Abstract
Microwave ablation is an emerging treatment option for many cancers, cardiac arrhythmias and other medical conditions. During treatment, microwaves are applied directly to tissues to produce rapid temperature elevations sufficient to produce immediate coagulative necrosis. The engineering design criteria for each application differ, with individual consideration for factors such as desired ablation zone size, treatment duration, and procedural invasiveness. Recent technological developments in applicator cooling, power control and system optimization for specific applications promise to increase the utilization of microwave ablation in the future. This article will review the basic biophysics of microwave tissue heating, provide an overview of the design and operation of current equipment, and outline areas for future research for microwave ablation.
Keywords: Thermal therapies, hyperthermia, microwave ablation
I. BACKGROUND
A. Thermal Therapies
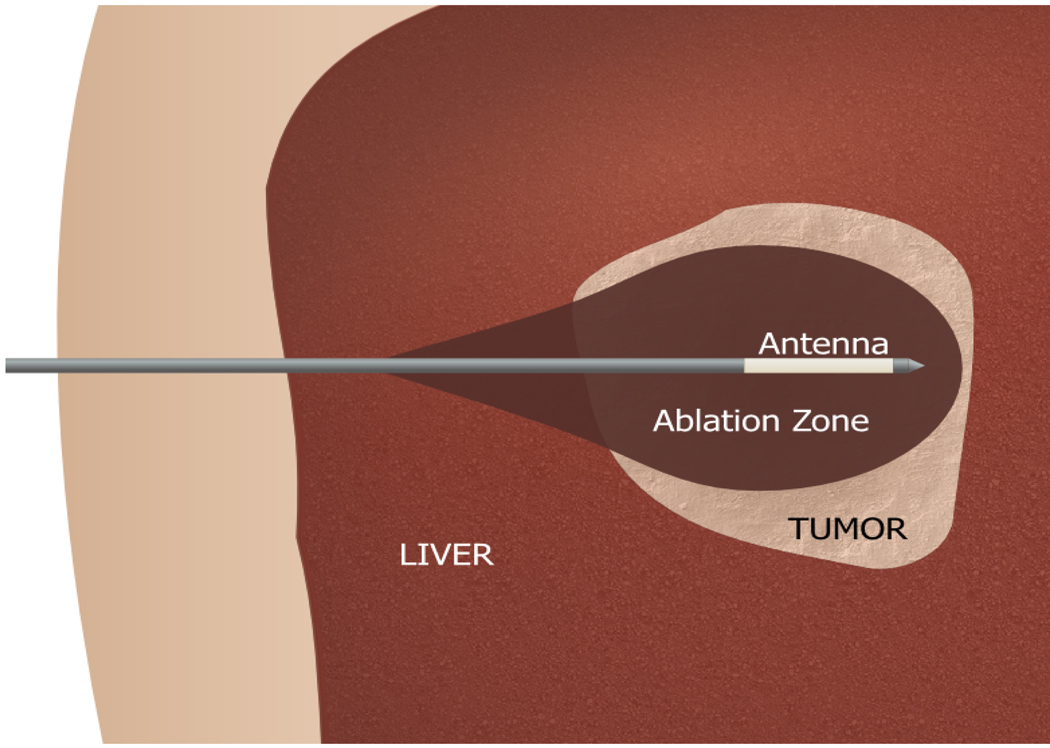
Thermal ablation is an emerging treatment option for several conditions, including cancer, cardiac arrhythmias, varicose veins and menorrhagia. Ablative procedures can be performed at open surgery, laparoscopically, using catheter-based applicators, percutaneously or transcutaneously. Minimally invasive ablations are typically performed using imaging as a diagnostic, guidance, monitoring and treatment assessment tool. Several energy sources are currently used for hyperthermic ablation, including radiofrequency electrical current, microwaves, laser light, and focused ultrasound. Each energy source is characterized by particular advantages and disadvantages in energy application and imaging appearance, which are often application specific. The focus of this review is on the physics, technology and application of microwave energy for thermal ablation therapy (Figure 1).
Figure 1.
Microwave ablation of a liver tumor. The antenna is placed percutaneously into the tumor mass before microwave energy is applied to heat the tumor to cytotoxic levels.
B. Microwave Energy For Tissue Ablation
Microwaves represent the portion of the electromagnetic spectrum between 300 MHz and 300 GHz. The Federal Communications Commission (FCC) or International Telecommunications Union (ITU) permit several unrestricted frequency bands for industrial, scientific and medical use in several regions, including those most commonly used for microwave ablation procedures: 915 MHz and 2.45 GHz. Other frequencies explored for therapeutic applications of microwaves include 433 MHz, and broadband pulses with the greatest spectral energy density between 1 GHz and 10 GHz.1, 2
Dielectric properties of biological tissues
The transmission of electromagnetic energy is determined by the dielectric permittivity and magnetic permeability of the media in which the waves propagate. The magnetic permeability of biological tissues is approximately the same as vacuum. However, the dielectric permittivity is significantly larger and contains both a real and imaginary component, which are used to define the more common terms: relative permittivity and conductivity. Relative permittivity, εr, is the real part of the complex permittivity and quantifies the ability to store electrical energy relative to vacuum. It is frequently referred to as dielectric constant; however, since permittivity is quite variable depending on frequency, temperature and other factors in biological tissues, the term relative permittivity will be used here for clarity.
The effective conductivity, σ, of a material is defined from the imaginary part of the complex permittivity and is used to describe how well a material absorbs microwave energy. It is important to note that effective conductivity describes contributions from moving charges (electrical current) and time-varying electric fields (displacement current), specifically the rotation of dipoles in the material as they attempt to align with and alternating electric field.3 The latter contribution dominates for most biological tissues in the microwave spectrum. This rotation of dipoles generates heat inside of lossy materials such as biological tissues, which will be described next.
Electromagnetic interactions with tissue
The most significant effect of an electromagnetic field applied to biological tissue is conversion of microwave energy to thermal energy. Heat transfer in tissue can be modeled using the so-called bioheat equation:4
| (1) |
where ρ is density (kg m−3), Cp is the specific heat capacity at constant pressure (J kg−1 m−3), T is temperature (K), t is time (s), kT is thermal conductivity (W/mK), Qh is the rate of heat applied (W m−3) and Qp is the rate of heat lost to blood perfusion (W m−3). The greatest constituent of most biological tissues is water, the atomic structure of which produces an electric dipole moment. Therefore, water molecules continually rotate to align with an applied electromagnetic field. This continual realignment increases kinetic energy, elevating temperature. The conversion of microwave energy to thermal energy follows the relationship:
| (2) |
where σ is effective conductivity (S m−1) and |E| is the applied electric field peak amplitude (V m−1). As described above, effective conductivity dictates how efficiently microwave energy is converted to heat, and is dependent primarily on the type of tissue, its water content, and the frequency of the applied field.
