx∣x) for the mutual information of y conditioned upon x. Then directionality is estimated by computing the ratio of mean conditional mutual information I(x; y + Δ y∣y) and I(y; x + Δ x∣x), averaged over the length of the data window and the total number of segments in each EEG signal:
| (5) |
where N is the number of data points within each EEG segment and K the total number of segments. This expression is equivalent to the two indices derived by [17] for a single data segment. Thus, if dx,y = 1 the coupling between signals is non-directional, whereas if dx,y > 1 the directionality is x → y and if dx,y < 1, y → x.
III. RESULTS
We first estimated baseline mutual information matrices for both healthy subjects and epilepsy patients in the entire frequency-range of interest (1-250 Hz). Results are summarized in Figures 1-3. In healthy controls, mutual information was low (μI=0.12, σI=0.03), and spontaneously and dynamically increased only in frontal regions, either bilaterally, e.g., top left panel in Figure 1, where increased inter-dependence between channels Fp1 and Fp2 are shown (I = 0.4), but low mutual information elsewhere, or between frontal channels within the same hemisphere, e.g., right top and bottom right panels in Figure 1. In contrast, significantly higher (μI = 0.29, σI = 0.05) but transiently increased mutual information was observed in all but one patient with epilepsy between random combinations of channels, e.g. top right and bottom left panels in Figure 2.
Fig. 1.
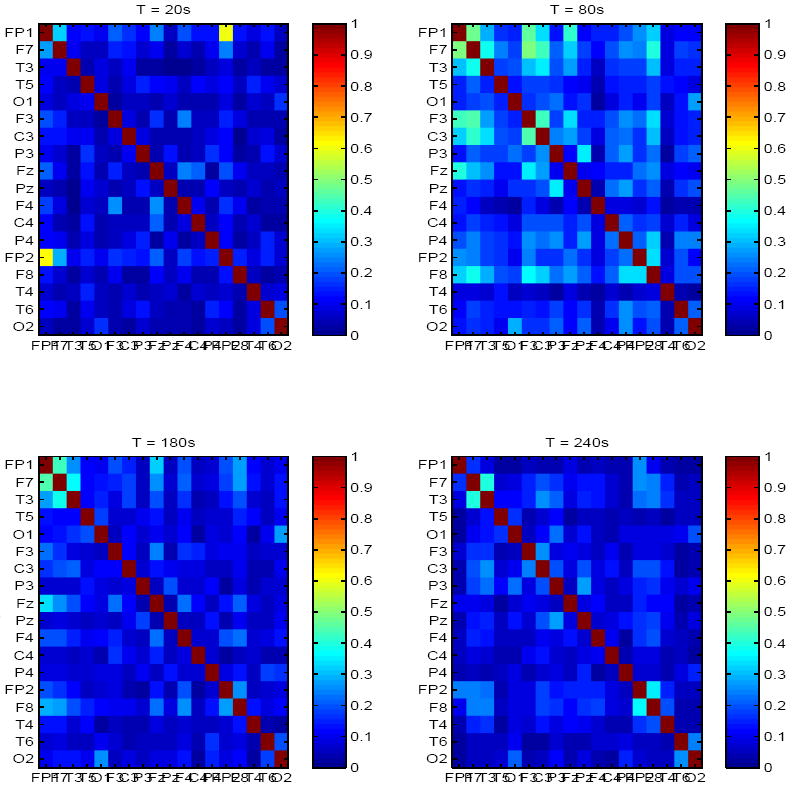
Mutual information matrix estimated from baseline EEG of a healthy adult, at 4 time points (on average 60s apart).
Fig. 3.
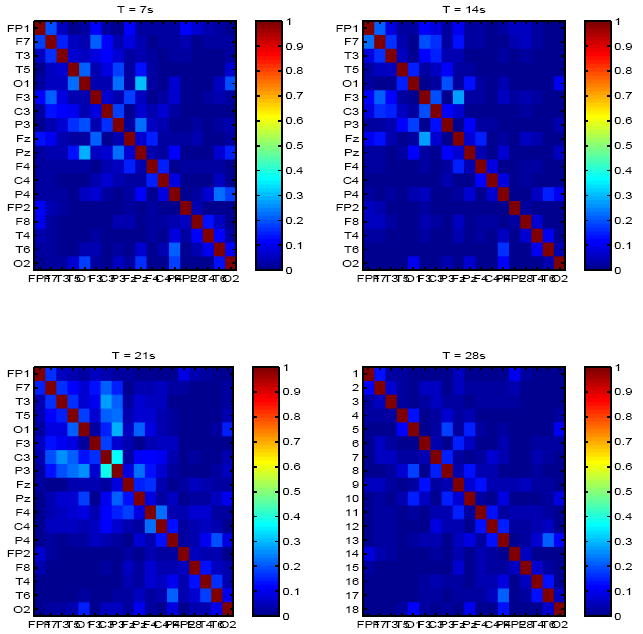
Mutual information matrix for a second patient with epilepsy and partial corpus callosotomy, at 4 time points (7s apart).
Fig. 2.
