Abstract
Purpose
To assess outcomes following endoscope-assisted pars plana vitrectomy with concurrent pars plana tube shunt placement.
Methods
Records of 18 adult patients (19 eyes) at one institution with uncontrolled chronic angle closure glaucoma (CACG) associated with corneal opacification or fibrosed pupils were retrospectively reviewed. All eyes underwent endoscope-assisted pars plana vitrectomy with Baerveldt tube shunt placement into the vitreous cavity between 1997 and 2005. Intraocular pressure (IOP) reduction, glaucoma medication reduction, complications, and visual acuity were analyzed.
Results
Mean follow-up duration was 62 months (range, 10–106 months). Mean preoperative IOP was 31.3±10.5 (SD) mmHg on 3.4±1.0 (SD) glaucoma medications. IOP was significantly reduced at each postoperative time point examined. In the 17 eyes without phthisis, IOP was significantly reduced at the final follow-up examination to a mean of 11.4±2.9 (SD) mmHg (P<0.0001) on 1.3±1.2 (SD) medications (P<0.0001). No complications occurred in 14 of 19 eyes. Postoperatively, best attained visual acuity improved in 14/19 eyes, remained unchanged in 4/19 eyes, and was reduced in 1/19 eye.
Conclusion
Combined endoscope-assisted pars plana vitrectomy with placement of a Baerveldt tube shunt into the vitreous cavity is a useful intervention in patients with uncontrolled CACG, media opacities, and limited surgical options.
Keywords: chronic angle closure glaucoma, complications, endoscope, IOP, outcomes, tube shunt, visual acuity, vitrectomy
Introduction
Glaucoma drainage tube shunts are typically used following failure of medical, laser, and conventional filtering surgery to adequately control intraocular pressure (IOP). They have been used to effectively manage patients with complicated glaucomas and have been shown to significantly reduce IOP.1–21 Reported indications for tube shunt placement include excessive conjunctival scarring diminishing the likely success of repeat trabeculectomy,4,6 abnormalities of the iridocorneal angle,2,6 neovascular glaucoma,1,5 the presence of a corneal graft,2,3,7 and inflammatory glaucoma.1 Despite a high incidence of success, tube shunts placed in the anterior segment can result in multiple complications. The rate of endothelial failure following anterior chamber tube shunt placement has been reported to be 17% to 35%,4,9,16 and tube-corneal endothelium touch has been observed in 5% to 23% of patients.1,4,6,8,16 The incidence of corneal graft failure, both immunologic and non-immunologic has been reported to be 8% to 46% in patients with a corneal graft and an anterior chamber tube shunt.2,3,6,7,10–12,16 Abnormalities of the iridocorneal angle in some cases of CACG, aphakia, or pseudophakia may make insertion of a tube into the anterior chamber difficult.14,17 Erosion of the tube portion of the shunt can result in poor vision and phthisis.1,9,16
Various solutions have been proposed to address complications related to anterior chamber tube shunt placement. Placement of the tube in the vitreous cavity with simultaneous pars plana vitrectomy (PPV) has been advocated in selected cases.5,9,13,14,18–21 While this is often an effective strategy, incomplete vitreous removal can result in subsequent tube obstruction and failure to adequately reduce IOP. Visualization of the peripheral vitreous with conventional viewing systems during PPV can be particularly difficult in the presence of corneal opacification or a fibrosed pupil that may be present in patients with advanced glaucoma who have undergone multiple prior surgeries (Figure 1). Placing the tube in the sulcus is an option, however; a vitrectomy is still necessary in aphakic eyes and eyes with an anterior chamber intraocular lens (IOL).17
Figure 1.
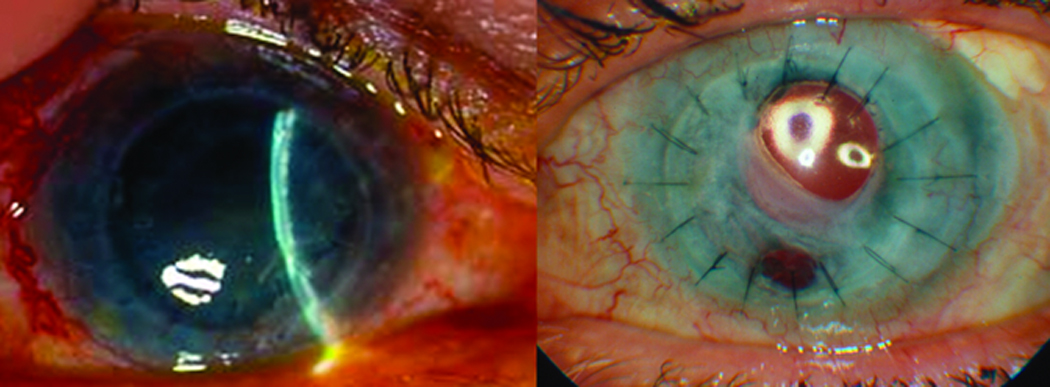
Patient #6 demonstrating an edematous, failed corneal graft (left) and patient # 9 demonstrating a fibrosed pupil (right).
The ocular endoscope has been reported to be a useful tool in ophthalmic surgery.22–38 It has been effectively used in the removal of peripheral vitreoretinal membranes,27,28 ciliary body photocoagulation,29,30 subretinal surgery,31 fluorescein angiography of the peripheral retina,32 visualization of intravitreal implants,33 removal of dislocated nuclear material,34 sulcus fixation of IOLs,35 removal of intraocular foreign bodies,36 retinal detachment repair,37 and endophthalmitis.38 Here we describe a new indication for the use of the ocular endoscope. We report a series of 19 consecutive eyes encountered over a 9-year period with corneal opacities or fibrosed pupils that underwent combined endoscope-assisted PPV and tube shunt placement in the vitreous cavity. All eyes had CACG and a failed corneal graft, corneal scarring, or a fibrosed pupil. The lens status was either aphakia or an anterior chamber, sulcus, or sutured posterior chamber IOL.