The frequency-dependent relative permittivities and conductivities of many normal and pathologic biological tissues are well-known at baseline conditions.5–9 Numerically modeling these properties for the purposes of electromagnetic simulation is accomplished using a multi-pole Cole-Cole model to account for large frequency spectra.6 Both relative permittivity and conductivity are also dependent on to other physical influences, such as water content and temperature.10–14 For the purposes of this discussion, these factors can typically be considered within a bulk mass of tissue rather than a cellular level. In most tissues the bulk dielectric properties can be considered isotropic, but in certain tissues such as muscle the properties may be mildly anisotropic.15 In addition, cellular and subcellular constituents such as protein structure and water binding can affect the bulk dielectric properties.13, 16
Penetration of a microwave field into a tissue medium is also dependent on the dielectric properties of the tissue. For a plane wave in a homogenous, isotropic medium, penetration depth, δ, of an electromagnetic field is defined as the distance required for the electric field of a plane wave to attenuate to 1/e (~37%) of its initial value, is:
| (3) |
where ω is angular frequency (rad/s; equal to 2 πf, where f is frequency in Hz), μ is magnetic permeability (H m−1), and ε is dielectric permittivity (F m−1). The penetration depth can be approximated by assuming the tissue is a good dielectric ([σ/ωε]2 ≪ 1):
| (4) |
which is a good assumption for most tissues and microwave ablation frequencies. Note that penetration depth is inversely related to conductivity, but heating rate is proportional to conductivity. Deeper penetration occurs at the expense of slower heating. This makes intuitive sense from a conservation of energy perspective: the primary cause of field attenuation is the conversion of microwave energy to heat. Balancing penetration depth and heat generation is important for evaluating which frequencies are most attractive for a given application. Lower frequencies with slower heating rates but deeper field penetration may be more desirable for large-volume heating applications such as regional hyperthermia.17 Higher frequencies may be desirable when rapid, controlled heating is desirable, such as ablation of the endometrium.18
One final point of consideration: much of the energy radiated by microwave antennas in lossy media such as biological tissues is absorbed in the near or Fresnel zones of the antenna and may not propagate as a plane wave. In this case, the aforementioned calculations of attenuation and penetration depth should be viewed as approximations. Electromagnetic simulation and, in particular, simulation of electromagnetic-thermal interactions can provide more accurate estimates of temporal heating when necessary.19
C. Microwaves Compared To Other Sources of Thermal Therapy
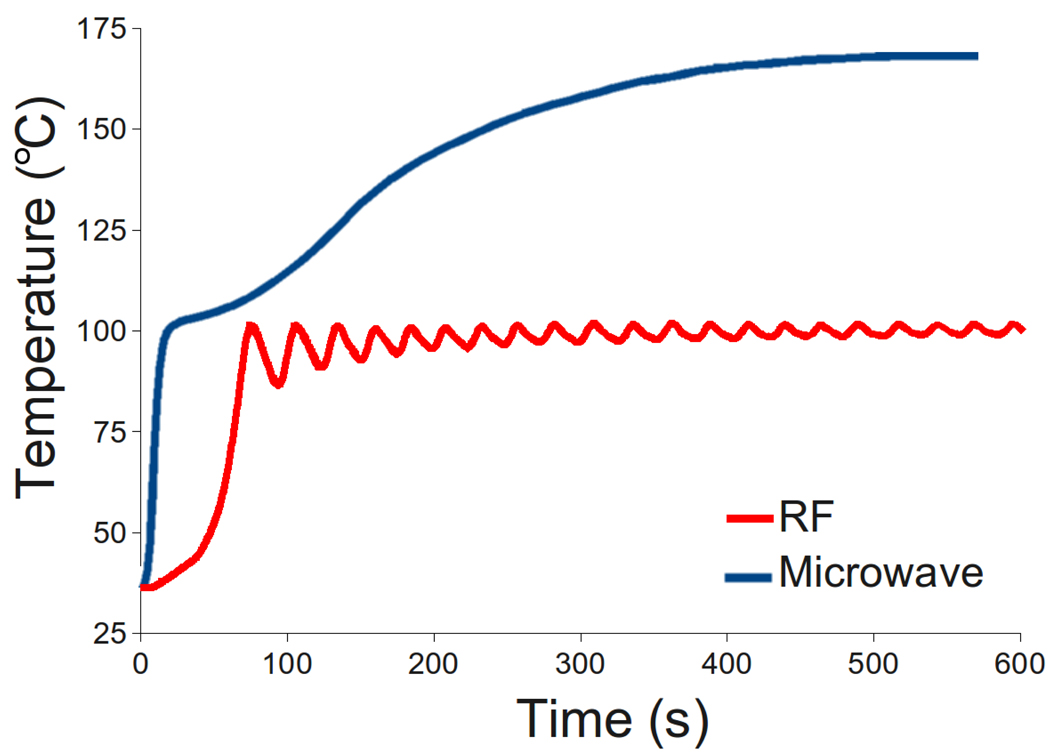
Microwave energy is distinct from other energies for thermal therapy in a number of ways. Perhaps most important is that microwaves propagate through all types of tissues and non-metallic materials, including water vapor, and dehydrated, charred and desiccated tissues created during the ablative process. RF, laser and ultrasound energies can be substantially affected by different tissue types, especially as a result of thermal ablation. For example, flow of RF current is hampered in aerated organs such as lung.20, 21 Rapid impedance elevation during RF ablation near 100 °C is also known to inhibit electrical current flow, effectively halting further application of RF energy unless the tissue is cooled and allowed to rehydrate, or artificially hydrated using ionic fluid infusion.22–24 A similar problem exists with laser and ultrasound energies, where high-temperature heating can inhibit further energy application.25, 26 By comparison, while dielectric properties can change substantially during treatment, microwave propagation is not hindered by these changes.14 Nor are microwaves prevented from transmitting through tissues with variable water content.12 As a result, microwaves are an attractive choice for thermal ablation (Figure 2).
Figure 2.
Temperatures measured 5 mm from a microwave (MW) and RF applicator during ablation of the renal cortex. Temperatures can exceed 100 °C during microwave ablation due to electromagnetic propagation through dehydrated, charred or desiccated tissue. Power was pulsed during RF ablation to prevent charring, limiting the maximum temperature to less than 100 °C. Heating rates during microwave ablation can also be more rapid than during RF ablation at the same power levels.
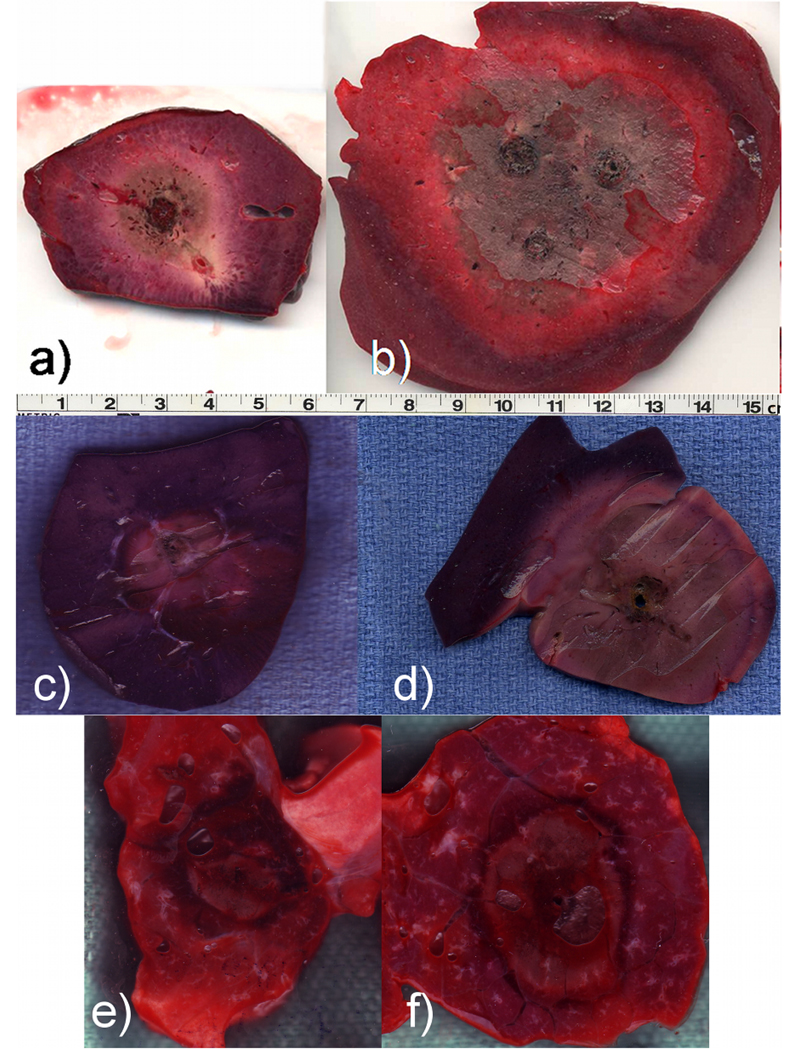
Microwaves may also offer more direct heating than other ablation energies, making them more potent in organs with high blood perfusion or near vascular heat sinks. In vivo studies have demonstrated that while RF ablation is relatively ineffective near vessels greater than 3 mm in diameter, microwaves will ablate up to and through similarly sized vessels.27–29 Recent comparisons of RF and microwave ablation have demonstrated improved performance with microwaves in liver, lung and kidney, even when the total energy applied was equivalent.30–33 Several studies have demonstrated hepatic microwave ablations 3–4 cm in diameter with a single applicator in vivo, with ablations up to 7 cm in diameter noted when using multiple antennas (Figure 3).34–36 Other studies have also shown that by using higher powers and shorter treatment times, microwaves may actually be more effective in vivo than ex vivo, an effect not noted with other ablation energy types to date.37
Figure 3.
Thermal ablations created in the liver (a-b), kidney (c-d) and lung (e-f). In the liver, both single (a) and three-antenna (b) microwave ablation were used, each delivering approximately 65 W for 10 min. In the kidney and lung, both RF (c,e) and microwave (d,f) are compared. In each tissue microwaves produced larger zones of ablation, especially with multiple antennas.30–32
The wavelength and penetration depth of microwaves in tissue is also suitable for a large variety of medical applications. At 915 MHz and 2.45 GHz, wave penetration is 2–4 cm in most tissues, which is often commensurate with the treatment target (eg, 2–4 cm tumors). By contrast, RF power deposition attenuates rapidly away from the RF electrode, making it an effective tool for electrocautery and small-volume thermal therapies. Similarly, the scattering and attenuation of lasers in tissue can be quite rapid and highly dependent on the wavelength of the light.38
Microwave thermal ablation is not without drawbacks, however. As mentioned earlier, the 2–4 cm wavelength penetration of microwave energy in tissue means that heating precision may not be as exquisite as with energies that are more rapidly absorbed. Rapid heating and high temperatures also still need more critical evaluation for safety when applied over a large volume. This is especially true from a monitoring standpoint, when rapid heating might be difficult to capture without realtime imaging.