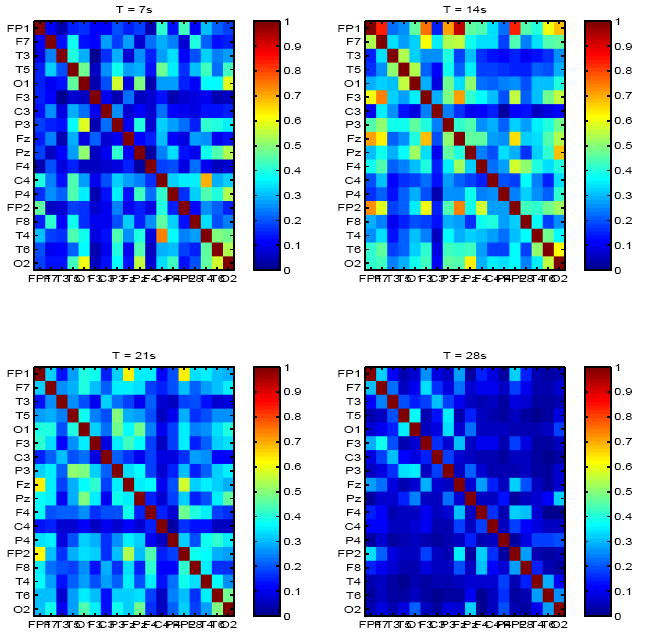
Mutual information matrix estimated from baseline EEG of a patient with epilepsy and , at 4 time points (7s apart).
Only one patient with epilepsy had a statistically distinct mutual information matrix. Inter-channel coupling (information) was significantly lower for this patient (μ = 0.075, σchan = 0.01) than all others, including healthy subjects. Mutual information values between bilateral frontal and temporal channels was specifically lower (< 0.05) than between other channel combinations. This patient had undergone partial corpus callosotomy and low information values between the two hemispheres may reflect loss of bilateral coupling following this procedure. Mutual information matrices for this patient at 4 time points are shown in Figure 3.
We also estimated the index of pairwise directional coupling described by Equation 5. In general, low coupling between regions appeared also approximately non-directional in healthy subjects, with increased directional information from the pre-frontal to frontal areas. In contrast, statistically higher directional coupling was observed from the pre-frontal cortex to frontal-/fronto-temporal and temporal regions bilaterally in patients with epilepsy. In addition, the flow of information was reversed between occipital and frontal regions, with the direction being from occipital to frontal regions (see minima in dx,y at curves at channel O1 in Figure 4). This was consistently the case in both healthy subjects and epilepsy patients. Finally, directionality of mutual information appeared non-specific in other regions. Directionality indices were averaged between subjects in each group and their range of variability is superimposed.
Fig. 4.

Directional coupling index between L prefrontal and all other regions and and L temporal and all others regions, respectively, in healthy subjects (left plots) and patients with epilepsy (right plots).
Based on estimated inter-channel mutual information and coupling direction, we also constructed network graphs for both healthy subjects and epilepsy patients. An example is shown in Figure 5. The connection strength, measured by mutual information is represented by the thickness of the edge, and the directionality, when significant, is also shown accordingly. Thicker lines correspond to higher mutual information between channels, dotted lines represent weak connections (I < 0.1). Higher coupling between pre-frontal, frontal and temporal areas were observed in patients with epilepsy, as well as additional couplings between pre-frontal/frontal and temporal and pre-frontal/frontal and parietal regions were estimated, predominantly in one hemisphere. These were transient and occurred only at some time-intervals but not others. Coupling strength for each pairs of channels was averaged over the entire EEG time interval.
Fig. 5.
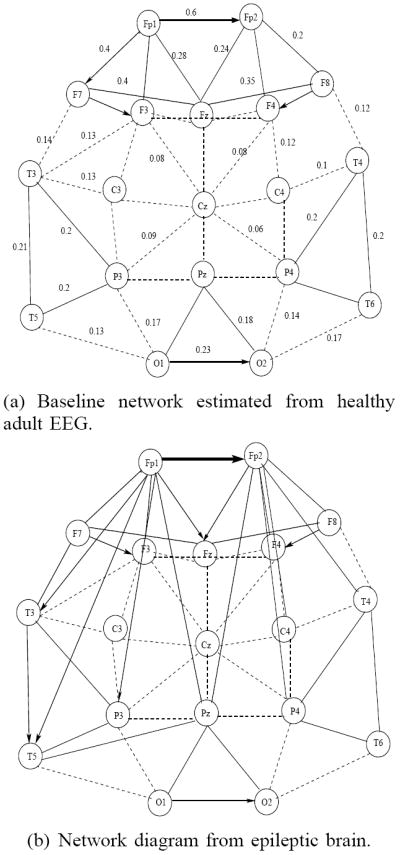
Inter-region connectivity network based on estimated coupling strength (in the range 0-1). Thicker lines indicate stronger connections, dashed lines indicate connections with low coupling strength.