Materials and Methods
This study included all adult subjects who underwent endoscope-assisted PPV for poor visualization (Figure 1) with concurrent Baerveldt-350mm2 glaucoma implant (Advanced Medical Optics, Irvine, California, USA) placement in the vitreous cavity between January 1, 1997 and December 30, 2005 at Vanderbilt Eye Institute. Surgeries were performed by a single glaucoma surgeon (KMJ) and a single vitreoretinal surgeon (AA). A retrospective chart review was performed. Data recorded included age, gender, number of prior surgeries, indication for tube shunt placement, indication for endoscope use, lens status, initial and final best corrected visual acuity (BCVA), best post-operative BCVA, initial and final IOP, initial and final number of glaucoma medications, and the occurrence of postoperative complications. The preoperative IOP, number of glaucoma medications, and BCVA were the last recorded values prior to surgery. Intraocular pressures at all postoperative examinations were measured by Goldmann applanation.
Patients did not have standardized follow-up, so for the purposes of this study the following postoperative ranges were used in IOP analysis: 1 month (30 days ± 15 days), 3 month (90 days ± 30 days), 6 month (180 days ± 60 days), 12 month (365 days ± 90 days), 24 month (24 months ± 120 days), 36 month (36 months ± 150 days), 48 month (48 months ± 150 days), and 60 month (60 months ± 150 days). Due to variable follow-up, particularly in patients with stable IOP several years following surgery, all patients did not have an examination in each time range. If patients had multiple IOP measurements in a given range, the mean value of these measurements was used in data analysis. Visual acuity was noted prior to surgery and at the most recent follow-up visit and was classified as stable (≤ 3 line loss or gain), improved (> 3 line gain), or significantly reduced (> 3 line loss). All patients included in the study had a postoperative follow-up duration of at least 10 months. All subjects underwent complete ocular examinations by the operating surgeons preoperatively and postoperatively at variable intervals. Medications were discontinued as IOP declined during postoperative examinations. Eleven patients agreed to undergo subsequent penetrating keratoplasty or keratoprosthesis placement.
Surgical Technique
A 270° conjunctival peritomy was made and a standard 20-gauge PPV was performed with removal of all vitreous and residual lens matter that could be safely removed with a conventional viewing system. The sclerostomies were then enlarged to accommodate the 19-gauge ocular endoscope (Endo-Optiks Inc. Little Silver, NJ). Prior to insertion, the orientation of the 12 o’clock position of the endoscope was determined by focusing on the text on a suture packet. Once oriented, the endoscope was inserted into the vitreous cavity through each sclerostomy. Any residual vitreous material, peripheral retinal tears, or bleeding was identified and the vitreous base was trimmed 360°, particularly in the superotemporal quadrant. A Baerveldt-350 mm2 glaucoma drainage implant was placed and secured to the patient's sclera with two interrupted 9-0 nylon sutures. The tube was ligated with a 7-0 Vicryl suture and inserted into the vitreous cavity through a 23-gauge ostomy placed 2.5 to 3 mm posterior to the limbus. A separate location from the superotemporal vitrectomy sclerostomy was chosen to avoid a leak and to decrease the risk of incarceration of residual vitreous strands at a port. The internal position of the tube was confirmed with the endoscope prior to closing the eye. Postoperative medications included topical antibiotics, cycloplegic, and steroids that were tapered based on the degree of intraocular inflammation. All subjects subsequently underwent complete postoperative ocular examinations by the glaucoma and vitreoretinal surgeons at variable intervals.
Statistical Analysis
A paired t-test (SigmaStat, SPSS, Inc., Chicago, IL) was used to compare the preoperative and postoperative IOP and number of glaucoma medications.
Outcomes
For the purpose of this report, the IOP success outcome measures of previous studies were used.5,6,9,10,18 A complete success was defined as a final IOP of ≤ 21 mmHg without medications; qualified success as a final IOP of ≤ 21mmHg with medications; qualified failure as a final IOP > 21mmHg with or without glaucoma medications; and failure as phthisis, loss of light perception, or the requirement for additional glaucoma surgery to control the IOP. Patient outcome determination was based on the subject’s last documented office visit.
Results
Included in this study were 19 eyes of 18 patients. Patient data characteristics are summarized in Table 1. Mean follow-up duration was 62 months (range 10–106 months). Indications for the procedure included uncontrolled angle-closure glaucoma in all eyes. Uveitic glaucoma with peripheral anterior synechiae (PAS) was present in 3/19 eyes, and traumatic glaucoma with PAS was present in 2/19 eyes. Indications for use of the endoscope during PPV included failed corneal graft in 13/19 eyes, corneal edema or scar in 4/19 eyes, band keratopathy in 1/19 eyes, and a fibrosed pupil in 1/19 eyes. Thirteen eyes were pseudophakic (anterior chamber IOL, sulcus IOL, sutured posterior chamber IOL) and 6 were aphakic. The mean number of prior surgical procedures was 3 ± 1.3 (SD, range 1–6). Two eyes had prior anterior chamber tube shunts that were repositioned into the posterior chamber due to anterior chamber shallowing.