Microwave energy can also overheat the cabling used to transfer power from the generator to the applicator. This internal cable heating must be offset either by limiting energy transmission, or by active cable cooling to prevent unwanted thermal damage to tissues along the cable length. Recent advances in internal antenna cooling solutions may help alleviate this problem in future microwave ablation technologies.39
Finally, microwave antenna design is critical to device performance. Trade-offs must often be made among the heating pattern produced by the device, its relative invasiveness (for percutaneous applicators), power handling and efficiency of energy coupling into the tissue. In addition, most antenna designs currently produce elongated ablations, which may not be desirable for many applications.40, 41 Technical challenges in antenna design and the application-specific nature of many systems increase the barrier to commercial development and widespread adoption. By contrast, RF and laser ablation systems may be simpler and less costly to develop.
II. MICROWAVE ABLATION TECHNOLOGY
The discussion of microwave ablation technologies is not complete without consideration of the entire power generation, distribution and delivery system. However, broad coverage of microwave power generation is beyond the scope of this article. A cursory overview of power generation and distribution will be presented first, with delivery through interstitial antennas to follow.
A. Power Generation and Distribution
Generator design
Microwave power in current systems is generated using either solid-state semiconductor sources, or vacuum-tube devices such as the magnetron. Magnetron sources have the advantage of relatively high power conversion efficiency (usually over 70%), high power output, and are robust devices. However, because the power source is monolithic and not phase controlled, microwave power produced by magnetrons is distributed to multiple antennas (if desired) by passive splitters. Solid state systems suffer from lower efficiency (usually less than 40%) and less output power per channel but are typically easier to control, and can have a cleaner output spectrum and a smaller footprint.42
Coaxial cable considerations
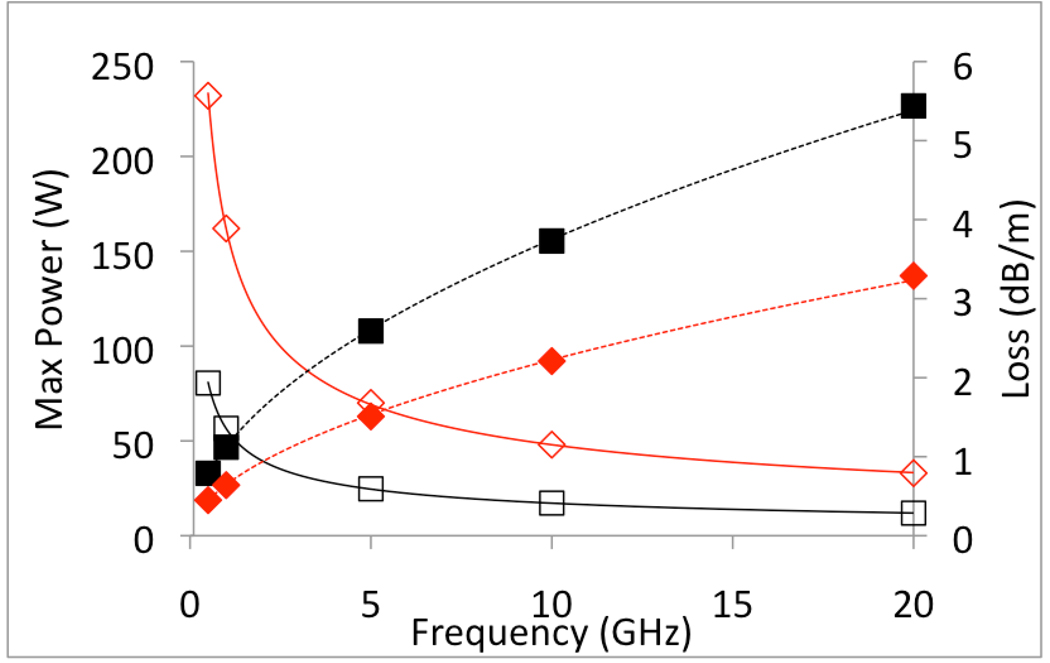
Microwave power is carried from the generator to the antenna through coaxial cables, due to their relative flexibility, transverse electric and magnetic wave propagation, and ease of connectivity. Cable flexibility is a function of cable diameter and construction materials, with improved flexibility in braided conductors and low-density dielectrics. Power handling – the ability of a cable to safely transfer power without overheating or failure – is related to these same factors, as well as the frequency of the applied microwave power (Figure 4). Therefore, the coaxial cables used to distribute power are typically more rigid than their counterparts in RF or laser ablation systems.
Figure 4.
Power handling ability (solid lines) and loss (dashed line, dB/m) of two semi-rigid coaxial cables: UT-47 (black, squares) and UT-85 (red, diamonds). Power loss increases with frequency and decreases with cable diameter, creating a trade-off between antenna invasiveness and power handling ability (www.micro-coax.com).
Coaxial cables also comprise the underlying structure of most interstitial microwave antennas. Most thermal ablation devices intended for percutaneous use are currently between 1.5 mm and 2.5 mm in diameter. Smaller antenna diameters are preferred for percutaneous applications since data from the biopsy literature suggests that larger needle diameters are associated with increased risk of complications such as bleeding and pneumothorax.43 However, the lower power-handling ability of small-diameter coaxial cables can be problematic. Increased power delivery has been associated with faster and potentially more effective treatments, particularly when targeting large tumors.36, 37, 44 At the same time, input powers exceeding the cable power rating can have detrimental effects on performance, and can lead to potentially dangerous heat generation in the flexible cables that carry power to the antenna, or within the antenna itself.45, 46 Workarounds such as time-limited power application, active antenna cooling, and antenna arrays can help increase energy delivery while using only small-diameter antennas (see research areas section).39
C. Microwave Ablation Antenna Design
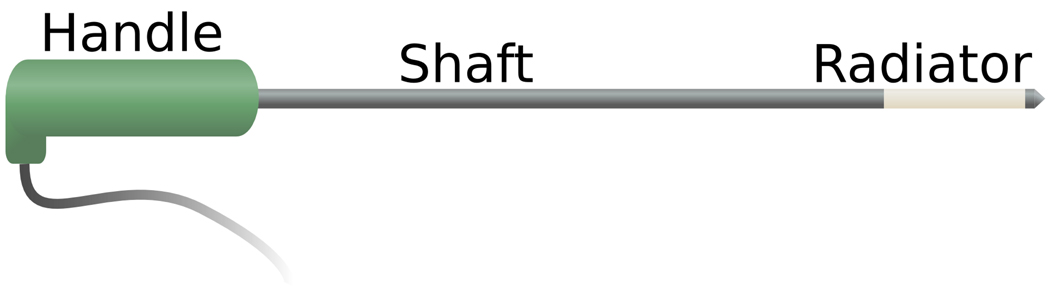
The microwave ablation can be defined in a number of different ways. In surgical and percutantous applications, the antenna is typically defined as the entire applicator beyond the flexible coaxial power delivery cable (Figure 5). Under this definition, the antenna then contains a normally rigid shaft and the radiating section at the distal end of the applicator. In catheter-based designs, the term antenna usually refers to the most distal radiating element, rather than the entire catheter.
Figure 5.
Cartoon schematic of a typical microwave ablation antenna. Coaxial cable runs the length of the shaft, with the radiating element at the distal end of the antenna. Energy is produced around the radiating element.