IV. DISCUSSION
We have presented initial results from a small study on baseline network dynamics in the healthy and epileptic brain at rest. We have estimated inter-region coupling and direction of information flow directly from EEGs, using measures of time-dependent mutual information and conditional mutual information, and a related index of directionality. We examined mutual information matrices as a function of time and observed low inter-dependence between channels in healthy subjects and even lower inter-region and inter-hemisphere coupling in one patient with partial corpus callosotomy. In addition, information flow measured by conditional mutual information appeared non-directional, possibly in part due to low coupling strength in all regions. In contrast, increased spontaneous and apparently random synchronization between brain regions at several time intervals, but only transiently, were observed in patients with epilepsy. Increased directionality of network interactions was also observed, particularly between pre-frontal, frontal and temporal channels. These results suggest that baseline network dynamics of the epileptic brain may be characterized by fluctuations between spontaneous coupling and decoupling, which may in turn facilitate seizure initiation and further inter-region hyper-synchronization in the pre-ictal and ictal intervals. A study that explicitly assesses the robustness of these measures in a larger number of patients and a set seizures with increased heterogeneity, is required to further validate these results. In addition, information measures may be compared to typically used correlation parameters, such as signal cross-coherence and/or cross-correlation, as well as other previously proposed measures of dynamic coupling [12][9].
Acknowledgments
This work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (NIH Award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health. This work was also supported by NIH Grant K23 NS049159.
Contributor Information
Catherine Stamoulis, Department of Neurology, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston MA 02215 cstamoul@bidmc.harvard.edu.
Lawrence J. Gruber, Neurophysiology Laboratory, Beth Israel Deaconess Medical Center, Boston MA 02215
Bernard S. Chang, Department of Neurology, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston MA 02215
References
- 1.Bettus G, Wendling F, Guye M, Valton L, Regis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81(1):58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Bowman AW. An alternative method of cross-validation for the smoothing of density estimates. Biometrika. 1984;71:353–60. [Google Scholar]
- 3.Breakspear M. Non-linear desynchronization in human electroencephalographic data. Human Brain Map. 2002;15:175–198. doi: 10.1002/hbm.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breakspear M. Dynamic connectivity in neural systems: theoretical and emprirical considerations. Neuroinformatics. 2004;2:205–226. doi: 10.1385/NI:2:2:205. [DOI] [PubMed] [Google Scholar]
- 5.Deco G, Jirsa V, McIntosh AR, Sporns O, Kotter R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Nat Acad Sci. 2007;106(25):10302–10307. doi: 10.1073/pnas.0901831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franaszczuk PJ, Bergery GK, Kaminski M. Analysis of mesial temporal seizure onset and propagation using the directed transfer function method. Electr Clin Neurophysiol. 1994;91:413–427. doi: 10.1016/0013-4694(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 7.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–438. [Google Scholar]
- 8.Kaminski M, Blinowska KJ. A new method for the description of information flow in brain structures. Biol Cybern. 1991;65:203–210. doi: 10.1007/BF00198091. [DOI] [PubMed] [Google Scholar]
- 9.Le Van Quyen, et al. Spatio-temporal characterization of non-linear changes in intracranial activities prior to human temporal lobe seizures. Eur J Neurosci. 2000;12:2124–34. doi: 10.1046/j.1460-9568.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 10.Liao W, et al. Altered functional connectivity & small-world networks in mesial temporal lobe epilepsy. PLoS One. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Nat Acad Sci. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinerie J, Adam C, Le Van Quyen M, Baulac M, Clemenceau S, Renault B, et al. Epileptic seizures can be anticipated by non-linear analysis. Nat Med. 1998;4:1173–1176. doi: 10.1038/2667. [DOI] [PubMed] [Google Scholar]
- 13.Scott DW, Terrell GR. Biased and unbiased cross-validation in density estimation. J Am Stat Assoc. 1987;82:1131–1146. [Google Scholar]
- 14.Stamoulis C, Chang BS. Application of matched-filtering to extract EEG features and decouple signal contributions from multiple seizure foci in brain malformations. IEEE Proceedings of the 4th International IEEE/EBMS Conference on Neural Engineering. 2009:514–517. doi: 10.1109/NER.2009.5109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamoulis C, Gruber LJ, Chang BS. Modulations of brain dynamics by seizure precursors: estimation using information measures. J Comp Neurosci. 2010 submitted. [Google Scholar]
- 16.Varela F, et al. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 17.Vejmelka M, Palus M. Inferring the directionality of coupling with conditional mutual information. Phys Rev E. 2008;77(026214):1–12. doi: 10.1103/PhysRevE.77.026214. [DOI] [PubMed] [Google Scholar]
- 18.Wilke C, Van Drongelen W, Kohrman M, He B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia. 2009:1–9. doi: 10.1111/j.1528-1167.2009.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Yang Z, Coote JH. Cross-sample entropy statistic as a measure of complexity and regularity of renal sympathetic nerve activity in the rat. Exp Physiol. 2007;92:659–669. doi: 10.1113/expphysiol.2007.037150. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, et al. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J Neurol. 2009;256:1705–1713. doi: 10.1007/s00415-009-5187-2. [DOI] [PubMed] [Google Scholar]


