Abstract
Acid sphingomyelinase (ASM) regulates the homeostasis of sphingolipids, including ceramides and sphingosine-1-phosphate (S1P). Because sphingolipids regulate AKT activation, we investigated the role of ASM in hepatic glucose and lipid metabolism. Initially, we overexpressed ASM in the livers of wild-type and diabetic db/db mice by adenovirus vector (Ad5ASM). In these mice, glucose tolerance was improved, and glycogen and lipid accumulation in the liver were increased. Using primary cultured hepatocytes, we confirmed that ASM increased glucose uptake, glycogen deposition, and lipid accumulation through activation of AKT and glycogen synthase kinase-3β. In addition, ASM induced up-regulation of glucose transporter 2 accompanied by suppression of AMP-activated protein kinase (AMPK) phosphorylation. Loss of sphingosine kinase-1 (SphK1) diminished ASM-mediated AKT phosphorylation, but exogenous S1P induced AKT activation in hepatocytes. In contrast, SphK1 deficiency did not affect AMPK activation. These results suggest that the SphK/S1P pathway is required for ASM-mediated AKT activation but not for AMPK inactivation. Finally, we found that treatment with high-dose glucose increased glycogen deposition and lipid accumulation in wild-type hepatocytes but not in ASM−/− cells. This result is consistent with glucose intolerance in ASM−/− mice. In conclusion, ASM modulates AKT activation and AMPK inactivation, thus regulating glucose and lipid metabolism in the liver.—Osawa, Y., Seki, E., Kodama, Y., Suetsugu, A., Miura, K., Adachi, M., Ito, H., Shiratori, Y., Banno, Y., Olefsky, J. M., Nagaki, M., Moriwaki, H., Brenner, D. A., Seishima, M. Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through AKT activation and AMP-activated protein kinase suppression.
Keywords: sphingosine-1-phosphate, glucose intolerance, glucose transporter 2, glycogen synthase kinase-3β
The liver is a key organ for glucose and lipid metabolism. During feeding, hepatocytes increase glucose uptake in response to elevated glucose levels in the portal vein and increase glycogen and triacylglyceride synthesis. AKT is a key molecule for insulin signaling and glucose metabolism. On AKT activation, glucose influx is stimulated by activation of glycogen synthesis through glycogen synthase kinase (GSK)-3β. The facilitative glucose transporter (GLUT) 2 is predominantly expressed in the liver (1). Hepatic GLUT2 is located at the plasma membrane even in the absence of insulin stimulation; glucose uptake by GLUT2 is therefore not directly affected by insulin. Glucose increases GLUT2 mRNA levels in hepatocytes (2), resulting in increased glucose influx. Constitutive activity of AMP-activated protein kinase (AMPK) decreases glycogen synthesis accompanied by down-regulation of GLUT2 expression (3). Therefore, both AKT and AMPK are involved in glucose metabolism.
Acid sphingomyelinase (ASM) deficiency leads to Niemann-Pick disease. ASM has been reported to be involved in various cellular functions (4), including glucose and lipid metabolism. ASM activity is increased in the serum of type 2 diabetic patients (5). Sphingomyelinase induces GLUT4 translocation to the plasma membrane (6, 7) and increases glucose uptake by adipocytes (7, 8). A high-fat, high-cholesterol diet does not induce hepatic triacylglyceride accumulation in ASM-deficient mice (ASM−/−) under an LDL receptor-deficient condition (9). ASM hydrolyzes sphingomyelin to ceramide and phosphorylcholine. Ceramide has been identified as a bioactive mediator in various cellular functions, including apoptosis and the cell cycle (10, 11). Ceramide accumulation contributes to the development of type 2 diabetes (12). Indeed, inhibition of ceramide synthesis by myriocin, serine palmitoyltransferase inhibitor, or dihydroceramide desaturase improves insulin resistance induced by glucocorticoid or saturated fat (13). Ceramide induces insulin resistance by inactivation of AKT through protein phosphatase-2A and PKC-ζ (14, 15) or by inhibition of AKT translocation to the plasma membrane (16). In addition, ceramide inhibits AMPK activation in hepatoma cells (17).
Not only ceramide but also its metabolite sphingosine-1-phosphate (S1P) is involved in glucose metabolism. Ceramide is hydrolyzed by ceramidase to sphingosine, which is further phosphorylated to S1P by sphingosine kinase (SphK; ref. 18). S1P stimulates AKT activation in human (19) and rodent hepatocytes (20) through the S1P receptor (S1PR). S1P signaling also increases glucose uptake via the insulin receptor and production of reactive oxygen species in C2C12 mouse myoblast cells (21). Because sphingolipids are important in glucose and lipid metabolism, we investigated the role of ASM in glucose and lipid metabolism in hepatocytes. In this study, we determined that ASM stimulates glucose uptake, glycogen deposition, and lipid accumulation via S1P formation and subsequent AKT activation. As a result, ASM deficiency causes glucose intolerance.
MATERIALS AND METHODS
Animals
The experiments were conducted in accordance with the institutional guidelines of Gifu University and Columbia University. Sprague-Dawley male rats and male wild-type (C57BL/6J), ASM−/−, SphK1-deficient (SphK1−/−; refs. 20, 22), and obese and diabetic db/db mice (C57BL/6 background) were used for this study.
Mice
ASM−/− mice (C57BL/6 background) and SphK1−/− mice (C57BL/6 background) (23), which lack the putative lipid kinase catalytic domain, were bred for studies. Obese and diabetic db/db mice (C57BL/6 background) were obtained from the Institute for Animal Reproduction (Ibaragi, Japan), and wild-type C57BL/6J mice were from Japan SLC (Shizuoka, Japan). Eight- to 10-wk-old male mice were used for in vivo studies.
Intraperitoneal glucose tolerance test (IPGTT)
The 8- to 10-wk-old male mice (wild-type, db/db, or ASM−/− mice) were deprived of food for 18 h. d-Glucose (2 mg/g body weight) was injected intraperitoneally, and blood glucose levels were monitored at 0, 30, 60, and 120 min after the injection using G checker (Sanko Junyaku, Tokyo, Japan). Values for area under the blood glucose curve after glucose loading were calculated. Serum insulin content was measured with a mouse insulin ELISA kit (Shibayagi, Gunma, Japan).