Antenna properties relevant to thermal ablation include both the radiation pattern and reflection coefficient, or return loss. In general, the lowest return loss is desirable to maximize energy transfer from the antenna into the tissue. Energy reflected from the antenna reduces tissue heating, while increasing unwanted heating of the antenna shaft. In extreme cases, high return loss may necessitate short ablation times to prevent thermal damage along the antenna shaft.47, 48
The desired radiation pattern is largely dependent on the clinical application. Most antennas in use currently radiate in the normal (broadside) mode, with propagation directed radially outward from the antenna. This is especially true of antennas designed for tumor ablation applications, where the ideal radiation pattern is focused and omni-directional to match the approximately spherical shape of many focal tumors. Antennas designed in the axial (end-fire) mode have been developed for cardiac applications to produce localized heating of a spot at the end of a catheter.49
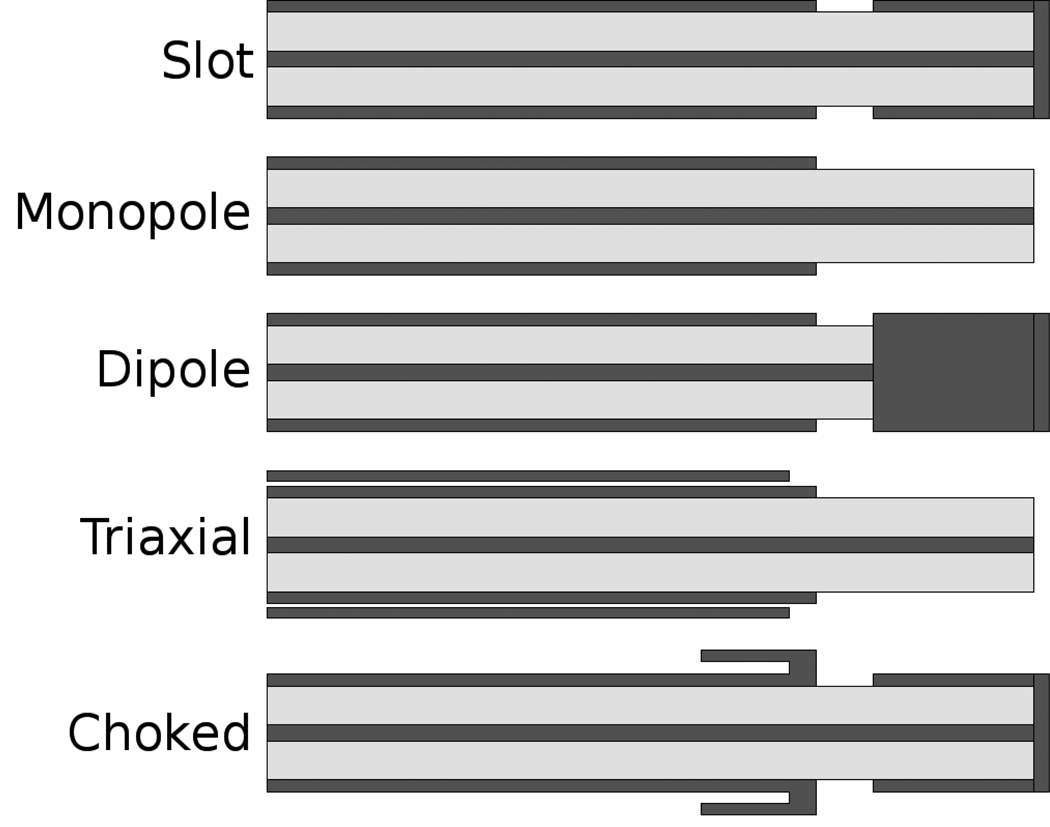
To achieve the goals of low return loss and focused energy radiation, several designs have been proposed. Broadly, these can be classified as designs that use a linear element, coaxial slot, loop, or helix as their primary mode of radiation (Figures 6–7). Designs primarily comprised of a linear element include monopoles, dipoles, and triaxial antennas.50–54 These antennas are highly efficient, coupling over 95% of input power into the tissue, with good broadside radiation patterns. However, they are relatively narrowband and radiation can be prone to hot-spots or elongated patterns. Tip loading can be used to increase the electrical length of an antenna or alter its heating characteristics.55, 56
Figure 6.
Schematic cross sections of five antenna designs for microwave ablation. From top to bottom: slot, monopole, dipole, triaxial and choked slot. Metallic components are represented in dark gray, dielectric insulators in light gray.
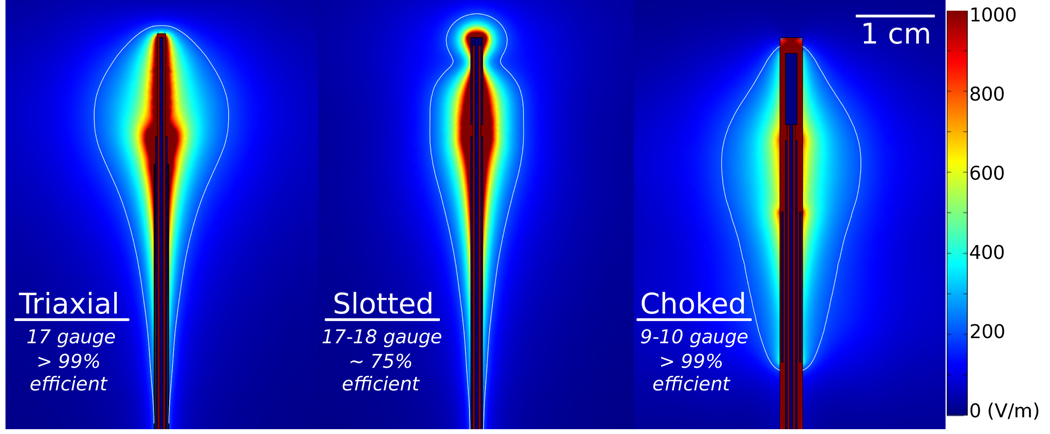
Figure 7.
Electric fields produced by three different antenna designs, representing designs using a linear element (triaxial, left), a single coaxial slot (center) and choked dipole (right). Approximate antenna diameter and power efficiency (1 – reflection coefficient) are noted to illustrate the design balance in each design.
Coaxial or annular slot antennas radiate from a smaller point, and can be designed to produce relatively low reflections with acceptable radiation patterns.57 Their smaller radiation point can be desirable for ablation of small volumes, but single-slot antennas (like many other microwave antenna designs) suffer from excessive backward heating along the antenna shaft without design modification. Coaxial chokes have therefore been proposed to reduce unwanted backward heating by enforcing the open boundary at the antenna’s feed point, thereby eliminating current on the outer surface of the coaxial cable.58–62 However, chokes add to the total antenna diameter, making them less attractive for use in percutaneous applications.
Looped or helical designs are less common in practice, but have been described in the literature . Notably, looped designs operating in the axial mode have been described for cardiac ablation, while helices have been proposed for microwave-assisted angioplasty.49, 63 Single and multi-loop designs have also been tested for microwave ablation in the liver.64
D. Challenges and future research
The major challenge to using microwaves for most clinical applications is controlling the heating zone for a desired clinical outcome without incidentally heating nearby tissues or causing complications. Primary focus has been given to antenna cooling and arrays as a means to safely deliver more power and produce larger ablations, but research has also continued in antenna design, frequency comparisons, and power application algorithms. Finally, clinical implementation of new systems for cardiac and oncologic interventions is ongoing. An overview of these developments and future directions are presented here.
Antenna Cooling
Antenna cooling is an obvious but somewhat challenging solution to the problem of limited power handling in small-diameter antennas. Air and water-cooled antenna designs have existed for several years, with water-cooled microwave ablation antennas recently described in widespread clinical use.39, 65 Sufficiently cooling the outer surface of the antenna shaft (ie, the outer conductor of a coaxial cable) prevents unwanted thermal damage that may be produced by heat conduction from inside the antenna shaft, heat conduction from the hot ablation zone along the antenna shaft, or backward heating from the radiating segment. By eliminating excessive heat produced by these three sources, higher powers can be passed through the antenna, resulting in larger ablation zones (Figure 8).
Figure 8.
Comparison of ablations created by using a triaxial antenna without cooling (above) and with cryogenic gas cooling (below). The uncooled antenna produces a tear-drop shaped ablation with thermal damage to the tissue surrounding the antenna shaft, but the cooled antenna produces a more spherical ablation without shaft heating.
Like coaxial chokes, cooling sleeves add to the overall diameter of the ablation antenna. Thinner cooling jackets limit antenna size, but are also characterized by less water flow and, therefore, reduced cooling capacity. Additional cooling power can be achieved by using cryogenic gas.66 Cryogenic cooling may also allow the antenna to be fixed in place by creating a small iceball at the distal end of the antenna, which alleviates the problem of applicator migration after applicator placement prior to energy application.67
Antenna Arrays for Microwave Ablation
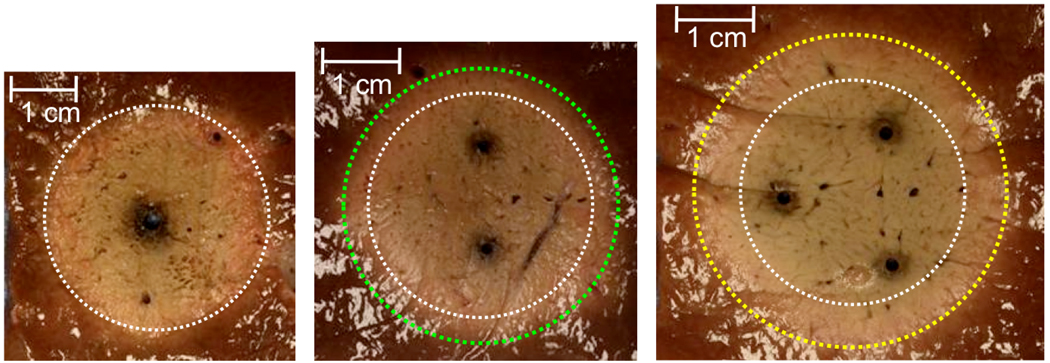
Due to a theoretical limit to the amount of power able to be distributed by a single antenna, antenna arrays are also being investigated. A recent study by Laeseke et al. demonstrated a fundamental advantage to using multiple antennas: power is more efficiently distributed by using an array of antennas, even when compared to a single antenna delivering the same total power (eg, 90 W in a single antenna versus 30 W in three antennas).68 A single antenna delivers power from a single source and much of the energy is spent heating tissues that have already been ablated. An antenna array distributes power to multiple sources so that less energy is wasted heating ablated tissue (Figure 9). In addition, heating produced simultaneously by multiple sources in proximity is known to produce ablation zones larger than might be expected from a sum of each source; an effect termed, “thermal synergy.”69–71 Several recent studies have confirmed the effect of thermal synergy when using arrays for microwave ablation, producing ablations up to 7 cm in diameter.35, 72, 73
Figure 9.