Patient Data Characteristics
| Eye | Age | Sex | # of Prior Surg | Surgery Indication |
Endoscope Indication |
Lens Status |
Initial BCVA, IOP, # Meds |
Final BCVA, IOP, # Meds |
Best BCVA |
Follow- up Duration (months) |
Postoperative Complications and Timing |
IOP Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | 2 | CACG | Failed graft | Aphakic | 1/200 | 2/200 | 20/300 | 36 | None | Success |
| 30 | 10 | |||||||||||
| 3 | 0 | |||||||||||
| 2 | 78 | M | 3 | Mixed Mech | Failed graft | Pseudo | 20/400 | 3/200 | 20/100 | 106 | None | Qualified |
| Glaucoma | 24 | 9 | Success | |||||||||
| 5 | 2 | |||||||||||
| 3 | 79 | M | 5 | Mixed Mech | Failed graft | Pseudo | 20/100 | 20/200 | 20/100 | 103 | Shunt retraction into suprachoroidal space requiring revision 2 months post-op |
Qualified |
| Glaucoma | 54 | 11 | Success | |||||||||
| 4 | 2 | |||||||||||
| 4 | 45 | F | 4 | Uveitic | Failed graft | Pseudo | 20/400 | 2/200 | 20/200 | 40 | Required CPC 3 years post-op | Failure |
| Glaucoma | 27 | 14 | ||||||||||
| 4 | 3 | |||||||||||
| 5 | 48 | F | 2 | Uveitic | Rejecting | Pseudo | 20/80 | 20/25 | 20/20 | 90 | None | Qualified |
| Glaucoma | graft | 21 | 8 | Success | ||||||||
| 4 | 1 | |||||||||||
| 6 | 80 | F | 2 | CACG | Failed graft | Pseudo | 20/400 | 20/400 | 20/80 | 70 | None | Qualified |
| 38 | 7 | Success | ||||||||||
| 3 | 2 | |||||||||||
| 7 | 42 | F | 5 | CACG | Failed graft | Aphakic | HM | HM | 2/200 | 93 | Required CPC 2 yrs post-surgery Retinal Detachment requiring repair 6 years post-op |
Failure |
| 50 | 8 | |||||||||||
| 4 | 0 | |||||||||||
| 8 | 32 | F | 2 | CACG | Band | Pseudo | 20/70 | 20/40 | 20/30 | 10 | Swollen lens matter blocked shunt requiring revision 2 months post-op |
Qualified |
| Keratopathy | 29 | 16 | Success | |||||||||
| 1 | 3 | |||||||||||
| 9 | 46 | F | 3 | Juvenile | Fibrosed | Aphakic | 20/200 | 20/150 | 20/150 | 87 | None | Qualified |
| CACG | Pupil | 21 | 12 | Success | ||||||||
| 3 | 2 | |||||||||||
| 10 | 51 | M | 2 | Traumatic | Failed graft | Pseudo | 20/200 | 1/200 | 20/70 | 87 | None | Success |
| Glaucoma | 27 | 15 | ||||||||||
| 4 | 0 | |||||||||||
| 11 | 72 | F | 2 | CACG | Corneal edema/scar |
Aphakic | 20/30 | HM | 20/40 | 62 | Choroidal hemorrhage requiring drainage 10 days post-op Band keratopathy after transplant |
Success |
| 36 | 11 | |||||||||||
| 4 | 0 | |||||||||||
| 12 | 60 | F | 3 | CACG/ | Failed graft | Pseudo | 1/200 | HM | 20/400 | 71 | Shunt blocked by vitreous requiring revision 1 month post-op Keratoprosthesis causing phthisis 5 years post-op |
Failure |
| Uveitic | 52 | Phthisis | ||||||||||
| Glaucoma | 3 | |||||||||||
| 13 | 75 | F | 3 | CACG | Corneal edema/scar |
Pseudo | 20/400 | 1/200 | 20/400 | 71 | None | Success |
| 26 | 12 | |||||||||||
| 3 | 0 | |||||||||||
| 14 | 35 | F | 6 | Traumatic | Failed graft | Aphakic | HM | NLP | 4/200 | 17 | Corneal transplant x 2 causing phthisis 1 year post-op |
Failure |
| Glaucoma | 38 | Phthisis | ||||||||||
| 4 | ||||||||||||
| 15 | 74 | M | 3 | CACG | Failed graft | Pseudo | 20/70 | 20/30 | 20/30 | 58 | Swollen lens matter blocked shunt requiring revision 3 months post-op |