Primary hepatocyte cultures
Sprague-Dawley male rats (200–250 g) or mice (4 wk old) were anesthetized with ketamine and xylazine administered by intraperitoneal injection. Hepatocytes were then isolated by a nonrecirculating in situ collagenase perfusion of livers cannulating through the portal vein of rats or inferior vena cava of mice. Livers were first perfused in situ with 0.5 mM EGTA containing calcium-free salt solution, followed by perfusion with solution containing 0.02% collagenase D (Roche Diagnostics, Indianapolis, IN, USA). The liver was then gently minced on a Petri dish and filtered with polyamide mesh (3-60/42; Sefar America Inc., New York, NY, USA). Hepatocytes were washed 3 times and centrifuged at 50 g for 1 min. Cell viability was consistently >90%, as determined by trypan blue exclusion. Cells were plated on dishes coated with rat collagen type I in Waymouth medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum with antibiotics (plating medium).
Cell treatment
After isolation from rat or mouse livers, hepatocytes were cultured in 10% FBS-containing medium for 4 h. Cells were then washed twice with PBS and changed to serum-free RPMI 1640 (Invitrogen) containing glucose (200 mg/dl) and antibiotics in the presence or absence of recombinant adenoviruses [10 plaque forming units (PFU)/cell]. After a 2-h incubation, the culture medium was changed to serum-free RPMI 1640 containing antibiotics, and the cells were incubated for an additional 16 h. Before stimulation with ASM (1 IU/ml; Sigma-Aldrich, St. Louis, MO, USA), SIP (1 μM; Sigma-Aldrich), or high-dose glucose (600 mg/dl), the cells were washed twice with PBS. The cells were pretreated with imipramine (50 μM; Sigma-Aldrich) for 1 h or with pertussis toxin (PTX) (Sigma-Aldrich) for 16 h in some experiments. The hepatocytes were then treated with or without ASM, SIP, or high-dose glucose, and incubated 8 h for glycogen, RNA, and protein extraction or 24 h for Oil Red O staining and triglyceride measurement. The experimental design is shown in Supplemental Fig. S1A.
Recombinant adenoviruses
The adenovirus 5 (Ad5) variants Ad5GFP, Ad5CA-AKT, Ad5DN-AKT, Ad5CA-AMPK, and Ad5DN-AMPK express green fluorescent protein (GFP), constitutively active (CA)-AKT encoding an amino-terminal myristylation signal, dominant-negative (DN)-AKT, c-myc-CA-AMPK, and c-myc-DN-AMPK, respectively (20, 24). The recombinant replication deficient adenovirus Ad5ASM expressing ASM was constructed by an AdEasy Adenoviral Vector System (Stratagene, La Jolla, CA, USA). In brief, the full length of human ASM DNA was subcloned into pTrack adenoviral vector. The plasmid DNA was prepared by the alkaline lysis method and transfected into BJ5183-AD-1 electroporation-competent cells. The virus was grown in HEK293 cells and purified by banding twice on CsCl gradients, then dialyzed and stored at −20°C. The pTrack plasmid contains a GFP sequence driven under a CMV promoter, and the Ad5ASM expresses both ASM and GFP. Mice were infected with the adenoviruses (5×108 PFU/mouse) by intravenous injection. IPGTT was performed on 3 d after the infection. The animals were humanely killed at 3 d for glycogen and protein extraction and at 7 d for Oil Red O staining and triglyceride measurement.
Glucose uptake
Primary cultured hepatocytes were treated with or without ASM for 2 h or infected with Ad5ASM or Ad5GFP, and then 2-deoxy-d-[1-3H]glucose (2 μCi/ml; Amersham Biosciences, Piscataway, NJ, USA) was added to the culture medium. After a further 1-h incubation, cells were washed 3 times with ice-cold PBS and lysed in 1% SDS and 200 mM NaOH. The amount of labeled glucose taken up was determined by scintillation counting.
Measurement of glycogen content
After the treatment of hepatocytes for the indicated periods, the dish was placed on ice, washed with ice-cold PBS twice, and then incubated with 30% KOH for 30 min at room temperature. For the measurement of glycogen content in liver tissue, frozen liver tissues were homogenized in 30% KOH. Then, ethanol was added to the lysate, and glycogen was precipitated by centrifugation. The pellet was dissolved with H2O, and glycogen content was determined by anthrone reagent (2 mg anthrone/ml sulfuric acid). Glucose solution was used as a standard.
Oil Red O staining
For lipid droplet staining, the cells were fixed with 10% formalin and stained with Oil Red O working solution. Hematoxylin was used for counterstaining. For liver tissue, the frozen liver sections were cut at a thickness of 5 μm on a cryostat and stained with Oil Red O.
Measurement of triglyceride
After the treatment, the primary cultured hepatocytes were washed with PBS and scraped with methanol. For the measurement of triglyceride in liver tissue, frozen liver tissues were homogenized in PBS, and methanol was added to the lysate. The lipids were extracted by the method of Bligh and Dyer (25), and triglyceride content was measured using a triglyceride kit (L-type TG M with lipid calibrator; Wako, Osaka, Japan).
Western blot
For the preparation of total cell proteins, cells or frozen liver tissues were homogenized in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 8.8; 150 mM NaCl; 10 mM EGTA; 1% Triton-X; 0.1% SDS; 1% deoxycholic acid; 0.3 mM PMSF; 1 mM sodium orthovanadate; 10 mM sodium fluoride; 0.1 mM sodium molybdate; and 0.5 mM 4-deoxypyridoxine). The proteins were separated by SDS-PAGE and were electrophoretically transferred onto nitrocellulose membrane. The membranes were first incubated with the primary antibodies, anti-phosphorylated-AKT (Ser-473; 9271; Cell Signaling Technology, Danvers. MA, USA), AKT (9272; Cell Signaling), phosphorylated-GSK3β (Ser-9, 9336; Cell Signaling), GSK3β (610201; BD Biosciences, San Jose, CA, USA), phosphorylated-AMPKα (Thr-172, 2531; Cell Signaling), AMPKα (2603; Cell Signaling), GLUT2 (sc-9116; Santa Cruz Biotechnology, Santa Cruz, CA, USA), ASM (sc-11352; Santa Cruz Biotechnology), and β-actin (Sigma-Aldrich) antibodies. Then, the membrane was incubated with the horseradish peroxidase-coupled secondary antibodies (Santa Cruz Biotechnology). Detection was performed with an ECL system (Amersham Biosciences), and the protein bands were quantified by densitometry using the ImageJ program (U.S. National Institutes of Health; http://rsb.info.nih.gov/ij/).