Ablations created using a total power of 90 W applied by a single antenna with 2.2 mm diameter (left), two antennas with 1.5 mm diameter each (45 W per antenna, center), or three antennas with 1.2 mm diameter (30 W per antenna, right). Simultaneous application of multiple antennas produces ablations with greater size and equivalent shape to a single antenna due to improved spatial distribution of energy.68
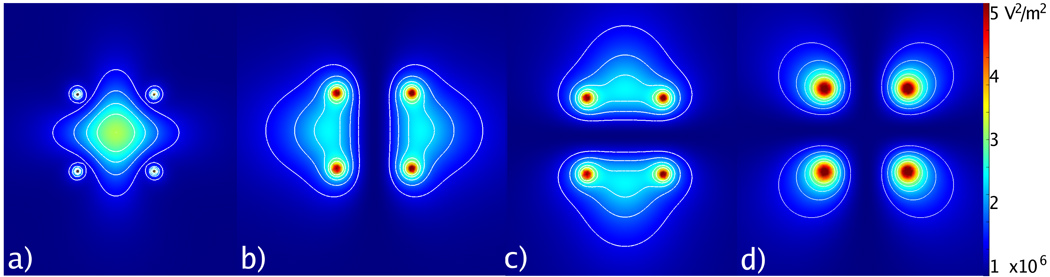
Seminal studies of phased-controlled antennas for microwave tissue heating have been described in the hyperthermia literature (Figure 10).74–81 The objective of many of these studies was to produce a precise zone of low-temperature hyperthermia (41–45 °C) in a target area without heating surrounding structures. Similar investigations for high-temperature microwave ablation are likely to follow. One potentially detrimental effect of antenna interference can occur if the relative phase is not known or controllable, as with multiple-generator multiple-antenna systems. Under this condition, the relative phase between antennas is unknown and the resulting interference may be largely constructive or destructive, leading to somewhat unpredictable results. It is currently unknown how much clinical impact random phase has on the final ablation zone. As an alternative, power may be switched between antennas in the array to eliminate interference, producing more predictable results.82 More study is needed to optimize and control power produced by antenna arrays for microwave ablation.
Figure 10.
Cross-section of electric fields produced by four antennas with phase control: a) all antennas at equal phase, b) left and right antennas 180° apart, c) top and bottom antennas 180° apart, and d) diagonal antennas 180° apart. Zones of constructive and destructive interference can be created with phase control of each channel.
Frequency Considerations For Microwave Ablation
As noted previously, microwave penetration and heating rates in tissue are highly dependent on frequency. Recent investigations comparing 915 MHz and 2.45 GHz for large-volume ablation in the liver have suggested that 915 MHz provides larger ablations.40 The hypothesis for this conclusion is that 915 MHz provides deeper field penetration and, thus, a greater volume of microwave heating. However, this study is currently without corroboration. In other studies, ablations produced by 915 MHz did not achieve similarly large ablations, so it is not yet clear how much impact is due to system design, implementation, tissue, frequency or other factors.83, 84 Higher frequencies have been used for shallow ablation of the endometrial lining of the uterus.18 Ongoing investigations should help define the role of frequency in individual applications.
III. APPLICATIONS
A. Cancer
The clinical indications for microwave ablation mirror those of RF ablation. The primary indication is for treatment of focal disease in those patients for whom surgery is not an option, who have refused or failed other treatments, or who require tissue-sparing procedures due to multiple tumor syndromes (eg, Von-Hippel-Lindau) or previous organ resection. The most common anatomic sites for microwave ablation worldwide are the liver, lung, kidney, prostate and bone.85 Other areas of interest include the breast, thyroid, brain and spleen.86–89
Liver
Both primary tumors such as hepatocellular carcinoma (HCC) and metastatic tumors originating from colorectal, lung, breast and other sites are treated in the liver. The primary benefits of using microwaves in the liver appear to be the ability to overcome large heat sinks inherent to this highly vascular organ, and the possibility to treat larger tumors with fewer applicator placements in less time than RF ablation.28 Faster heating provided by microwaves has also reduced treatment time when compared to RF ablation (Figure 2).
Early reports of a survival advantage when using microwave ablation against small HCC encouraged additional engineering and clinical development of clinical technique in Asia in the early 1990s.47, 48, 90, 91 A decade later, larger-scale studies demonstrated favorable results similar to RF ablation without significant complications.92 Similar trends have been observed in the treatment of colorectal metastases.93 One randomized comparison between microwave ablation and surgery found no significant difference in three-year survival, but reduced blood loss with microwave ablation.94 All of these studies were performed with a 2.45 GHz system using relatively small generator powers (~60 W) and short treatment times (~1–2 min).
Advances in technology have allowed increased use of microwave ablation, and more recent studies are confirming early results with some studies reporting three-year survival near 90 percent in small HCC.95, 96 Microwaves also appear to be effective against larger tumors (> 3 cm diameter), which have historically plagued RF ablation. Recent reports by Yu and Yin support the use of microwaves, especially with multiple applicators, against medium and large tumors in the liver.97, 98 Newer microwave ablation systems utilize both 915 MHz and 2.45 GHz, with most systems now employing dipole antennas.40
Lung
The greatest opportunity for microwave ablation may be in the treatment of lung cancer due to improved microwave penetration in lung when compared to other organs, or RF ablation. Preclinical studies have noted faster heating and larger ablations when using microwave devices optimized for lung tissue, and in direct comparison to RF ablation.31, 99, 100 Clinical evidence supporting the use of microwaves in the lung are sparse, but early data suggests that microwaves provide good local control with minimal complications in the treatment of lung tumors.46
Kidney
Percutaneous treatment for kidney tumors is rapidly increasing in popularity due to recent studies noting reduced cost and morbidity when compared to surgery or laparoscopic treatments.101 Preclinical studies in normal kidney have echoed the results of liver and lung studies: microwaves appear to produce more rapid heating and larger ablations than RF in the kidney.30, 84, 102 Emerging clinical data support the use of microwave ablation for treating renal-cell carcinoma, but additional study is needed to verify these promising results.103
Bone
RF ablation for osteoid ostoemas is now widely accepted, but can be somewhat problematic due to the high impedance and low thermal conductivity in bone. There is reason to suspect that microwaves could improve upon RF ablation treatments, but only a few reports of microwave ablation for osteoid osteomas exist.85 Larger series in the treatment of bone tumors and metastatic disease have been reported, with encouraging results.104–106
Prostate
One of the earliest uses for microwave thermal therapies was to treat malignancies and benign hyperplasia in the prostate.107 Some success has been noted in reducing tumor bulk and delaying growth of the primary tumor.108 However, enthusiasm for microwave ablation in the prostate has waned in recent years, in part due to challenges in avoiding thermal damage to nearby nerves and collagenous tissues such as the uretha, difficulty in visualizing the ablation zone at imaging, and improvements in other therapies such as minimally invasive resection, hormone therapy, brachytherapy and cryoablation.109–112
B. Cardiac Arrhythmias
As in cancer treatment, microwave ablation can be used in many of the same ways as RF ablation for the treatment of certain cardiac arrhythmias. With a theoretically deeper penetration of energy, microwaves would seem to again be a logical choice for cardiac ablation as well. Indeed, preclinical and early clinical data have supported the use of microwaves for the treatment of atrial fibrillation, especially when using an ablative approach to the Cox-Maze procedure or pulmonary vein isolation.113–116 However, there are some difficulties in getting reliable energy transmission between a microwave applicator and beating heart, and avoiding collateral damaging by stray or fringe fields around the microwave applicator. As a result, some recent reports have deemphasized the role of microwave ablation in cardiac ablation.117 Additional clinical testing and technology development will be necessary to further elucidate the role of microwaves in cardiac ablation.
C. Other
Microwave energy has been proposed and used as a possible treatment for other clinical conditions, most notably for endometrial ablation. During endometrial ablation, an antenna is introduced transvaginally into the uterus. Microwave energy is applied typically at a frequency of 9.2 GHz to produce coagulative necrosis of the endometrial lining. The technique has been shown to be safe, effective and relatively simple means to treat excessive uterine bleeding in nonsurgical patients.118 Other applications of microwave energy which have been investigated but have received mixed clinical attention include microwave-assisted angioplasty, where plaque is warmed using microwave energy to make enlargement easier;119 warming and reshaping of collagenous tissues in the shoulder or knee to reduce the need for surgery;120 and vascular ablation to treat varicose veins.121
CONCLUSIONS
In conclusion, microwave ablation is playing an increasingly important role in the treatment of cancer, cardiac arrhythmias and other diseases. In most cases, devices and equipment used clinically are first or second generation. Recent developments including cooled systems to increase power delivery, antenna arrays to distribute power spatially, and optimization of technology and technique based on the specific tissues have already impacted the clinical utilization of microwave ablation. Additional developments are likely to continue this trend. As technologies improve, increased clinical utilization of this exciting technology is expected.