Qualified |
| 22 | 12 | Success | ||||||||||
| 4 | 1 | |||||||||||
| 16 | 70 | F | 4 | CACG | Failed graft | Pseudo | 20/200 | 20/150 | 20/40 | 57 | Required CPC 2 years post-op | Failure |
| 25 | 8 | |||||||||||
| 3 | 2 | |||||||||||
| 17 | 21 | F | 1 | CACG | Corneal edema/ scar |
Aphakic | 20/400 | 20/400 | 20/200 | 37 | None | Qualified |
| 28 | 15 | Success | ||||||||||
| 4 | 3 | |||||||||||
| 18 | 65 | F | 2 | CACG | Corneal edema/ scar |
Pseudo | HM | 20/40 | 20/40 | 39 | None | Qualified |
| 26 | 15 | Success | ||||||||||
| 1 | 1 | |||||||||||
| 19 | 48 | F | 3 | CACG | Failed graft | Pseudo | 20/400 | 20/400 | 20/400 | 35 | None | Success |
| 21 | 10 | |||||||||||
| 3 | 0 |
BCVA: Best Corrected Visual Acuity; CACG: Chronic Angle Closure Glaucoma; CPC: Cyclophotocoagulation; F: Female; HM: Hand Motion; IOP: Intraocular Pressure LP: Light Perception; M: Male; NLP: No Light Perception OD: Right eye; OS: Left eye; POAG: Primary Open Angle Glaucoma; Surg: Surgeries
Preoperative IOP averaged 31.3 ± 10.5 mmHg (SD, range 21–54) on 3.4 ± 1.0 (SD, range 1–5) glaucoma medications. Analysis was performed on IOP preoperatively and at several postoperative time points. Mean and standard deviation measurements at each time point are displayed in Figure 2. At each postoperative time point examined, there was a statistically significant reduction in IOP (P<0.0001, paired t-test). Intraocular pressure ± standard deviation and the number of patients with an IOP measurement at each time point in the 19 eyes were as follows: preoperative (31.3 mmHg ± 10.5, n=19), 1 month (18.3 mmHg ± 4.8, n=19), 3 month (14.8 mmHg ± 4.3, n=15), 6 month (14.4 mmHg ± 5.0, n=18), 12 month (12.6 mmHg ± 3.6, n=17), 24 month (11.6 mmHg ± 2.6, n=15), 36 month (13.0 mmHg ± 4.8, n=16), 48 month (11.6 mmHg ± 2.4, n=9), and 60 month (11.0 mmHg ± 2.3, n=10). In the 17 eyes that did not undergo phthisis, IOP was significantly reduced at final follow up with a mean of 11.4 ± 2.9 mmHg (SD, range 7–16) (P<0.0001, paired t-test). The number of glaucoma medications required in these eyes at final follow up examination was also significantly reduced with a mean of 1.3 ± 1.2 (SD, range 0–3) (P<0.0001, paired t-test). Based on the previously described outcome measures, 5/19 eyes were classified as a complete success with final IOP ≤ 21 on no glaucoma medications, 9/19 were classified as a qualified success with final IOP ≤ 21 on 1 or more glaucoma medications, 0/19 were classified as a qualified failure with final IOP ≥ 21mmHg with or without glaucoma medications, and 5/19 were classified as a failure with phthisis, loss of light perception, or the requirement for additional glaucoma surgery to control the IOP. A Kaplan-Meier cumulative probability curve of complete or qualified success is illustrated in Figure 3.
Figure 2.
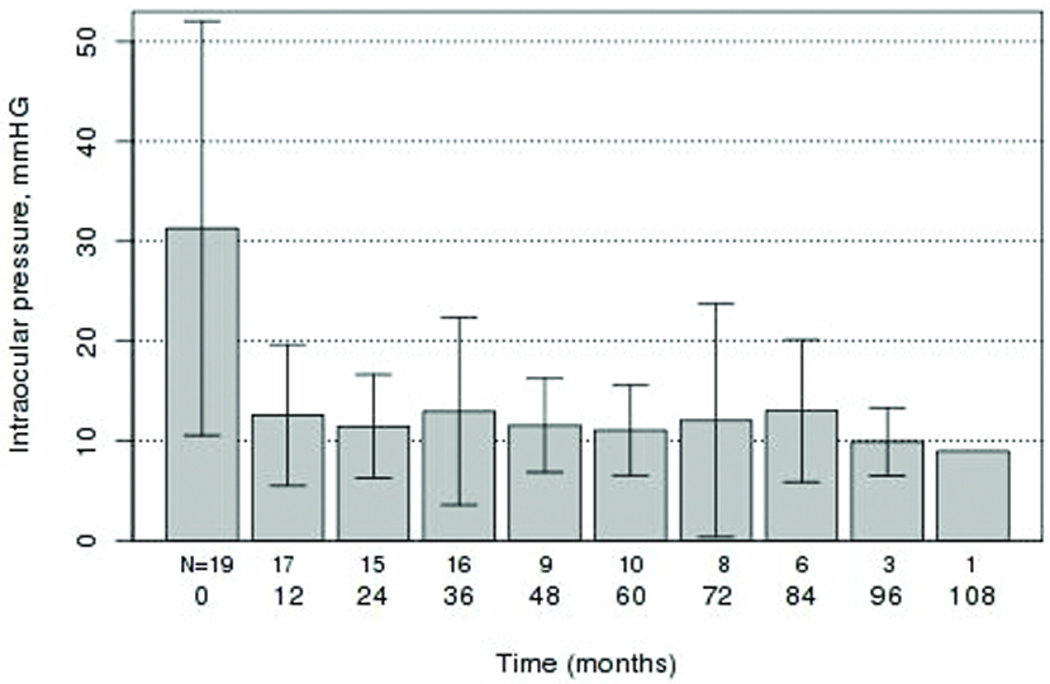
Mean intraocular pressure (mmHg) values with standard deviation at baseline and at postoperative follow-up intervals.
Figure 3.
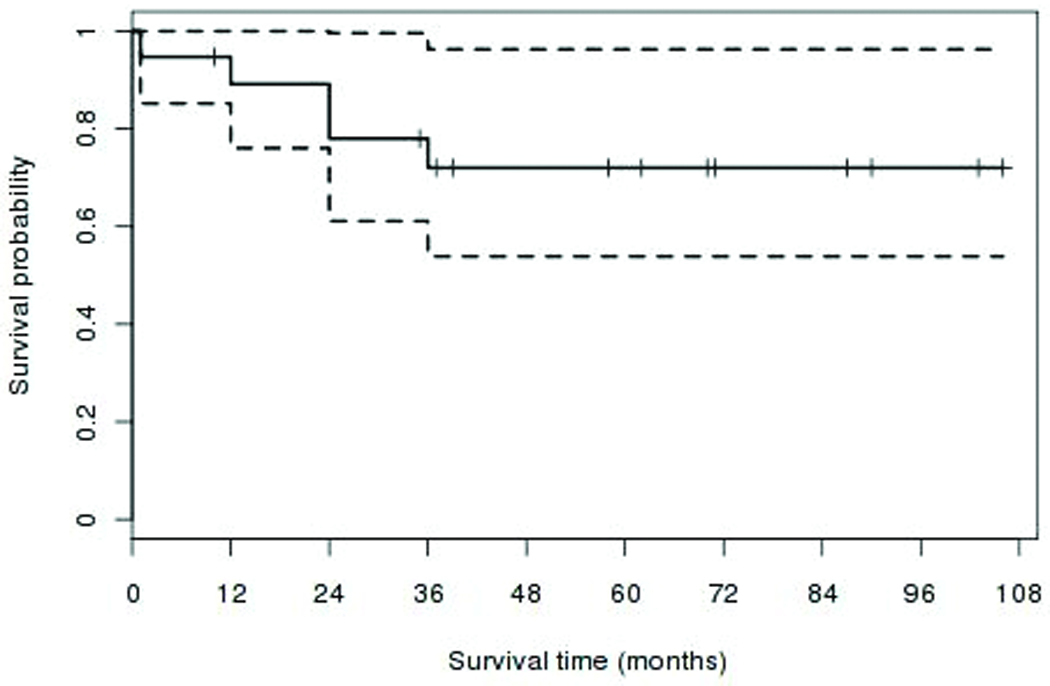
Kaplan-Meier cumulative probability curve of success (with or without medications) for intraocular pressure (IOP) ≤21 mmHg.