Quantitative real-time RT-PCR
Extracted RNA from the cells was reverse-transcribed, and quantitative real-time PCR with probe-primer sets of GLUT2 and 18S ribosomal RNA (Applied Biosystems, Foster City, CA, USA) was performed using TaqMan analysis (ABI Prism 7000; Applied Biosystems). The changes were normalized based on 18S.
Mass spectrometric analysis of sphingolipids
Electrospray ionization tandem mass spectrometry analysis was performed on a Thermo Finnegan TSQ 7000 triple quadruple mass spectrometer (Thermo Finnegan, San Jose, CA, USA), operating in multiple reaction monitoring positive ionization mode, as reported previously (26).
S1P formation
Primary rat hepatocytes were incubated with [3H]serine (2 μCi/ml)-containing medium for 12 h. Then, the medium was changed to serum-free RPMI 1640 medium containing [3H]serine with subsequent incubation with adenoviruses for an additional 18 h. The cellular lipids were extracted by the method of Bligh and Dyer (25) and separated on TLC plates in the solvent system of 1-butanol/acetic acid/water (60:20:20, v/v). The radiolabeled S1P spot was scraped off from the plates, and the radioactivity was measured in a liquid scintillation counter.
Statistical analysis
The results shown are representative of ≥3 independent experiments. Data are expressed as means ± sd from ≥4 independent experiments. Data between groups were analyzed by Student's t test. Values of P < 0.05 were considered statistically significant.
RESULTS
Exogenous ASM improves glucose metabolism in mice
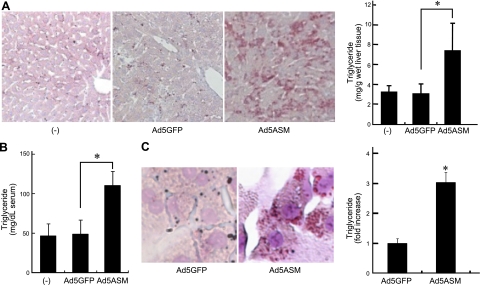
To explore the role of hepatic ASM on glucose and lipid metabolism, an adenovirus vector expressing human ASM, Ad5ASM, was used in this study. On administration of Ad5ASM into mice, ASM was preferentially expressed in the liver but not in muscle and adipose tissue (Supplemental Fig. S1B, C). As a result, ASM activity in the liver was increased (Supplemental Fig. S1D). Sufficient expression and activity of ASM in Ad5ASM-infected primary cultured hepatocytes were confirmed. This activity efficiently suppressed the level of sphingomyelins in hepatocytes (Supplemental Fig. S1E and Table 1). Initially, we investigated the effect of ASM in a glucose tolerance test and demonstrated that blood glucose levels were decreased in both wild-type (Fig. 1A) and diabetic db/db mice (Fig. 1B) in which ASM was overexpressed by Ad5ASM, indicating that ASM improves glucose tolerance. Then, we used primary cultured hepatocytes to investigate the effect of ASM on glucose uptake. Exogenous administration or adenoviral introduction of ASM increased glucose uptake in hepatocytes (Fig. 1C), suggesting that ASM improves glucose tolerance by increased hepatic glucose uptake. Next, we examined the role of ASM in glycogen synthesis. Exogenous ASM expression increased hepatic glycogen content in vivo (Fig. 1D) and in vitro (Fig. 1E) using primary cultured hepatocytes. We further examined the role of ASM in lipid metabolism. Exogenous ASM introduction increased hepatic lipid content and serum triglycerides (Fig. 2A, B) in vivo. We verified that ASM induces accumulation of lipid droplets and triglyceride in primary cultured hepatocytes (Fig. 2C). These results indicate that ASM contributes not only to glucose uptake but also to the synthesis of glycogen and triglyceride in the liver.
Table 1.
Changes in the sphingolipid profile in Ad5ASM-infected hepatocytes
| Species | Ad5GFP | Ad5ASM |
|---|---|---|
| C14-SphM | 31.5 ± 0.1 | 22.2 ± 1.8* |
| C16-SphM | 6626.5 ± 261.3 | 4376.0 ± 210.8* |
| C18-SphM | 2053.2 ± 65.0 | 1108.7 ± 53.4* |
| C18:1-SphM | 260.9 ± 5.8 | 168.7 ± 5.1* |
| C20-SphM | 1062.2 ± 20.0 | 564.4 ± 84.2* |
| C20:1-SphM | 130.0 ± 2.6 | 79.3 ± 5.5* |
| C22-SphM | 8397.5 ± 538.7 | 5223.5 ± 688.8* |
| C22:1-SphM | 1221.8 ± 60.2 | 696.0 ± 109.1* |
| C24-SphM | 24,630.7 ± 115.6 | 16,310.1 ± 2479.5* |
| C24:1-SphM | 17,194.4 ± 630.6 | 10,981.6 ± 1385.0* |
| C14-Cer | 5.8 ± 0.6 | 7.1 ± 1.5 |
| C16-Cer | 181.5 ± 7.5 | 185.3 ± 4.3 |
| C18-Cer | 39.3 ± 5.5 | 50.4 ± 1.3* |
| C-18:1-Cer | 8.4 ± 0.9 | 10.1 ± 0.1* |
| C20-Cer | 33.0 ± 5.1 | 42.8 ± 1.5 |
| C24-Cer | 919.8 ± 70.5 | 1140.7 ± 82.5* |
| C24:1-Cer | 547.0 ± 70.3 | 598.4 ± 3.8 |
| Sph | 140.3 ± 19.0 | 248.6 ± 14.5* |
Primary cultured rat hepatocytes were infected with Ad5GFP or Ad5ASM. Sphingomyelin and the different ceramide species were examined by mass spectrometric analysis. Results are expressed as picomoles lipid per milligram protein; they represent means ± sd from 3 independent experiments. SphM, sphingomyelin; Cer, ceramide; Sph, sphingosine.
P < 0.05.
Figure 1.
Ad5ASM improves glucose tolerance in mice. A, B) Wild-type (A) or diabetic db/db mice (B) were infected with Ad5GFP or Ad5ASM (5×108 PFU/mouse), then deprived of food for 18 h, and IPGTT was performed by administering a glucose load of 2 mg/g body weight 3 d after the infection. Values of area under the blood glucose curve were calculated (right panels). C) Primary cultured rat hepatocytes were treated with ASM (1 U/ml) for 2 h (left panel) or infected with Ad5GFP or Ad5ASM (10 PFU/cell; right panel), followed by measurement of 2-deoxy-d-[1-3H]glucose uptake after a 1-h incubation period. D) Hepatic glycogen content in wild-type mice was determined under food-deprivation conditions at 3 d after the adenoviral infection. E) Glycogen content was determined in rat hepatocytes at 8 h after ASM treatment (left panel) or normal (200 mg/dl) and high-dose glucose (600 mg/dl) (right panel) treatment with Ad5GFP or Ad5ASM infection. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
Figure 2.
