Abstract
Shear stress is a ubiquitous environmental cue experienced by stem cells when they are being differentiated or expanded in perfusion cultures. However, its role in modulating self-renewing stem cell phenotypes is unclear, since shear is usually only studied in the context of cardiovascular differentiation. We used a multiplex microfluidic array, which overcomes the limitations of macroperfusion systems in shear application throughput and precision, to initiate a comprehensive, quantitative study of shear effects on self-renewing mouse embryonic stem cells (mESCs), where shear stresses varying by >1000 times (0.016–16 dyn/cm2) are applied simultaneously. When compared with static controls in the presence or absence of a saturated soluble environment (i.e., mESC-conditioned medium), we ascertained that flow-induced shear stress specifically up-regulates the epiblast marker Fgf5. Epiblast-state transition in mESCs involves heparan sulfate proteoglycans (HSPGs), which have also been shown to transduce shear stress in endothelial cells. By disrupting (with sulfation inhibitors and heparinase) and partially reconstituting (with heparin) HSPG function, we show that mESCs also mechanically sense shear stress via HSPGs to modulate Fgf5 expression. This study demonstrates that self-renewing mESCs possess the molecular machinery to sense shear stress and provides quantitative shear application benchmarks for future scalable stem cell culture systems.—Toh, Y.-C., Voldman, J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction.
Keywords: microfluid, HSPG, mESC
Conveying shear stress with perfusion culture is useful for mimicking in vivo flow environments (e.g., in cardiac and vascular tissues) to facilitate shear-dependent differentiation of stem cells (1, 2). At the same time, shear stress can be a by-product of stem cell processing, such as pluripotent stem cell expansion in bioreactors for subsequent clinical applications (3, 4). Despite the increasing use of perfusion culture in stem cell research, it is unclear how changes in the stem cell microenvironment caused by fluid flow, in particular the application of shear stress, affect pluripotent stem cell phenotypes (4). This is especially so when perfusion is applied in the context of self-renewal, since the majority of the studies investigating perfusion-mediated shear stress effects are conducted in the context of stem cell differentiation (1, 2, 5). Characterization of stem cells in a perfused self-renewing environment is mostly performed in bioreactors aimed at scaling up stem cell cultures (6–10). These studies have reported the maintenance or increase of pluripotency markers in mouse embryonic stem cells (mESCs) at shear stresses ranging from 0.47 to 10 dyn/cm2 (6–8). However, the exact relationship between shear stress and pluripotency markers remains vague. The only mESC phenotype that has been reported to be dependent on shear stress magnitudes is cell proliferation (6, 7). Given that there is increasing evidence that shear stress can activate stem cell signaling pathways during differentiation (11), it is unlikely that stem cells that are subjected to shear in self-renewing perfused cultures exhibit phenotypes equivalent to that of static cultures. One challenge, however, is that a number of shear transduction mechanisms have been identified in model systems (e.g., endothelial cells), which may or may not be active in self-renewing stem cells, so there is no clear basis to determine a priori the shear-sensing mechanisms that may exist in these self-renewing cells.
To systematically investigate the role of shear stress in self-renewing stem cells, we undertook an approach to first screen for shear-responsive phenotypes and then targeted the related potential molecular pathways for further mechanistic studies. Screening for shear-responsive phenotypes in self-renewing stem cells requires perfusion experiments for shear application to be able to decouple shear stress from other alterations in the microenvironment, such as soluble factor removal, caused by medium flow. Furthermore, one would ideally want to apply shear stress across a range of magnitudes to determine the efficacy of shear stress as a mediator of a particular phenotype. Tailoring to these needs, we employed microfluidic perfusion to precisely apply multiple shear stresses to stem cells in a self-renewing environment. Microfluidic perfusion surpasses macroscale perfusion in precision and throughput of shear application because of more defined flow characteristics (12, 13) and minute reagent consumption (14–16). Thus, stem cell phenotypes that respond specifically to shear stresses in fluid flow can be distinguished more readily from other flow-induced phenotypic alteration.