Five of 19 eyes had complications related to this procedure. All 5 required repeat surgical intervention. Four of these eye required tube shunt revision. Two of these 4 eyes (eye # 8 and 15) developed a swollen Soemmering’s ring blocking the tube, 1 eye (eye #12) had retained vitreous blocking the tube, and 1 eye (eye #3) developed shunt retraction into the suprachoroidal space. Even though patients (eye #3, 8, 12,and 15) required subsequent surgery within the postoperative period to permit their drainage tube to function, that event was not considered as a qualifying additional glaucoma surgery criterion for ‘failure’ in this study. One eye (eye #11) developed a hemorrhagic choroidal detachment that required surgical drainage. This eye regained 20/40 visual acuity following a penetrating keratoplasty that eventually failed and resulted in significantly decreased vision. This patient declined a repeat penetrating keratoplasty.
Of the 5 eyes classified as failures, 3 eyes (eye #4, 7, and 16) required supplemental transscleral diode cyclophotocoagulation for additional IOP control 2–3 years following surgery. Two eyes (eye # 12 and 14) developed phthisis following subsequent corneal surgery due to a sclera melt around a keratoprosthesis placed in eye #12 and following a penetrating keratoplasty in eye #14.
Postoperatively, best attained visual acuity improved in 14/19 eyes, remained unchanged in 4/19 eyes, and was reduced in 1/19 eye. All 17 eyes that did not undergo phthisis retained vision at the most recent follow-up examination. Final visual acuity remained stable (≤ 3 line loss or gain) in 9/17 eyes, improved (> 3 line gain) in 3/17 eyes, and was reduced (> 3 line loss) in 5/17 eyes.
Discussion
The surgical management of advanced, uncontrolled angle-closure glaucomas in patients with corneal opacification or a fibrosed pupil is complex, especially if aphakia or an anterior chamber intraocular lens is present. These patients are typically on maximal medical therapy, have undergone several prior procedures without adequate IOP reduction, and have scarred conjunctiva. Vitrectomy with concurrent pars plana tube shunt placement is often a final surgical option for IOP control. A thorough peripheral vitrectomy is critical for long-term success of shunts placed in the vitreous cavity and can be particularly difficult to achieve in eyes with significant media opacities. Residual vitreous can occlude the tube with subsequent IOP increase or exert traction on the retina resulting in a tear. The ocular endoscope was used to aid visualization during peripheral vitreous removal and tube shunt placement to reduce the risk of tube obstruction with residual vitreous. This series describes our experience using the ocular endoscope to complete PPV prior to tube shunt placement in the vitreous cavity in 19 consecutive eyes with advanced, uncontrolled angle-closure glaucoma and corneal opacities or a fibrosed pupil. Long-term IOP was significantly reduced from a preoperative mean of 31.3 mmHg on 3.4 glaucoma medications to a final postoperative mean of 11.4 mmHg on 1.3 glaucoma medications. During follow-up, IOP was significantly reduced at all postoperative time intervals examined. Postoperative complications related to this procedure occurred in 5 of 19 eyes. Only one of these 5 eyes had a significant decrease in final visual acuity. Two eyes developed phthisis, but this appeared to be secondary to subsequent corneal surgeries. Only one eye developed postoperative tube obstruction with residual vitreous despite all eyes having media opacities making complete removal of peripheral vitreous impossible with a convention viewing system. Obstruction of 2 tubes occurred following partial removal of a Sommering’s ring and postoperative hydration of residual lens material. In subsequent surgeries, if lens material was not easily accessible for complete removal and was not felt to be visually significant, it was left in place. Final visual acuity over long-term follow up remained stable in 10/17 eyes, improved in 2/17 eyes, and was reduced in 5/17 eyes.