ASM stimulates lipid accumulation. A) Wild-type mice were infected with Ad5GFP or Ad5ASM (5×108 PFU/mouse). Hepatic lipid content was assessed by Oil Red O staining (left panel; original view ×200) and triglyceride measurement (right panel) in food-deprived wild-type mice at 7 d after the adenovirus infection. B) Serum triglycerides were determined in food-deprived wild-type mice at 7 d after the infection. C) Primary cultured rat hepatocytes were infected with Ad5GFP or Ad5ASM (10 PFU/cell). Lipid droplets were assessed by Oil Red O staining (original view ×800). Triglyceride level in hepatocytes was determined (right panel). Data are means ± sd from ≥4 independent experiments. *P < 0.05.
ASM induces AKT activation
AKT is a key molecule in insulin signaling and glucose metabolism. We hypothesized that AKT is involved in ASM-mediated glucose, glycogen, and lipid metabolism. To prove this, we initially investigated whether AKT affects glucose and lipid metabolism in hepatocytes using adenoviral overexpression of DN- or CA-AKT (Ad5DN-AKT and Ad5CA-AKT, respectively). DN-AKT inhibited insulin-induced phosphorylation of AKT, thereby inhibiting glycogen deposition and lipid accumulation by insulin or high-dose glucose in primary cultured hepatocytes (data not shown). In contrast, a constitutively active form of AKT strongly phosphorylated GSK3β (Supplemental Fig. S2A), resulting in increased glycogen deposition and lipid accumulation (Supplemental Fig. S2B and data not shown). AKT is therefore crucial for glucose and lipid metabolism in hepatocytes. On the basis of the fundamental roles of AKT in glucose and lipid metabolism, we investigated the role of AKT in ASM-mediated glucose, glycogen, and lipid metabolism. First, we examined whether ASM alters AKT signaling. Food intake stimulates AKT and GSK3β phosphorylation in mouse liver (Fig. 3A). Introduction of exogenous ASM increased AKT activation under food-deprivation conditions and further increased under feeding conditions (Fig. 3A) without increasing serum insulin levels (data not shown) in comparison with control virus-infected animals. We further tested the requirement for insulin in ASM-induced AKT activation. In insulin-free conditions, introduction of ASM still increased AKT and GSK3β phosphorylation in primary cultured rat hepatocytes (Fig. 3B, left panel). High-dose glucose did not affect AKT and GSK3β phosphorylation in primary hepatocytes (Fig. 3B, left panel). These results suggest that ASM-induced AKT activation is not mediated by insulin or a response to increased intracellular glucose. In contrast, overexpression of DN-AKT abolished ASM-mediated AKT and GSK3β activity (Fig. 3B, right panel). DN-AKT overexpression also abolished ASM-induced glycogen deposition and lipid accumulation (Fig. 3C, D). These results suggest that ASM regulates glucose, glycogen, and lipid metabolism via activation of AKT.
Figure 3.
DN-AKT eliminates the effect of ASM introduction. A) Wild-type mice were infected with Ad5GFP or Ad5ASM (5×108 PFU/mouse), and the animals were killed in both nonfeeding (fasting; 18 h food deprivation) and feeding conditions at 3 d after the adenoviral infection. Protein extracts from liver tissue were subjected to immunoblot for phosphorylated (p)-AKT, AKT, p-GSK3β, and GSK3β. Relative densitometric intensity of p-AKT and p-GSK3β was determined for each protein band and normalized to AKT and GSK3β, respectively (bottom panels). B) Primary cultured rat hepatocytes infected with Ad5GFP or Ad5ASM (10 PFU/cell) were further infected with Ad5GFP or Ad5DN-AKT or treated with or without high-dose glucose (600 mg/dl; normal glucose, 200 mg/dl) for 8 h. Protein extracts from hepatocytes were subjected to immunoblot for p-AKT, AKT, p-GSK3β, and GSK3β. C) Primary cultured rat hepatocytes infected with Ad5GFP or Ad5ASM plus Ad5GFP or Ad5DN-AKT were treated with or without high-dose glucose. Glycogen content was determined in hepatocytes at 8 h after the high-dose glucose treatment. D) Primary cultured rat hepatocytes were infected with Ad5GFP or Ad5ASM plus Ad5GFP or Ad5DN-AKT. Lipid droplets were assessed by Oil Red O staining in hepatocytes (original view ×800). Results shown are representative of ≥3 independent experiments. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
ASM decreases AMPK phosphorylation and increases GLUT2 expression in hepatocytes
Because AMPK is an important component in glucose metabolism, the effects of ASM on AMPK signaling were examined. Glycogen deposition induced by high-dose glucose was increased by DN-AMPK and reduced by CA-AMPK (Supplemental Fig. S2C, D) with no observed effects on AKT and GSK3β phosphorylation (data not shown), indicating that AMPK activation reduced glycogen synthesis; this result is consistent with previous studies (3). Then, we assessed the role of AMPK in ASM-mediated effects. In the control virus-infected livers, food intake did not affect AMPK phosphorylation (Fig. 4A), consistent with a previous report (27). In contrast, introduction of ASM reduced AMPK phosphorylation in response to food intake (Fig. 4A). ASM also increased food intake-related GLUT2 protein expression in the liver (Fig. 4A). A previous study gave indirect evidence that high glycogen content represses AMPK activation in skeletal muscle (28). However, our data demonstrated that AMPK phosphorylation was reduced by ASM in primary cultured hepatocytes under normal glucose conditions (Fig. 4B), in which the glycogen content was lower than that in Ad5GFP-infected cells cultured in high-dose glucose medium (Fig. 1E). These results suggest that the AMPK inactivation by ASM is not mediated by a response to increased intracellular glycogen. In primary cultured hepatocytes, high-dose glucose slightly reduced AMPK phosphorylation (Fig. 4B), as shown in a previous report in HepG2 hepatoblastoma cells (29), suggesting that the refractoriness of AMPK by food intake was probably due to systemic mediators. In addition to AMPK, GLUT2 protein and mRNA levels were increased in ASM-expressing primary hepatocytes (Fig. 4B, C). CA-AMPK partially inhibited glycogen deposition and lipid accumulation induced by ASM (Fig. 4D, E). These results suggest that reduced AMPK activation is also crucial for ASM-mediated glucose metabolism.