We designed a multiplex logarithmic microfluidic array to directly screen perfusion effects across a wide range of flow rates, corresponding to 1000 times variation in applied shear stress in a single experiment. When experiments across different flow rate ranges were combined, shear magnitudes spanning from 6.3 × 10−5 to 16 dyn/cm2 can be studied using this device. We assessed mESC phenotypes under these varying shear conditions and determined that perfusion resulted in decreased growth of the mESC colonies and expression of the pluripotency marker Nanog, with a concomitant up-regulation of the epiblast marker Fgf5. Further investigation by shearing the cells in a saturated soluble environment suggests that only Fgf5 is modulated by shear stress. By targeting signaling mechanisms involved in epiblast transition, we then identified that heparan sulfate proteoglycan (HSPG), a known shear-sensing element in endothelial cells (17) and modulator of Fgf signaling in stem cells (18), is also involved in shear stress sensing in mESCs. This is the first instance that we are aware of that demonstrates the modulation of self-renewing mESC phenotypes by shear stress via a specific molecular element, providing new insights into how shear affects mESCs in a self-renewing environment.
MATERIALS AND METHODS
ESC lines and culture
The mESC line with an Oct4-GFP reporter (ABJ1; kindly provided by George Daley, Harvard Medical School, Boston, MA, USA) was maintained in stem cell (SC) medium consisting of high glucose DMEM (10313039; Invitrogen, Carlsbad, CA, USA) supplemented with 15% FCS (16141079; Invitrogen), 4 mM L-glutamine (25030081; Invitrogen), 1 mM nonessential amino acid (11140050; Invitrogen), 50 U/ml penicillin, 50 μg/ml streptomycin (15140122; Invitrogen), 100 μM β-mercaptoethanol (M7522; Sigma, St. Louis, MO, USA), and 10 ng/ml leukemia inhibitory factor (LIF; ESG1107; Chemicon, Temecula, CA, USA). For routine maintenance, the cells were passaged every 2 d with fresh medium added daily.
Conditioned medium (CM) preparation
mESC-CM was prepared by plating mESCs at a density of 5 × 103 cells/cm2 on 140-mm culture dishes in 20 ml of SC medium. After 2 d of culture, the medium was collected and filtered through a 3-kDa filter unit (UFC900324; Millipore, Bedford, MA, USA) at 3200 g for 45 min to concentrate the high-molecular-mass secreted soluble factors. The concentrate was then diluted in 20 ml of fresh SC medium to replenish depleted nutrients.
Multiplex logarithmic microfluidic array design and fabrication
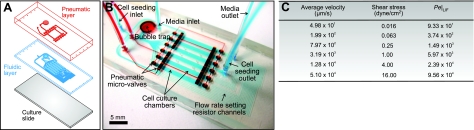
The 1 × 6 logarithmic microfluidic array is a 2-layered polydimethylsiloxane (PDMS; Slygard 184; Dow Corning, Midland, MI, USA) device, which consists of a fluidic layer that is controlled by microvalves actuated via a second pneumatic layer, similar to that reported by Blagović et al. (19) (Fig. 1A). The fluidic layer includes six 10-mm (length) × 1.25-mm (width) × 250-μm (height) cell culture chambers with 100-μm (width) × 100-μm (height) flow-setting resistor channels at the outlet of the chambers. The lengths of the flow-setting resistor channels in each culture chamber were designed based on fluidic resistance calculation such that the flow rates in each culture chamber vary by a factor of 4. The pneumatic layer consists of 250-μm pneumatic lines connecting 2 sets of 150-μm pneumatically actuated displacement chambers, which control fluid paths during cell seeding and medium perfusion. The fluidic and pneumatic layers are separated by a 250-μm PDMS membrane, which forms part of the fluidic layer slab.
Figure 1.