REFERENCES
- 1.Bakker JF, Paulides MM, Westra AH, Schippers H, Van Rhoon GC. Design and test of a 434 MHz multi-channel amplifier system for targeted hyperthermia applicators. Int J Hyperthermia. 2010;26:158–170. doi: 10.3109/02656730903341191. [DOI] [PubMed] [Google Scholar]
- 2.Converse M, Bond EJ, Van Veen BD, Hagness SC. A computational study of ultra-wideband versus narrowband microwave hyperthermia for breast cancer treatment. IEEE Trans Microw Theory Tech. 2006;54:2169–2180. [Google Scholar]
- 3.Balanis CA. Advanced engineering electromagnetics. New York: John Wiley & Sons; 1989. [Google Scholar]
- 4.Pennes HH. Temperature of skeletal muscle in cerebral hemiplegia and paralysis agitans. Arch Neurol Psychiatry. 1949;62:269–279. doi: 10.1001/archneurpsyc.1949.02310150016002. [DOI] [PubMed] [Google Scholar]
- 5.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: ii. measurements in the frequency range 10 hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: iii. parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996;41:2271–2293. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- 7.Schwan HP, Li K. Measurements of materials with high dielectric constant and conductivity at ultrahigh frequencies. AIEE Trans Comm Electronics. 1955;74:603–607. [Google Scholar]
- 8.Brady MM, Symons SA, Stuchly SS. Dielectric behavior of selected animal tissues in vitro at frequencies from 2 to 4 GHz. IEEE Trans Biomed Eng. 1981;28:305–307. doi: 10.1109/TBME.1981.324707. [DOI] [PubMed] [Google Scholar]
- 9.Stuchly MA, Athey TW, Stuchly SS, Samaras GM, Taylor G. Dielectric properties of animal tissues in vivo at frequencies 10 MHz--1 GHz. Bioelectromagnetics. 1981;2:93–103. doi: 10.1002/bem.2250020202. [DOI] [PubMed] [Google Scholar]
- 10.Duck FA. Physical properties of tissue: a comprehensive reference book. Academic Press; 1990. [Google Scholar]
- 11.Lazebnik M, Converse MC, Booske JH, Hagness SC. Ultrawideband temperature-dependent dielectric properties of animal liver tissue in the microwave frequency range. Phys Med Biol. 2006;51:1941–1955. doi: 10.1088/0031-9155/51/7/022. [DOI] [PubMed] [Google Scholar]
- 12.Schepps JL, Foster KR. The uhf and microwave dielectric properties of normal and tumour tissues: variation in dielectric properties with tissue water content. Phys Med Biol. 1980;25:1149–1159. doi: 10.1088/0031-9155/25/6/012. [DOI] [PubMed] [Google Scholar]
- 13.Chin L, Sherar M. Changes in dielectric properties of ex vivo bovine liver at 915 MHz during heating. Phys Med Biol. 2001;46:197–211. doi: 10.1088/0031-9155/46/1/314. [DOI] [PubMed] [Google Scholar]
- 14.Brace CL. Temperature-dependent dielectric properties of liver tissue measured during thermal ablation: toward an improved numerical model. Conf Proc IEEE Eng Med Biol Soc. 2008;1:230–233. doi: 10.1109/IEMBS.2008.4649132. [DOI] [PubMed] [Google Scholar]
- 15.Epstein BR, Foster KR. Anisotropy in the dielectric properties of skeletal muscle. Med Biol Eng Comput. 1983;21:51–55. doi: 10.1007/BF02446406. [DOI] [PubMed] [Google Scholar]
- 16.Bircan C, Barringer S. Determination of protein denaturation of muscle foods using the dielectric properties. J Food Sci. 2002;67:202–205. [Google Scholar]
- 17.Turner PF, Tumeh A, Schaefermeyer T. Bsd-2000 approach for deep local and regional hyperthermia: physics and technology. Strahlenther Onkol. 1989;165:738–741. [PubMed] [Google Scholar]
- 18.Feldberg I, Cronin N. A 9.2 GHz microwave applicator for the treatment of menorrhagia. IEEE MTT-S. 1998;2:755–758. [Google Scholar]
- 19.Bertram JM, Yang D, Converse MC, Webster JG, Mahvi DM. Antenna design for microwave hepatic ablation using an axisymmetric electromagnetic model. Biomed Eng Online. 2006;5:15. doi: 10.1186/1475-925X-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed M, Liu Z, Afzal KS, Weeks D, Lobo SM, Kruskal JB, Lenkinski RE, et al. Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;230:761–767. doi: 10.1148/radiol.2303021801. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Youk JH, Kim YK, Han YM, Chung GH, Lee SY, Kim CS. Radio-frequency thermal ablation with hypertonic saline solution injection of the lung: ex vivo and in vivo feasibility studies. Eur Radiol. 2003;13:2540–2547. doi: 10.1007/s00330-003-1876-x. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 23.Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, Pellicanò S, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205–210. doi: 10.1148/radiology.202.1.8988212. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg SN, Stein MC, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-radiofrequency technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999;10:907–916. doi: 10.1016/s1051-0443(99)70136-3. [DOI] [PubMed] [Google Scholar]
- 25.Orth K, Russ D, Duerr J, Hibst R, Steiner R, Beger HG. Thermo-controlled device for inducing deep coagulation in the liver with the nd:yag laser. Lasers Surg Med. 1997;20:149–156. doi: 10.1002/(sici)1096-9101(1997)20:2<149::aid-lsm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Roberts WW, Hall TL, Ives K, Wolf JSJ, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–738. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- 27.Lu DSK, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 28.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DSK. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–1092. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009;41:168–172. doi: 10.1080/00313020802579292. [DOI] [PubMed] [Google Scholar]
- 30.Laeseke PF, Lee FTJ, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20:1224–1229. doi: 10.1016/j.jvir.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brace CL, Hinshaw JL, Laeseke PF, Sampson LA, Lee FTJ. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and ct findings in a swine model. Radiology. 2009;251:705–711. doi: 10.1148/radiol.2513081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FTJ. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 33.Andreano A, Huang Y, Meloni F, Lee FT, Jr, Brace CL. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2009;37:2967–2973. doi: 10.1118/1.3432569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FTJ. Microwave ablation with a single small-gauge triaxial antenna: in vivo porcine liver model. Radiology. 2007;242:435–440. doi: 10.1148/radiol.2422051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FTJ. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology. 2007;244:151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 36.Strickland AD, Clegg PJ, Cronin NJ, Swift B, Festing M, West KP, Robertson GSM, et al. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2002;89:1003–1007. doi: 10.1046/j.1365-2168.2002.02155.x. [DOI] [PubMed] [Google Scholar]
- 37.Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, Goldberg SN. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 38.Skinner MG, Iizuka MN, Kolios MC, Sherar MD. A theoretical comparison of energy sources--microwave, ultrasound and laser--for interstitial thermal therapy. Phys Med Biol. 1998;43:3535–3547. doi: 10.1088/0031-9155/43/12/011. [DOI] [PubMed] [Google Scholar]
- 39.Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, Xu ZF, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna--experimental and clinical studies. Radiology. 2007;242:914–924. doi: 10.1148/radiol.2423052028. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Wang Y, Ni X, Gao Y, Shao Q, Liu L, Liang P. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. AJR Am J Roentgenol. 2009;192:511–514. doi: 10.2214/AJR.07.3828. [DOI] [PubMed] [Google Scholar]
- 41.Tse H, Liao S, Siu C, Yuan L, Nicholls J, Leung G, Ormsby T, et al. Determinants of lesion dimensions during transcatheter microwave ablation. Pacing Clin Electrophysiol. 2009;32:201–208. doi: 10.1111/j.1540-8159.2008.02203.x. [DOI] [PubMed] [Google Scholar]
- 42.Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD. Ct-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 2003;229:475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- 44.Brace CL, Laeseke PF, van der Weide DW, Lee FT. Microwave ablation with a triaxial antenna: results in ex vivo bovine liver. IEEE Trans Microw Theory Tech. 2005;53:215–220. doi: 10.1109/TMTT.2004.839308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009 doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 46.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, ct findings, and safety in 50 patients. Radiology. 2008;247:871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 47.Sato M, Watanabe Y, Ueda S, Iseki S, Abe Y, Sato N, Kimura S, et al. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110:1507–1514. doi: 10.1053/gast.1996.v110.pm8613057. [DOI] [PubMed] [Google Scholar]