Figure 4.
ASM decreases AMPK phosphorylation and increases GLUT2 expression. A, B) Wild-type mice were infected with Ad5GFP or Ad5ASM (5×108 PFU/mouse), and the animals were killed in both nonfeeding (fasting; 18 h food deprivation) and feeding conditions at 3 d after the adenoviral infection. Primary cultured rat hepatocytes infected with Ad5GFP or Ad5ASM (10 PFU/cell) were cultured in normal (200 mg/dl) or high-dose glucose (600 mg/dl) conditions for 8 h. Left panels: protein extracts from the liver tissue (A) or the hepatocytes (B) were subjected to immunoblot for phosphorylated (p)-AMPK, AMPK, GLUT2, and β-actin. Right panels: relative densitometric intensity of p-AMPK and GLUT2 was determined for each protein band and normalized to AMPK and β-actin, respectively. C). mRNA levels of GLUT2 in hepatocytes were determined by quantitative real-time RT-PCR and normalized by 18S ribosomal RNA. D) Primary cultured rat hepatocytes infected with Ad5GFP or Ad5CA-AMPK were further infected with Ad5GFP or Ad5ASM. Cells were then incubated in normal or high-dose glucose conditions for 8 h. Glycogen content in hepatocytes was determined. E) Primary cultured rat hepatocytes were infected with Ad5GFP, Ad5CA-AMPK, and/or Ad5ASM. Lipid droplets in hepatocytes were assessed by Oil Red O staining (original view ×800). Results shown are representative of ≥3 independent experiments. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
ASM activates AKT through the SphK/S1P pathway
ASM hydrolyzes sphingomyelin into ceramide, which is further hydrolyzed and phosphorylated by ceramidase and SphK to form S1P. Because S1P activates AKT in hepatocytes (20), we investigated the contribution of S1P generation to ASM-mediated glucose and lipid metabolism. The ASM-induced phosphorylation of AKT and GSK3β was reduced in SphK1−/− hepatocytes (Fig. 5A). Of note, S1P formation was inhibited in SphK1−/− cells (Fig. 5B, left panels). Overexpression of neutral ceramidase (NCD) or exogenous S1P activated AKT (Fig. 5B, middle and right panels) in hepatocytes, as we reported previously (20). These results suggest that S1P generated by the breakdown of sphingomyelin contributes to ASM-induced AKT activation. We then examined the involvement of S1PR. Hepatocytes were treated with PTX because S1PRs coupled with Gi are sensitive to PTX. ASM-mediated AKT activation was reduced by PTX treatment (Fig. 5C), indicating that ASM generates S1P, which further activates AKT, at least partially via S1PRs. ASM-induced glycogen deposition and lipid accumulation were also reduced in SphK1−/− hepatocytes (Fig. 6A, B), whereas the sphingomyelin and ceramide levels were similar to those in wild-type hepatocytes (data not shown). Moreover, SphK1−/− mice did not show hepatic steatosis by exogenous ASM introduction (Fig. 6C). In contrast to AKT, AMPK was not affected by S1P administration or NCD overexpression (Fig. 5B, middle panel). As in wild-type mice, SphK1−/− mice expressing exogenous ASM showed decreased phosphorylation of AMPK (Fig. 5A), indicating that the SphK/S1P pathway is not required for ASM-induced AMPK down-regulation.
Figure 5.
ASM-induced AKT phosphorylation is suppressed in SphK1−/− hepatocytes. A) Primary cultured mouse hepatocytes isolated from SphK1+/+ or SphK1−/− mice were infected with Ad5GFP or Ad5ASM (10 PFU/cell). Protein extracts from the hepatocytes were subjected to immunoblot for phosphorylated (p)-AKT, AKT, p-GSK3β, GSK3β, p-AMPK, AMPK, and β-actin. B) [3H]Serine-labeled hepatocytes from SphK1+/+ or SphK1−/− mice were infected with Ad5GFP or Ad5ASM. Radiolabeled S1P was measured (left panels). Primary cultured rat hepatocytes were treated with or without S1P (1 μM) for 8 h (middle top panel). Primary cultured rat hepatocytes were infected with Ad5GFP or adenovirus expressing human neutral ceramidase (Ad5NCD) (middle bottom panel). Protein extracts from the hepatocytes were subjected to immunoblot for p-AKT, p-AMPK, and β-actin. C) Primary cultured rat hepatocytes were infected with Ad5GFP or Ad5ASM (10 PFU/cell), and incubated for 2 h. Then, cells were treated with or without the indicated concentration of PTX for 24 h. Top panel: protein extracts from hepatocytes were subjected to immunoblot for p-AKT and AKT. Relative densitometric intensity of p-AKT and p-AMPK was determined for each protein band of the sample and normalized to AKT and AMPK, respectively (A, B, right panels; C, bottom panel). Results shown are representative of ≥3 independent experiments. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
Figure 6.