Multiplex logarithmic microfluidic array for multiple shear application. A) Schematic illustration of device assembly. Fluidic layer (in blue) is controlled by microvalves actuated by pneumatic lines (in red). Fluidic and pneumatic layers are plasma bonded and clamped onto a polystyrene culture slide. B) Micrograph of an assembled device, showing the 6 cell culture chambers. Flow-setting resistor channels at chamber outlets control the flow rate, which determines the shear stress. C) Theoretical calculations of the average velocity, wall shear stress, and Pe for LIF.
Both the fluidic and pneumatic layers were designed using AutoCAD (AutoDesk, San Rafael, CA, USA) and translated into plastic molds via stereolithographic rapid prototyping (Prototherm 12120; Fineline Prototyping, Raleigh, NC, USA). PDMS replicas were obtained by pouring degassed PDMS mixture (10:1 ratio of prepolymer to curing agent) onto the plastic molds and allowing it to cure at 60°C overnight. To obtain a precise thickness of 250 μm for the fluidic layer, the mold was clamped between 2 aluminum plates during PDMS curing. Connections were made in the pneumatic layer with a 1.5-mm-diameter corer (Harris Uni-core; Ted Pella Inc., Redding, CA, USA) before plasma bonding to the fluidic layer. Fluidic connections were then obtained by coring through the entire device.
Perfusion culture of mESCs
The microfluidic array device and auxiliary fluidic connections (UpChurch Scientific, Oak Harbor, WA, USA) were autoclaved, assembled, and coated with 0.1% gelatin (ES-006-B; Millipore) as described previously (19). Then, 1 × 106 cells/ml of mESCs were suspended in SC medium (used for routine maintenance) + 60 mM HEPES buffer (15630080; Invitrogen), seeded into the cell culture chambers, and incubated at 37°C overnight to allow for cell attachment. Perfusion culture was initiated by circulating 25 ml of SC medium or mESC-CM with a peristaltic pump (RP-1; Ranin Instrument, Oakland, CA, USA) over 72 h to ensure that the medium sink was sufficiently large compared with the device volume (∼20 μl). The flow rates were set to achieve the desired range of shear stresses, estimated from the wall shear stress between 2 parallel plates: τ = 6μQ/wh2, where τ is the wall shear stress, μ is the medium viscosity, Q is the flow rate, h is the chamber height, and w is the chamber width. Open dish cultures were used as static controls, where mESCs were seeded at an areal density equivalent to that of perfusion culture (i.e., 0.85×104 cells/cm2) on a gelatin-coated polystyrene slide (260225; Ted Pella Inc.) used in perfusion culture. A silicone gasket (C24727; Molecular Probes, Eugene, OR, USA) was used to create a medium reservoir where 0.8 ml of fresh SC medium was added daily.
Quantification of mESC colony growth
The growth of the mESC colonies was estimated by quantifying the fold increase in cell attachment area at a particular time point over the initial cell attachment area after seeding. Phase images across the entire cell culture chamber of the microfluidic array were acquired using a ×10 objective on an inverted microscope (Zeiss Axiovert 200; Carl Zeiss, Thornwood, NY, USA) with an automated stage (Ludl MAC 5000; Ludl Electronic Products, Inc., Hawthorne, NY, USA). Matlab (Mathworks, Natick, MA, USA) was then used to perform image processing (extract bright morphological features, adjust contrast, thresholding, convert to binary image, and fill holes) to calculate the cell attachment area in each image. The average cell attachment area was obtained by averaging over the entire field of the cell culture chamber. Quantification of colony growth in static cultures was performed by acquiring images from a field area equivalent to that of perfusion culture and processed with similar algorithms.