- 48.Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 49.Gu Z, Rappaport CM, Wang PJ, VanderBrink BA. A 2 1/4-turn spiral antenna for catheter cardiac ablation. IEEE Trans Biomed Eng. 1999;46:1480–1482. doi: 10.1109/10.804576. [DOI] [PubMed] [Google Scholar]
- 50.Brace CL, van der Weide DW, Lee FT, Laeseke PF, Sampson L. Analysis and experimental validation of a triaxial antenna for microwave tumor ablation. IEEE MTTS Int Microw Symp. 2004;3:1437–1440. doi: 10.1109/MWSYM.2004.1338842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labonte S, Blais A, Legault S, Ali H, Roy L. Monopole antennas for microwave catheter ablation. IEEE Trans Microwave Theory Tech. 1996;44:1832–1840. [Google Scholar]
- 52.de Sieyes DC, Douple EB, Strohbehn JW, Trembly BS. Some aspects of optimization of an invasive microwave antenna for local hyperthermia treatment of cancer. Med Phys. 1981;8:174–183. doi: 10.1118/1.594930. [DOI] [PubMed] [Google Scholar]
- 53.Hurter W, Reinbold F, Lorenz W. A dipole antenna for interstitial microwave hyperthermia. IEEE Trans Microwave Theory Tech. 1991;39:1048–1054. [Google Scholar]
- 54.Schaller G, Erb J, Engelbrecht R. Field simulation of dipole antennas for interstitial microwave hyperthermia. IEEE Trans Microwave Theory Tech. 1996;44:887–895. [Google Scholar]
- 55.Ahn H, Lee K. Interstitial antennas tipped with reactive load. IEEE Microwave Wireless Comp Lett. 2005;15:215–220. [Google Scholar]
- 56.Hamada L, Saito K, Yoshimura H, Ito K. Dielectric-loaded coaxial-slot antenna for interstitial microwave hyperthermia: longitudinal control of heating patterns. Int J Hyperthermia. 2000;16:219–229. doi: 10.1080/026567300285240. [DOI] [PubMed] [Google Scholar]
- 57.Ito K, Hyodo M, Shimura M, Kasai H. Thin applicator having coaxial ring slots for interstitial microwave hyperthermia. Ant Prop Soc Int Sym. 1990;3:1233–1236. [Google Scholar]
- 58.Longo I, Gentili G, Cerretelli M, Tosoratti N. A coaxial antenna awith miniaturized choke for minimally invasive interstitial heating. IEEE Trans Microwave Theory Tech. 2003;50:82–88. doi: 10.1109/TBME.2002.807320. [DOI] [PubMed] [Google Scholar]
- 59.Pisa S, Cavagnaro M, Bernardi P, Lin JC. A 915-MHz antenna for microwave thermal ablation treatment: physical design, computer modeling and experimental measurement. IEEE Trans Biomed Eng. 2001;48:599–601. doi: 10.1109/10.918599. [DOI] [PubMed] [Google Scholar]
- 60.Lin JC, Wang YJ. The cap-choke catheter antenna for microwave ablation treatment. IEEE Trans Biomed Eng. 1996;43:657–660. doi: 10.1109/10.495286. [DOI] [PubMed] [Google Scholar]
- 61.Wong TZ, Trembly BS. A theoretical model for input impedance of interstitial microwave antennas with choke. Int J Radiat Oncol Biol Phys. 1994;28:673–682. doi: 10.1016/0360-3016(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 62.Yang D, Bertram JM, Converse MC, O'Rourke AP, Webster JG, Hagness SC, Will JA, et al. A floating sleeve antenna yields localized hepatic microwave ablation. IEEE Trans Biomed Eng. 2006;53:533–537. doi: 10.1109/TBME.2005.869794. [DOI] [PubMed] [Google Scholar]
- 63.Liu P, Rappaport CM. A helical microwave antenna for welding plaque during balloon angioplasty. IEEE Trans Microw Theory Tech. 1996;44:1819–1831. [Google Scholar]
- 64.Shock SA, Meredith K, Warner TF, Sampson LA, Wright AS, Winter TC3, Mahvi DM, et al. Microwave ablation with loop antenna: in vivo porcine liver model. Radiology. 2004;231:143–149. doi: 10.1148/radiol.2311021342. [DOI] [PubMed] [Google Scholar]
- 65.Yeh MM, Trembly BS, Douple EB, Ryan TP, Hoopes PJ, Jonsson E, Heaney JA. Theoretical and experimental analysis of air cooling for intracavitary microwave hyperthermia applicators. IEEE Trans Biomed Eng. 1994;41:874–882. doi: 10.1109/10.312095. [DOI] [PubMed] [Google Scholar]
- 66.Knavel EM, Sampson LA, Lubner M, Hinshaw JL, Andreano A, Lee FT, Jr, Brace CL. High-powered gas-cooled microwave ablation: Shaft cooling creates an effective stich zone without preserving peri-antenna tissue; World Conference on Interventional Oncology; Jun 9–12, 2010; Philadelphia, PA. [Google Scholar]
- 67.Steinke K, King J, Glenn DW, Morris DL. Percutaneous radiofrequency ablation of lung tumors with expandable needle electrodes: tips from preliminary experience. AJR Am J Roentgenol. 2004;183:605–611. doi: 10.2214/ajr.183.3.1830605. [DOI] [PubMed] [Google Scholar]
- 68.Laeseke P, Sampson L, Lee FJ, Brace C. Spatially distributing power improves thermal profiles and reduces invasiveness. J Intervent Oncol. 2009;2:13–18. [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, Choi BI. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 70.Laeseke PF, Sampson LA, Frey TM, Mukherjee R, Winter TC3, Lee FTJ, Brace CL. Multiple-electrode radiofrequency ablation: comparison with a conventional cluster electrode in an in vivo porcine kidney model. J Vasc Interv Radiol. 2007;18:1005–1010. doi: 10.1016/j.jvir.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Laeseke PF, Sampson LA, Haemmerich D, Brace CL, Fine JP, Frey TM, Winter TC3, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241:116–124. doi: 10.1148/radiol.2411051271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright AS, Lee FTJ, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 73.Simon CJ, Dupuy DE, Iannitti DA, Lu DSK, Yu NC, Aswad BI, Busuttil RW, et al. Intraoperative triple antenna hepatic microwave ablation. AJR Am J Roentgenol. 2006;187:W333–W340. doi: 10.2214/AJR.05.0804. [DOI] [PubMed] [Google Scholar]
- 74.Jones KM, Mechling JA, Strohbehn JW, Trembly BS. Theoretical and experimental sar distributions for interstitial dipole antenna arrays used in hyperthermia. IEEE Trans Microw Theory Tech. 1989;37:1200–1209. [Google Scholar]
- 75.Furse CM, Iskander MF. Three-dimensional electromagnetic power deposition in tumors using interstitial antenna arrays. IEEE Trans Biomed Eng. 1989;36:977–986. doi: 10.1109/10.40798. [DOI] [PubMed] [Google Scholar]
- 76.Lyons BE, Britt RH, Strohbehn JW. Localized hyperthermia in the treatment of malignant brain tumors using an interstitial microwave antenna array. IEEE Trans Biomed Eng. 1984;31:53–62. doi: 10.1109/TBME.1984.325370. [DOI] [PubMed] [Google Scholar]
- 77.Turner PF. Regional hyperthermia with an annular phased array. IEEE Trans Biomed Eng. 1984;31:106–114. doi: 10.1109/tbme.1984.325376. [DOI] [PubMed] [Google Scholar]
- 78.Magin RL, Peterson AF. Noninvasive microwave phased arrays for local hyperthermia: a review. Int J Hyperthermia. 1989;5:429–450. doi: 10.3109/02656738909140470. [DOI] [PubMed] [Google Scholar]
- 79.Trembly BS. The effects of driving frequency and antenna length on power deposition within a microwave antenna array used for hyperthermia. IEEE Trans Biomed Eng. 1985;32:152–157. doi: 10.1109/TBME.1985.325436. [DOI] [PubMed] [Google Scholar]
- 80.Turner PF. Interstitial equal-phased arrays for em hyperthermia. IEEE Trans Microw Theory Tech. 1986;34:572–578. [Google Scholar]
- 81.Trembly BS, Wilson AH, Sullivan MJ, Stein AD, Wong TZ, Strohbehn JW. Control of the sar pattern withing an interstitial microwave array through variation of antenna driving phase. IEEE Trans Microw Theory Tech. 1986;34:568–571. [Google Scholar]
- 82.Brace C, Laeseke P, Sampson L, van der Weide D, Lee FJ. Switched-mode microwave ablation: less dependence on tissue properties leads to more consistent ablations than phased arrays; Radiological society of north america annual meeting; chicago, il: 2007. [Google Scholar]
- 83.Oshima F, Yamakado K, Nakatsuka A, Takaki H, Makita M, Takeda K. Simultaneous microwave ablation using multiple antennas in explanted bovine livers: relationship between ablative zone and antenna. Radiat Med. 2008;26:408–414. doi: 10.1007/s11604-008-0251-x. [DOI] [PubMed] [Google Scholar]
- 84.Hope WW, Schmelzer TM, Newcomb WL, Heath JJ, Lincourt AE, Norton HJ, Heniford BT, et al. Guidelines for power and time variables for microwave ablation in an in vivo porcine kidney. J Surg Res. 2008 doi: 10.1016/j.jss.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 85.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25 Suppl 1:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 86.Duan YQ, Gao YY, Ni XX, Wang Y, Feng L, Liang P. Changes in peripheral lymphocyte subsets in patients after partial microwave ablation of the spleen for secondary splenomegaly and hypersplenism: a preliminary study. Int J Hyperthermia. 2007;23:467–472. doi: 10.1080/02656730701474533. [DOI] [PubMed] [Google Scholar]