ASM-induced glycogen deposition and lipid accumulation are suppressed in SphK1−/− hepatocytes. A) Primary cultured hepatocytes isolated from SphK1+/+ or SphK1−/− mice were infected with Ad5GFP or Ad5ASM. Glycogen content in hepatocytes was determined. B) Lipid droplets in SphK1+/+or SphK1−/− hepatocytes infected with Ad5GFP or Ad5ASM were assessed by Oil Red O staining (original view ×800). C) SphK1+/+ or SphK1−/− mice were infected with Ad5GFP or Ad5ASM, followed by assessment for hepatic lipid content by Oil Red O staining (left panel; original view ×200) and triglyceride measurement (right panel) in food-deprivation conditions at 7 d after the infection (original view ×200). Results shown are representative of ≥3 independent experiments. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
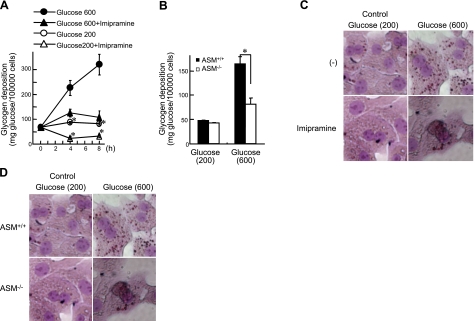
ASM deficiency inhibits glucose uptake, glycogen deposition, and lipid accumulation in hepatocytes
Finally, we tested whether ASM deficiency causes glucose intolerance. Sphingomyelin accumulation and ceramide reduction were observed in hepatocytes isolated from ASM−/− mice (Supplemental Table S1). Blood glucose levels in ASM−/− mice were higher than those in ASM+/+ mice throughout the IPGTT period without any changes in insulin levels (Fig. 7). To further investigate the specific role of ASM in hepatocytes, primary hepatocytes were isolated and treated with high-dose glucose (600 mg/dl), which increased glycogen levels (Fig. 8A). Imipramine, a tricyclic antidepressant, causes proteolysis of the active 72-kDa ASM form, thus inhibiting ASM activity (30). Pretreatment with imipramine inhibited high-dose glucose-induced glycogen deposition (Fig. 8A), as did the knockout of ASM in mouse hepatocytes (Fig. 8B). Moreover, high-dose glucose increased lipid droplets in primary hepatocytes, and imipramine or ASM deficiency suppressed this effect (Fig. 8C, D). These results suggest that high-dose glucose increases glucose uptake, resulting in glycogen deposition and lipid accumulation in hepatocytes. In contrast, ASM deficiency inhibits glucose uptake, glycogen deposition, and lipid accumulation, resulting in glucose intolerance. In ASM−/− mice, similar levels of GSK3β phosphorylation were induced by food intake, whereas GLUT2 induction was reduced in comparison to the livers of ASM+/+ mice (Fig. 9A). Reduction of AMPK phosphorylation and induction of GLUT2 by high-dose glucose were inhibited in ASM−/− hepatocytes (Fig. 9B), whereas high-dose glucose did not affect AKT phosphorylation (data not shown). These results suggest that glucose intolerance in ASM−/− mice was due, at least in part, to insufficient reduction of AMPK activity and induction of GLUT2.
Figure 7.
ASM−/− mice were glucose intolerant. A) ASM+/+ and ASM−/− mice were deprived of food for 18 h, and IPGTT was performed by administering a glucose load of 2 mg/g body weight. Serial glucose (left panel) and insulin (right panel) were measured at the indicated time points. Blood glucose values for area under the curve (AUC) were calculated (middle panel). Results shown are representative of ≥3 independent experiments. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
Figure 8.
Inhibition of ASM impairs glucose uptake and glycogen deposition in primary cultured hepatocytes. A) Primary cultured rat hepatocytes were treated with normal (200 mg/dl) or high-dose glucose (600 mg/dl) with or without imipramine for the indicated periods of time. B) Primary cultured mouse hepatocytes from ASM+/+ and ASM−/− mice were treated with normal or high-dose glucose for 8 h. Glycogen content in hepatocytes was measured. C) Primary cultured rat hepatocytes were treated with normal or high-dose glucose with or without imipramine for 24 h. D) Primary cultured hepatocytes from ASM+/+ or ASM−/− mice were treated with normal or high-dose glucose for 24 h. Lipid droplets were assessed by Oil Red O staining in hepatocytes (original view ×800). Results shown are representative of ≥3 independent experiments. Data are means ± sd from ≥4 independent experiments. *P < 0.05.
Figure 9.
Inhibition of ASM suppresses GLUT2 induction. A) ASM+/+ and ASM−/− mice were killed in both nonfeeding (fasting; 18 h food deprivation) and feeding conditions. Protein extracts from liver tissue were subjected to immunoblot for phosphorylated (p)-GSK3β, GSK3β, GLUT2, and β-actin (left panel). Relative densitometric intensity of p-GSK3β (middle panel) and GLUT2 (right panel) was determined for each protein band of the liver tissue sample and normalized to GSK3β and β-actin, respectively. B) Primary cultured hepatocytes from ASM+/+ and ASM−/− mice were treated with normal (200 mg/dl) or high-dose glucose (600 mg/dl) for 8 h. Protein extracts from hepatocytes were subjected to immunoblot for p-AMPK, AMPK, GLUT2, and β-actin. Results shown are representative of ≥3 independent experiments. *P < 0.05.
DISCUSSION
The present study addresses the contributions of ASM to glucose and lipid metabolism in the liver. The sphingomyelin/ceramide/S1P pathway is involved in glucose metabolism (6–9, 12–14, 21). Food intake reduced most sphingomyelin species in the mouse liver (Supplemental Table S2). Likewise, high-dose glucose reduced sphingomyelin species in primary cultured hepatocytes (Supplemental Table S2), suggesting that this pathway is related to glucose metabolism in hepatocytes. In our study using hepatocytes, ASM induced insulin-like effects such as glucose uptake, glycogen deposition, and lipid accumulation via S1P-mediated AKT activation. Ceramide attenuates insulin signaling, including AKT activation (14–16), thereby contributing to insulin resistance through multiple pathways (13, 15). In primary cultured hepatocytes, the effect of ASM introduction on ceramide level was smaller than that in sphingomyelin degradation, suggesting that the generated ceramide is further converted to S1P. Ceramide and S1P often exert opposing effects; thus, the balance between these two sphingolipids may be important for AKT activation. During ASM activation, the balance is inclined toward S1P, resulting in AKT activation. Indeed, ASM did not activate AKT in SphK1−/− hepatocytes, suggesting that ASM mediates insulin-like effects via S1P formation. S1P acts as a ligand for the S1PRs and also as an intracellular second messenger (31, 32). Among S1PRs, Gi-associated S1PR1 (33) is mainly expressed in the liver (34), and PTX pretreatment inhibited exogenous S1P-induced AKT phosphorylation in primary cultured hepatocytes (data not shown), suggesting that S1P may activate AKT through predominantly S1PR1. PTX treatment partially inhibits ASM-induced AKT activation, suggesting that the generated S1P activates AKT at least partially via S1PRs and may also activate AKT through receptor-independent mechanisms.