Numerous prior studies have described outcomes following combined PPV and glaucoma drainage implant placement in the vitreous cavity in patients with complicated glaucomas and poorly controlled IOP. 5,13,14,18–21 In these studies, patients did not have media opacities and PPV was performed with a conventional viewing system. Lloyd, et al first described 10 patients that underwent combined PPV and Molteno implant into the vitreous cavity for treatment of neovascular glaucoma.5 Average follow-up duration was 18 months. Six patients achieved final intraocular pressures less than 22 mmHg. Tube blockage with residual vitreous occurred in 1 patient. Sheppard, et al reported 7 patients with inflammatory glaucoma who underwent PPV and Molteno implant into the vitreous cavity.13 Follow-up ranged from 3 to 18 months. Mean intraocular pressure decreased from 41 mm Hg preoperatively to 12 mm Hg postoperatively. Varma, et al reported 13 patients who underwent PPV and glaucoma drainage implant placement in the vitreous cavity for glaucoma associated with shallow anterior chamber or vitreous prolapse and pseudophakia or aphakia.14 Mean follow-up duration was 18 months. Mean IOP decreased from 35 mm Hg preoperatively to 14 mm Hg postoperatively. Luttrull, et al reported 50 eyes that underwent pneumatically stented Baerveldt drainage device implantation modified for pars plana insertion as treatment of complicated glaucomas.18 Mean follow-up duration was 18 months. The mean preoperative IOP was 44 mmHg on 3.2 glaucoma medications. The mean final postoperative IOP was 14 mmHg on 0.6 glaucoma medications with a final IOP ≤ 22 in 47/50 eyes. They reported one eye with a semi-opaque failed corneal graft and impaired visualization at the time of pars plana vitrectomy with subsequent tube blockage with residual vitreous postoperatively. Scott, et al reported 40 eyes that underwent PPV and glaucoma drainage implant placement in the vitreous cavity.19 The tube was placed through the pars plana in 26/40 eyes. Follow up ranged from 7 to 86 months with a median of 16 months. Mean preoperative IOP was 34 mmHg and the median number of glaucoma medications was 2. At 1 year postoperatively, mean IOP was 13 mmHg and the median number of glaucoma medications was 0. No case of tube obstruction was reported. Joos, et al reported 9 eyes that underwent repositioning of Baerveldt aqueous implants from the anterior chamber into the vitreous cavity as management of anterior chamber tube-related complications.20 Mean follow-up duration was 17 months. IOP remained controlled in all eyes with a mean of 14.3 mmHg. Progression of the anterior segment problem, which prompted the revision, was halted in 3 of 5 eyes with corneal decompensation and shallow anterior chambers and in all 4 eyes with recurrent tube erosion. deGuzman, et al reported 33 eyes that underwent PPV and glaucoma drainage implant placement in the vitreous cavity.21 Mean follow-up duration was 32 months. Mean preoperative IOP was 33 mmHg on 3.6 glaucoma medications and was reduced to a mean of 13.4 on 0.6 glaucoma medications. Three cases of tube blockage (2 vitreous, 1 iris) requiring surgical correction occurred. Most cases in the above series were without significant media opacities.
In patients with advanced glaucoma and coexisting corneal disease, an alternate technique to achieve IOP control is to perform a penetrating keratoplasty (PK) at the time of vitrectomy and pars plana tube shunt placement.39–41 Three prior studies have examined patients that have undergone combined PPV using a temporary corneal prosthesis, placement of a tube shunt in the vitreous cavity, and PK. In the largest study by Ritterband, et al, 26/82 eyes (31.7%) at 1 month, 19/80 eyes (23.8%) at 3 months, and 3/62 eyes (4.8%) at 12 months following surgery had an IOP ≥ 22.41 Sustained elevated IOP and surgical trauma at the time of PK are known risk factors for graft failure.42–44 In order to optimize graft survival, IOP could be controlled prior to performing PK. In our series, only 3/19 at 1 month, 1/15 at 3 months, and 0/17 at 12 months had an IOP ≥ 22. In eyes with visual potential, waiting to perform PK until IOP is well controlled may delay visual recovery, but a staged approach may provide adequate IOP control and minimize the inflammatory response following a subsequent corneal graft. A potential advantage of using a surgical corneal prosthesis during PPV and performing a PK at the time of pars plana tube shunt placement is improved visualization during vitrectomy allowing more complete removal of peripheral vitreous. A disadvantage is the additional operating room time to place the surgical keratoprosthesis not typically used in a PK alone. The use of the ocular endoscope in our study was aimed at enhancing visualization of the peripheral vitreous allowing adequate removal and ensuring proper tube placement at the conclusion of the case. In our series, 1/19 eyes had postoperative vitreous obstruction of the tube. This patient underwent repeat pars plana vitrectomy and achieved IOP control for 3.5 years before developing phthisis following keratoprosthesis placement.
This study supports prior evidence that pars plana tube shunt placement is a useful option when managing patients with uncontrolled angle-closure glaucoma on maximal tolerated medical therapy with scarred conjunctiva. In addition, this study describes a new surgical technique to utilize the ocular endoscope to assist removal of vitreous in patients when visualization is compromised by media opacities to permit placement of a pars plana shunt without a concurrent corneal surgical prosthesis or corneal graft procedure.
Acknowledgments
This study was supported by the Joseph Ellis Family Glaucoma Research Fund, and an unrestricted departmental grant from Research to Prevent Blindness Inc., NY. A NIH/NEI travel grant was awarded to Dr. Tarantola for ARVO presentation. The authors acknowledge the statistical analysis consultation provided by Chun Li, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This material was presented in part as a paper at the American Society of Retinal Specialists Annual Meeting, Maui, Hawaii, October 15, 2008, and a poster at the Association for Research in Vision and Ophthalmology Annual Meeting, Ft. Lauderdale, FL, May 3, 2009.
None of the authors has any financial/conflicting interests to disclose
Summary Statement:
This retrospective interventional case series assessed outcomes following endoscope-assisted PPV with pars plana tube shunt placement. In eyes that have undergone prior procedures on maximal tolerated medical therapy, this procedure resulted in a significant reduction in intraocular pressure and decreased the number of glaucoma medications required over long-term follow-up.