- 87.Lin J, Hirai S, Chiang C, Hsu W, Su J, Wang Y. Computer simulation and experimental studies of sar distributions of interstitial arrays of sleeved-slot microwave antennas for hyperthermia treatment of brain tumors. IEEE Trans Microwave Theory Tech. 2000;48:2191–2198. [Google Scholar]
- 88.Tsutsui H, Usuda J, Kubota M, Yamada M, Suzuki A, Shibuya H, Miyajima K, et al. Endoscopic tumor ablation for laryngotracheal intraluminal invasion secondary to advanced thyroid cancer. Acta Otolaryngol. 2008;128:799–807. doi: 10.1080/00016480701714285. [DOI] [PubMed] [Google Scholar]
- 89.Singletary SE. Minimally invasive ablation techniques in breast cancer treatment. Ann Surg Oncol. 2002;9:319–320. doi: 10.1007/BF02573863. [DOI] [PubMed] [Google Scholar]
- 90.Abe T, Shinzawa H, Wakabayashi H, Aoki M, Sugahara K, Iwaba A, Haga H, et al. Value of laparoscopic microwave coagulation therapy for hepatocellular carcinoma in relation to tumor size and location. Endoscopy. 2000;32:598–603. doi: 10.1055/s-2000-9016. [DOI] [PubMed] [Google Scholar]
- 91.Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72 Suppl 1:124–131. doi: 10.1159/000111718. [DOI] [PubMed] [Google Scholar]
- 92.Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 93.Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, et al. Percutaneous microwave coagulation therapy for solitary metastatic liver tumors from colorectal cancer: a pilot clinical study. Am J Gastroenterol. 1999;94:322–327. doi: 10.1111/j.1572-0241.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 94.Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276–284. [PubMed] [Google Scholar]
- 95.Shiomi H, Naka S, Sato K, Demura K, Murakami K, Shimizu T, Morikawa S, et al. Thoracoscopy-assisted magnetic resonance guided microwave coagulation therapy for hepatic tumors. Am J Surg. 2008;195:854–860. doi: 10.1016/j.amjsurg.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 96.Lu M, Xu H, Xie X, Yin X, Chen J, Kuang M, Xu Z, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054–1060. doi: 10.1007/s00535-005-1671-3. [DOI] [PubMed] [Google Scholar]
- 97.Yu NC, Lu DSK, Raman SS, Dupuy DE, Simon CJ, Lassman C, Aswad BI, et al. Hepatocellular carcinoma: microwave ablation with multiple straight and loop antenna clusters--pilot comparison with pathologic findings. Radiology. 2006;239:269–275. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 98.Yin X, Xie X, Lu M, Xu H, Xu Z, Kuang M, Liu G, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914–1923. doi: 10.1002/cncr.24196. [DOI] [PubMed] [Google Scholar]
- 99.Furukawa K, Miura T, Kato Y, Okada S, Tsutsui H, Shimatani H, Kajiwara N, et al. Microwave coagulation therapy in canine peripheral lung tissue. J Surg Res. 2005;123:245–250. doi: 10.1016/j.jss.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 100.Durick NA, Laeseke PF, Broderick LS, Lee FTJ, Sampson LA, Frey TM, Warner TF, et al. Microwave ablation with triaxial antennas tuned for lung: results in an in vivo porcine model. Radiology. 2008;247:80–87. doi: 10.1148/radiol.2471062123. [DOI] [PubMed] [Google Scholar]
- 101.Pandharipande PV, Gervais DA, Mueller PR, Hur C, Gazelle GS. Radiofrequency ablation versus nephron-sparing surgery for small unilateral renal cell carcinoma: cost-effectiveness analysis. Radiology. 2008;248:169–178. doi: 10.1148/radiol.2481071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kigure T, Harada T, Yuri Y, Fujieda N, Satoh Y. Experimental study of microwave coagulation of a vx-2 carcinoma implanted in rabbit kidney. Int J Urol. 1994;1:23–27. doi: 10.1111/j.1442-2042.1994.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 103.Clark PE, Woodruff RD, Zagoria RJ, Hall MC. Microwave ablation of renal parenchymal tumors before nephrectomy: phase i study. AJR Am J Roentgenol. 2007;188:1212–1214. doi: 10.2214/AJR.05.2190. [DOI] [PubMed] [Google Scholar]
- 104.Simon CJ, Dupuy DE. Image-guided ablative techniques in pelvic malignancies: radiofrequency ablation, cryoablation, microwave ablation. Surg Oncol Clin N Am. 2005;14:419–431. doi: 10.1016/j.soc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 105.Carrafiello G, Laganà D, Pellegrino C, Fontana F, Mangini M, Nicotera P, Petullà M, et al. Percutaneous imaging-guided ablation therapies in the treatment of symptomatic bone metastases: preliminary experience. Radiol Med. 2009;114:608–625. doi: 10.1007/s11547-009-0395-5. [DOI] [PubMed] [Google Scholar]
- 106.Fan Q, Ma B, Zhou Y, Zhang M, Hao X. Bone tumors of the extremities or pelvis treated by microwave-induced hyperthermia. Clin Orthop Relat Res. 2003:165–175. doi: 10.1097/01.blo.0000037439.23683.9c. [DOI] [PubMed] [Google Scholar]
- 107.Bolmsjö M, Wagrell L, Hallin A, Eliasson T, Erlandsson BE, Mattiasson A. The heat is on--but how? a comparison of tumt devices. Br J Urol. 1996;78:564–572. doi: 10.1046/j.1464-410x.1996.17213.x. [DOI] [PubMed] [Google Scholar]
- 108.Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. J Endourol. 1996;10:565–573. doi: 10.1089/end.1996.10.565. [DOI] [PubMed] [Google Scholar]
- 109.Armstrong N, Vale L, Deverill M, Nabi G, McClinton S, N'Dow J, Pickard R. Surgical treatments for men with benign prostatic enlargement: cost effectiveness study. BMJ. 2009;338:b1288. doi: 10.1136/bmj.b1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu X, Elliott SP, Wilt TJ, McBean AM. Practice patterns in benign prostatic hyperplasia surgical therapy: the dramatic increase in minimally invasive technologies. J Urol. 2008;180:241–245. doi: 10.1016/j.juro.2008.03.039. discussion 245. [DOI] [PubMed] [Google Scholar]
- 111.Ellis DS, Manny TBJ, Rewcastle JC. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology. 2007;70:9–15. doi: 10.1016/j.urology.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 112.Barqawi AB, Crawford ED. The current use and future trends of focal surgical therapy in the management of localized prostate cancer. Cancer J. 2007;13:313–317. doi: 10.1097/PPO.0b013e318156eb99. [DOI] [PubMed] [Google Scholar]
- 113.Melby SJ, Zierer A, Kaiser SP, Schuessler RB, Damiano RJJ. Epicardial microwave ablation on the beating heart for atrial fibrillation: the dependency of lesion depth on cardiac output. J Thorac Cardiovasc Surg. 2006;132:355–360. doi: 10.1016/j.jtcvs.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 114.Molloy TA. Midterm clinical experience with microwave surgical ablation of atrial fibrillation. Ann Thorac Surg. 2005;79:2115–2118. doi: 10.1016/j.athoracsur.2004.06.104. [DOI] [PubMed] [Google Scholar]
- 115.Comas GM, Imren Y, Williams MR. An overview of energy sources in clinical use for the ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2007;19:16–24. doi: 10.1053/j.semtcvs.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Bakir I, Casselman FP, Brugada P, Geelen P, Wellens F, Degrieck I, Van Praet F, et al. Current strategies in the surgical treatment of atrial fibrillation: review of the literature and onze lieve vrouw clinic's strategy. Ann Thorac Surg. 2007;83:331–340. doi: 10.1016/j.athoracsur.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 117.Vicol C, Kellerer D, Petrakopoulou P, Kaczmarek I, Lamm P, Reichart B. Long-term results after ablation for long-standing atrial fibrillation concomitant to surgery for organic heart disease: is microwave energy reliable? J Thorac Cardiovasc Surg. 2008;136:1156–1159. doi: 10.1016/j.jtcvs.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 118.Sharma B, Preston J, Ray C. Microwave endometrial ablation for menorrhagia: outcome at 2 years--experience of a district general hospital. J Obstet Gynaecol. 2004;24:916–919. doi: 10.1080/01443610400019005. [DOI] [PubMed] [Google Scholar]
- 119.Nardone DT, Smith DL, Martinez-Hernandez A, Consigny PM, Kosman Z, Rosen A, Walinsky P. Microwave thermal balloon angioplasty in the atherosclerotic rabbit. Am Heart J. 1994;127:198–203. doi: 10.1016/0002-8703(94)90525-8. [DOI] [PubMed] [Google Scholar]
- 120.Richards RG, Kääb MJ. Microwave-enhanced fixation of rabbit articular cartilage. J Microsc. 1996;181:269–276. doi: 10.1046/j.1365-2818.1996.125406.x. [DOI] [PubMed] [Google Scholar]
- 121.Subwongcharoen S, Praditphol N, Chitwiset S. Endovenous microwave ablation of varicose veins: in vitro, live swine model, and clinical study. Surg Laparosc Endosc Percutan Tech. 2009;19:170–174. doi: 10.1097/SLE.0b013e3181987549. [DOI] [PubMed] [Google Scholar]