In addition to AKT, AMPK also contributes to glucose metabolism. AMPK is a serine/threonine protein kinase that senses the immediate availability of cellular energy. Activated AMPK switches on catabolic pathways and switches off protein, carbohydrate, and lipid biosynthesis (anabolic pathways; ref. 35). Activation of AMPK in the liver reduces glycogen synthesis and lipogenesis as well as expression of GLUT2 (3). Activation of AMPK by 5-amino-4-imidazolecarboxamide riboside diminishes GLUT2 expression in primary cultured hepatocytes (36). Indeed, DN-AMPK increased glycogen deposition and lipid accumulation in primary hepatocytes and HepG2 cells (data not shown and ref. 37). Thus, in addition to increased AKT activation, reduced AMPK activation may be associated with another anabolic effect of ASM. Ceramide decreases (38) but S1P increases (39) AMPK phosphorylation in endothelial cells. In our study, ASM-induced AMPK inhibition in hepatocytes was independent of S1P. Thus, the decrease in AMPK activity may be due to ceramide processed by ASM.
Ad5ASM-infected mice showed improved glucose tolerance. Because adenovirus preferentially infects hepatocytes, the decrease in blood glucose was due to a variation of hepatic glucose metabolism, although sphingolipids have roles in glucose metabolism in muscle (40) and adipocytes (6–8). The ASM-induced decrease in blood glucose may be attributed to increased uptake of hepatic glucose rather than decreased production, because introduction of ASM did not affect the activity of adenylate cyclase, the glucagon target enzyme, in primary cultured rat hepatocytes (data not shown). The effects of ASM on hepatocytes are comparable to those of insulin. However, the introduction of hepatic ASM improved hyperglycemia in db/db mice with type 2 diabetes without increasing insulin levels (data not shown), indicating that ASM may reduce blood glucose regardless of the presence of insulin resistance. Instead of decreasing blood glucose, ASM increased hepatic triglyceride content, suggesting that ASM stimulates lipogenesis, in which transported glucose is converted to triacylglycerol (41). The effects of exogenous ASM in the liver are similar to the effects of deletion of hepatic phosphatase and tension homolog on chromosome 10 (PTEN), which is a negative regulator of AKT. Deletion of PTEN in the liver results in hyperphosphorylation of hepatic AKT and improves glucose tolerance but induces fatty liver in mice (42).
ASM−/− mice are glucose intolerant. The abnormality is not due to inactivation of the AKT/GSK3β pathway but is due, in part, to insufficient reduction of AMPK activity and induction of GLUT2. In addition, crosstalk may occur between ASM and various signaling pathways, including those of glucose and lipid metabolism, because ASM regulates sphingolipids in lipid raft microdomains that function as platforms for signal transduction and protein sorting (18). For example, disruption of lipid raft microdomains or chronic ethanol exposure inhibits localization of TC10 to lipid rafts, which induces GLUT4 translocation and glucose intake without affecting phosphatidylinositol-3 kinase/AKT signaling in adipocytes (43, 44). Pretreatment with imipramine inhibited high-dose glucose-induced lipid accumulation in rat hepatocytes, as did the knockout of ASM in mouse hepatocytes. As reported previously, the ASM inhibitor desipramine attenuates triglyceride increase by high-dose palmitic acid treatment in HepG2 cells (9). Moreover, a high-fat, high-cholesterol diet does not induce hepatic triacylglyceride accumulation in ASM−/− mice under LDL receptor-deficient conditions (9). These observations further support the role of ASM as a regulator of triglyceride synthesis in addition to glucose uptake. Further studies will determine the precise mechanism underlying the anabolic effects of ASM and its activation and ceramide/S1P regulation (45). The hypothetical roles of ASM are schematically summarized in Fig. 10.
Figure 10.
Hypothetical signals induced by ASM. Acid sphingomyelinase regulates glucose and lipid metabolism through AKT activation and AMPK suppression in hepatocytes. AKT activation is induced through S1P formation. AMPK suppression increases GLUT2 expression.
In conclusion, overexpression of ASM in the liver improves glucose tolerance in both wild-type and diabetic db/db mice. ASM stimulates glucose uptake, glycogen deposition, and lipid accumulation in hepatocytes via AKT and GSK3β phosphorylation, which requires S1P formation. In addition, ASM decreases AMPK phosphorylation through ceramides, leading to GLUT2 up-regulation. Indeed, inactivation of ASM produces glucose intolerance in hepatocytes. Targeting ASM may become a new therapeutic strategy in type 2 diabetes.
Supplementary Material
Acknowledgments
The authors thank Dr. Jacek Bielawski (Medical University of South Carolina, Charleston, SC, USA) for sphingolipid measurement, Dr. Yoko Sugiyama (Gifu University Graduate School of Medicine, Gifu, Japan) for measurement of adenylate cyclase activity, and Dr. Kenneth Walsh (Boston University, Boston, MA, USA) for adenoviruses encoding CA-AMPK and DN-AMPK.
This work was supported by grants from Mitsubishi Pharma Research Foundation and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (19790478 and 21790657 to Y.O. and 21390179 to M.S.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. (1988) Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and β-pancreatic islet cells. Cell 55, 281–290 [DOI] [PubMed] [Google Scholar]
- 2. Rencurel F., Waeber G., Antoine B., Rocchiccioli F., Maulard P., Girard J., Leturque A. (1996) Requirement of glucose metabolism for regulation of glucose transporter type 2 (GLUT2) gene expression in liver. Biochem. J. 314(Pt. 3), 903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foretz M., Ancellin N., Andreelli F., Saintillan Y., Grondin P., Kahn A., Thorens B., Vaulont S., Viollet B. (2005) Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 4. Smith E. L., Schuchman E. H. (2008) The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 22, 3419–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorska M., Baranczuk E., Dobrzyn A. (2003) Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm. Metab. Res. 35, 506–507 [DOI] [PubMed] [Google Scholar]
- 6. Liu P., Leffler B. J., Weeks L. K., Chen G., Bouchard C. M., Strawbridge A. B., Elmendorf J. S. (2004) Sphingomyelinase activates GLUT4 translocation via a cholesterol-dependent mechanism. Am. J. Physiol. Cell Physiol. 286, C317–C329 [DOI] [PubMed] [Google Scholar]
- 7. David T. S., Ortiz P. A., Smith T. R., Turinsky J. (1998) Sphingomyelinase has an insulin-like effect on glucose transporter translocation in adipocytes. Am. J. Physiol. 274, R1446–1453 [DOI] [PubMed] [Google Scholar]
- 8. Al-Makdissy N., Younsi M., Pierre S., Ziegler O., Donner M. (2003) Sphingomyelin/cholesterol ratio: an important determinant of glucose transport mediated by GLUT-1 in 3T3–L1 preadipocytes. Cell. Signal. 15, 1019–1030 [DOI] [PubMed] [Google Scholar]