References
- 1.Sherwood MB, Joseph NH, Hitchings RA. Surgery for refractory glaucoma. Results and complications with a modified Schocket technique. Arch Ophthalmol. 1987;105:562–569. doi: 10.1001/archopht.1987.01060040132051. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell PJ, Robin JB, Schanzlin DJ, et al. Molteno implant for control of glaucoma in eyes after penetrating keratoplasty. Ophthalmology. 1988;95:364–369. doi: 10.1016/s0161-6420(88)33187-8. [DOI] [PubMed] [Google Scholar]
- 3.Kirkness CM, Ling Y, Rice NS. The use of silicone drainage tubing to control post-keratoplasty glaucoma. Eye. 1988;2:583–590. doi: 10.1038/eye.1988.109. [DOI] [PubMed] [Google Scholar]
- 4.Hill RA, Heuer DK, Baerveldt G, Minckler DS, Martone JF. Molteno implantation for glaucoma in young patients. Ophthalmology. 1991;98:1042–1046. doi: 10.1016/s0161-6420(91)32179-1. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd MA, Heuer DK, Baerveldt G, et al. Combined Molteno implantation and pars plana vitrectomy for neovascular glaucomas. Ophthalmology. 1991;98:1401–1405. doi: 10.1016/s0161-6420(91)32120-1. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd MA, Sedlak T, Heuer DK, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Update of a pilot study. Ophthalmology. 1992;99:679–687. doi: 10.1016/s0161-6420(92)31910-4. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood MB, Smith MF, Driebe WT, Jr, Stern GA, Beneke JA, Zam ZS. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthalmic Surg. 1993;24:185–189. [PubMed] [Google Scholar]
- 8.Netland PA, Walton DS. Glaucoma drainage implants in pediatric patients. Ophthalmic Surg. 1993;24:723–729. [PubMed] [Google Scholar]
- 9.Gandham SB, Costa VP, Katz LJ, et al. Aqueous tube-shunt implantation and pars plana vitrectomy in eyes with refractory glaucoma. Am J Ophthalmol. 1993;116:189–195. doi: 10.1016/s0002-9394(14)71284-x. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd MA, Baerveldt G, Fellenbaum PS, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994;101:1456–1463. doi: 10.1016/s0161-6420(94)31152-3. discussion 1463-4. [DOI] [PubMed] [Google Scholar]
- 11.Hodkin MJ, Goldblatt WS, Burgoyne CF, Ball SF, Insler MS. Early clinical experience with the Baerveldt implant in complicated glaucomas. Am J Ophthalmol. 1995;120:32–40. doi: 10.1016/s0002-9394(14)73756-0. [DOI] [PubMed] [Google Scholar]
- 12.Price FW, Jr, Wellemeyer M. Long-term results of Molteno implants. Ophthalmic Surg. 1995;26:130–135. [PubMed] [Google Scholar]
- 13.Sheppard JD, Shrum KR. Pars plana Molteno implantation in complicated inflammatory glaucoma. Ophthalmic Surg. 1995;26:218–222. [PubMed] [Google Scholar]
- 14.Varma R, Heuer DK, Lundy DC, Baerveldt G, Lee PP, Minckler DS. Pars plana Baerveldt tube insertion with vitrectomy in glaucomas associated with pseudophakia and aphakia. Am J Ophthalmol. 1995;119:401–407. doi: 10.1016/s0002-9394(14)71224-3. [DOI] [PubMed] [Google Scholar]
- 15.Sidoti PA, Dunphy TR, Baerveldt G, et al. Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology. 1995;102:1107–1118. doi: 10.1016/s0161-6420(95)30904-9. [DOI] [PubMed] [Google Scholar]
- 16.Siegner SW, Netland PA, Urban RC, Jr, et al. Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology. 1995;102:1298–1307. doi: 10.1016/s0161-6420(95)30871-8. [DOI] [PubMed] [Google Scholar]
- 17.Rumelt S, Rehany U. Implantation of glaucoma drainage implant tube into the ciliary sulcus in patients with corneal transplants. Arch Ophthalmol. 1998;116:685–687. doi: 10.1001/archopht.116.5.685. [DOI] [PubMed] [Google Scholar]
- 18.Luttrull JK, Avery RL, Baerveldt G, Easley KA. Initial experience with pneumatically stented Baerveldt implant modified for pars plana insertion for complicated glaucoma. Ophthalmology. 2000;107:143–149. doi: 10.1016/s0161-6420(99)00034-2. discussion 149-50. [DOI] [PubMed] [Google Scholar]
- 19.Scott IU, Alexandrakis G, Flynn HW, Jr, et al. Combined pars plana vitrectomy and glaucoma drainage implant placement for refractory glaucoma. Am J Ophthalmol. 2000;129:334–341. doi: 10.1016/s0002-9394(99)00363-3. [DOI] [PubMed] [Google Scholar]
- 20.Joos KM, Laviña AM, Tawansy KA, Agarwal A. Posterior repositioning of glaucoma implants for anterior segment complications. Ophthalmology. 2001;108:279–284. doi: 10.1016/s0161-6420(00)00521-2. [DOI] [PubMed] [Google Scholar]
- 21.de Guzman MH, Valencia A, Farinelli AC. Pars plana insertion of glaucoma drainage devices for refractory glaucoma. Clin Experiment Ophthalmol. 2006;34:102–107. doi: 10.1111/j.1442-9071.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 22.Norris JL, Cleasby GW, Nakanishi AS, Martin JL. Intraocular endoscopic surgery. Am J Ophthalmol. 1981;91:603–606. doi: 10.1016/0002-9394(81)90058-1. [DOI] [PubMed] [Google Scholar]
- 23.Volkov VV, Danilov AV, Vassin LN, Frolov YA. Flexible endoscopies. Ophthalmoendoscopic techniques and case reports. Arch Ophthalmol. 1990;108:956–957. doi: 10.1001/archopht.1990.01070090058039. [DOI] [PubMed] [Google Scholar]
- 24.Eguchi S, Araie M. A new ophthalmic electronic videoendoscope system for intraocular surgery. Arch Ophthalmol. 1990;108:1778–1781. doi: 10.1001/archopht.1990.01070140132046. [DOI] [PubMed] [Google Scholar]
- 25.Leon CS, Leon JA. Microendoscopic ocular surgery: a new intraoperative, diagnostic and therapeutic strategy I: Endoscopic equipment/methodology applied to cataract surgery with intraocular lens implantation. J Cataract Refract Surgery. 1991;17:568–572. doi: 10.1016/s0886-3350(13)81042-5. [DOI] [PubMed] [Google Scholar]
- 26.Leon CS, Leon JA. Microendoscopic ocular surgery: a new intraoperative, diagnostic and therapeutic strategy II: Preliminary results from the study of glaucomatous eyes. J Cataract Refract Surgery. 1991;17:573–576. doi: 10.1016/s0886-3350(13)81043-7. [DOI] [PubMed] [Google Scholar]
- 27.Ciardella AP, Fisher YL, Carvalho C, et al. Endoscopic vitreoretinal surgery for complicated proliferative diabetic retinopathy. Retina. 2001;21:20–27. doi: 10.1097/00006982-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Faude F, Wiedemann P. Vitreoretinal endoscope for the assessment of the peripheral retina and the ciliary body after large retinectomies in severe anterior PVR. Int Ophthalmol. 2004;25:53–56. doi: 10.1023/b:inte.0000018550.36179.9e. [DOI] [PubMed] [Google Scholar]
- 29.Valmaggia C, de Smet M. Endoscopic laser coagulation of the ciliary processes in patients with severe chronic glaucoma. Klin Monatsbl Augenheilkd. 2004;221:343–346. doi: 10.1055/s-2004-812870. [DOI] [PubMed] [Google Scholar]
- 30.Kawai K. The microendoscope for ciliary process photocoagulation in neovascular glaucoma. Tokai J Exp Clin Med. 2002;27:27–34. [PubMed] [Google Scholar]
- 31.Koch FH, Luloh KP, Augustin AJ, et al. Subretinal microsurgery with gradient index endoscopes. Ophthalmologica. 1997;211:283–287. doi: 10.1159/000310809. [DOI] [PubMed] [Google Scholar]
- 32.Terasaki H, Miyake Y, Mori M, Suzuki T, Kondo M. Fluorescein angiography of extreme peripheral retina and rubeosis iridis in proliferative diabetic retinopathy. Retina. 1999;19:302–308. doi: 10.1097/00006982-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Koch FH, Gumbel HO, Hattenbach LO, Ohrloff C. [Intravitreal endoscopic visualization of intraocular ganciclovir devices: improved long-term treatment of CMV retinitis] Klin Monatsbl Augenheilkd. 1999;214:107–111. doi: 10.1055/s-2008-1034759. [DOI] [PubMed] [Google Scholar]
- 34.Boscher C, Lebuisson DA, Lean JS, Nguyen-Khoa JL. Vitrectomy with endoscopy for management of retained lens fragment and/or posteriorly dislocated intraocular lens. Graefes Arch Clin Exp Ophthalmol. 1998;236:115–121. doi: 10.1007/s004170050051. [DOI] [PubMed] [Google Scholar]
- 35.Sasahara M, Kiryu J, Yoshimura N. Endoscope assisted transscleral suture fixation to reduce the incidence of intraocular lens dislocation. J Cataract Refract Surg. 2005;31:1777–1780. doi: 10.1016/j.jcrs.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Norris JL, Cleasby GW. Intraocular foreign body removal by endoscopy. Ann Ophthalmol. 1982;14:371–372. [PubMed] [Google Scholar]
- 37.Ben-nun J. Cornea sparing by endoscopically guided vitreoretinal surgery. Ophthalmology. 2001;108:1465–1470. doi: 10.1016/s0161-6420(01)00642-x. [DOI] [PubMed] [Google Scholar]
- 38.de Smet MD, Carlborg EA. Managing severe endophthalmitis with the use of an endoscope. Retina. 2005;25:976–980. doi: 10.1097/00006982-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Johnston RH, Nguyen R, Jongsareejit A, Lee BR, Patel S, Chong LP. Clinical study of combined penetrating keratoplasty, pars plana vitrectomy with temporary keratoprosthesis, and pars plana seton implant. Retina. 1999;19:116–121. doi: 10.1097/00006982-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Sidoti PA, Mosny AY, Ritterband DC, Seedor JA. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108:1050–1058. doi: 10.1016/s0161-6420(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 41.Ritterband DC, Shapiro D, Trubnik V, et al. Cornea Glaucoma Implant Study Group (COGIS). Penetrating keratoplasty with pars plana glaucoma drainage devices. Cornea. 2007;26:1060–1066. doi: 10.1097/ICO.0b013e3181342835. [DOI] [PubMed] [Google Scholar]
- 42.Ayyala RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol. 2000;45:91–105. doi: 10.1016/s0039-6257(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 43.Rapuano CJ, Schmidt CM, Cohen EJ, et al. Results of alloplastic tube shunt procedures before, during, or after penetrating keratoplasty. Cornea. 1995;14:26–32. [PubMed] [Google Scholar]
- 44.Beebe WE, Starita RJ, Fellman RL, Lynn JR, Gelender H. The use of Molteno implant and anterior chamber tube shunt to encircling band for the treatment of glaucoma in keratoplasty patients. Ophthalmology. 1990;97:1414–1422. doi: 10.1016/s0161-6420(90)32393-x. [DOI] [PubMed] [Google Scholar]