- 9. Deevska G. M., Rozenova K. A., Giltiay N. V., Chambers M. A., White J., Boyanovsky B. B., Wei J., Daugherty A., Smart E. J., Reid M. B., Merrill A. H., Jr., Nikolova-Karakashian M. (2009) Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem. 284, 8359–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenkins R. W., Canals D., Hannun Y. A. (2009) Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell. Signal. 21, 836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hannun Y. A. (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274, 1855–1859 [DOI] [PubMed] [Google Scholar]
- 12. Summers S. A., Nelson D. H. (2005) A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing's syndrome. Diabetes 54, 591–602 [DOI] [PubMed] [Google Scholar]
- 13. Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- 14. Holland W. L., Summers S. A. (2008) Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 29, 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wymann M. P., Schneiter R. (2008) Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 9, 162–176 [DOI] [PubMed] [Google Scholar]
- 16. Stratford S., DeWald D. B., Summers S. A. (2001) Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem. J. 354, 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liangpunsakul S., Sozio M. S., Shin E., Zhao Z., Xu Y., Ross R. A., Zeng Y., Crabb D. W. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G1004–G1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tani M., Ito M., Igarashi Y. (2007) Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 19, 229–237 [DOI] [PubMed] [Google Scholar]
- 19. Osawa Y., Banno Y., Nagaki M., Brenner D. A., Naiki T., Nozawa Y., Nakashima S., Moriwaki H. (2001) TNF-α-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J. Immunol. 167, 173–180 [DOI] [PubMed] [Google Scholar]
- 20. Osawa Y., Uchinami H., Bielawski J., Schwabe R. F., Hannun Y. A., Brenner D. A. (2005) Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-α. J. Biol. Chem. 280, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 21. Rapizzi E., Taddei M. L., Fiaschi T., Donati C., Bruni P., Chiarugi P. (2009) Sphingosine 1-phosphate increases glucose uptake through trans-activation of insulin receptor. Cell. Mol. Life Sci. 66, 3207–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osawa Y., Hannun Y. A., Proia R. L., Brenner D. A. (2005) Roles of AKT and sphingosine kinase in the antiapoptotic effects of bile duct ligation in mouse liver. Hepatology 42, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 23. Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 24. Adachi M., Brenner D. A. (2008) High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology 47, 677–685 [DOI] [PubMed] [Google Scholar]
- 25. Bligh E.G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 26. Pettus B. J., Bielawski J., Porcelli A. M., Reames D. L., Johnson K. R., Morrow J., Chalfant C. E., Obeid L. M., Hannun Y. A. (2003) The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-α. FASEB J. 17, 1411–1421 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez A. A., Kumar R., Mulligan J. D., Davis A. J., Weindruch R., Saupe K. W. (2004) Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am. J. Physiol. Endocrinol. Metab. 287, E1032–E1037 [DOI] [PubMed] [Google Scholar]
- 28. Wojtaszewski J. F., Jorgensen S. B., Hellsten Y., Hardie D. G., Richter E. A. (2002) Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51, 284–292 [DOI] [PubMed] [Google Scholar]
- 29. Zang M., Zuccollo A., Hou X., Nagata D., Walsh K., Herscovitz H., Brecher P., Ruderman N. B., Cohen R. A. (2004) AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J. Biol. Chem. 279, 47898–47905 [DOI] [PubMed] [Google Scholar]
- 30. Grassme H., Gulbins E., Brenner B., Ferlinz K., Sandhoff K., Harzer K., Lang F., Meyer T. F. (1997) Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91, 605–615 [DOI] [PubMed] [Google Scholar]
- 31. Pyne S., Pyne N. J. (2002) Sphingosine 1-phosphate signalling and termination at lipid phosphate receptors. Biochim. Biophys. Acta 1582, 121–131 [DOI] [PubMed] [Google Scholar]
- 32. Olivera A., Spiegel S. (1993) Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365, 557–560 [DOI] [PubMed] [Google Scholar]
- 33. Siehler S., Manning D. R. (2002) Pathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptors. Biochim. Biophys. Acta 1582, 94–99 [DOI] [PubMed] [Google Scholar]
- 34. Yang A. H., Ishii I., Chun J. (2002) In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta 1582, 197–203 [DOI] [PubMed] [Google Scholar]
- 35. Hardie D. G. (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 36. Leclerc I., Lenzner C., Gourdon L., Vaulont S., Kahn A., Viollet B. (2001) Hepatocyte nuclear factor-4α involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes 50, 1515–1521 [DOI] [PubMed] [Google Scholar]
- 37. Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., Cohen R. A., Zang M. (2008) SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283, 20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Y., Song P., Xu J., Zhang M., Zou M. H. (2007) Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J. Biol. Chem. 282, 9777–9788 [DOI] [PubMed] [Google Scholar]
- 39. Igarashi J., Shoji K., Hashimoto T., Moriue T., Yoneda K., Takamura T., Yamashita T., Kubota Y., Kosaka H. (2009) Transforming growth factor-β1 down-regulates caveolin-1 expression and enhances sphingosine 1-phosphate signaling in cultured vascular endothelial cells. Am. J. Physiol. Cell Physiol 297, C1263–C1274 [DOI] [PubMed] [Google Scholar]
- 40. Adams J. M., 2nd, Pratipanawatr T., Berria R., Wang E., DeFronzo R. A., Sullards M. C., Mandarino L. J. (2004) Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53, 25–31 [DOI] [PubMed] [Google Scholar]
- 41. Glimcher L. H., Lee A. H. (2009) From sugar to fat: How the transcription factor XBP1 regulates hepatic lipogenesis. Ann. N. Y. Acad. Sci. 1173(Suppl. 1), E2–E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stiles B., Wang Y., Stahl A., Bassilian S., Lee W. P., Kim Y.-J., Sherwin R., Devaskar S., Lesche R., Magnuson M. A., Wu H. (2004) Live-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc. Natl. Acad. Sci. U. S. A. 101, 2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watson R. T., Shigematsu S., Chiang S. H., Mora S., Kanzaki M., Macara I. G., Saltiel A. R., Pessin J. E. (2001) Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 154, 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sebastian B. M., Nagy L. E. (2005) Decreased insulin-dependent glucose transport by chronic ethanol feeding is associated with dysregulation of the Cbl/TC10 pathway in rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 289, E1077–E1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeidan Y. H., Hannun Y. A. (2010) The acid sphingomyelinase/ceramide pathway: biomedical significance and mechanisms of regulation. Curr. Mol. Med. 10, 454–466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.