Quantitative real-time PCR (qRT-PCR)
To harvest cells from the microfluidic array, the device was disassembled in sterile PBS to retrieve the polystyrene slide where cells were attached. A PDMS device consisting of 6 chambers, each with a footprint equal to that of a cell culture chamber in the microfluidic array, was aligned and vacuum-sealed onto the polystyrene slide. This setup fluidically isolated the cell culture chambers such that 50 μl of cell lysate could be collected individually from each chamber. Fresh cell lysis buffer was added to cell lysate from each cell culture to a final volume of 350 μl for subsequent processing. Cells from static controls were harvested by adding 0.7 ml of cell lysis buffer. Total RNA was isolated using RNeasy Plus Microkit (74034; Qiagen, Valencia, CA, USA). cDNA was synthesized with DyNAmo cDNA synthesis kit (F-470; Finnzymes, Espoo, Finland) according to manufacturer's instructions. qRT-PCR reactions were set up using DyNAmo SYBR Green qPCR kits (F-400; Finnzymes) and performed on an MJ Opticon 2 real-time PCR machine (MJ Research, Waltham, MA, USA). Quantification of transcript amounts was based on a standard curve established with cDNA converted from Stratagene qPCR mouse reference total RNA (750600; Agilent Technologies, Germantown, MD, USA). The transcript level of each gene was normalized to Gapdh level for a given sample. To compare gene expression between perfused and static cultures, Gapdh-normalized transcript levels in perfusion samples were normalized to that of static samples. The primers used are listed in Table 1.
Table 1.
qRT-PCR primer sequences
| Gene | Primers |
|---|---|
| Nanog | CTGCTCCGCTCCATAACTTC |
| TTTCCCTAGTGGCTTCCAAA | |
| Rex1 | CCCCCTGGAAGTGAGTCATA |
| CCACTTGTCTTTGCCGTTTT | |
| Oct4 | CACGAGTGGAAAGCAACTCA |
| TTCATGTCCTGGGACTCCTC | |
| Fgf5 | GAAAAGACAGGCCGAGAGTG |
| GAAGTGGGTGGAGACGTGTT | |
| Gata4 | TCTCACTATGGGCACAGCAG |
| GGGACAGCTTCAGAGCAGAC | |
| Sox1 | CCTGAAAATGATGCTGCTGA |
| GGAGTAGCTGTGGGTGTGGT | |
| Brachyury (T) | CAGCCCACCTACTGGCTCTA |
| GAGCCTGGGGTGATGGTA | |
| Gapdh | CACTGAGCATCTCCCTCACA |
| GTGGGTGCAGCGAACTTTAT |
Disruption of HSPG function
HSPG function in mESCs was disrupted by either inhibiting ATP-sulfurylase activity or digesting heparan sulfate chains from the core glycoproteins. To inhibit ATP-sulfurylase activity, 20 mM of sodium chlorate (NaClO3; 403016; Sigma) was added in SC medium (for static control) or mESC-CM (for perfusion cultures). Digestion of heparan sulfate chains from the glycoproteins was achieved by treating cells with 15 mU/ml of heparinase III (H8891; Sigma) in SC medium or mESC-CM for static and perfusion cultures, respectively. Since heparinase III activity has a half-life of <24 h (20), fresh heparinase III was added to the medium reservoir of the perfusion system every 24 h to a final concentration of 15 mU/ml. In static controls, a fresh working solution of heparinase III was changed every 24 h. To rescue the ligand-receptor binding function of HSPG, we added 1 μg/ml heparin (H3149; Sigma) to 20 mM NaClO3.
Statistical analysis
Data for each experimental condition were the average of ≥3 independent experiments. Student's t test was performed to determine the statistical significance of pairwise comparisons between perfusion and static cultures. Linear regression between phenotypic responses and shear stress magnitudes was performed (OriginPro; OriginLab, Northampton, MA, USA) to determine correlation. The significance of the correlation of determination (R2) was determined by f test (ANOVA), and t test was performed to determine whether the regression slope was significantly different from 0.
RESULTS
Perfusion alters mESC phenotypes in self-renewing conditions
Our approach to comprehensively study shear stress effects on self-renewing mESCs was to employ a multiplex logarithmic microfluidic array to conduct parallel experiments at multiple shear magnitudes, which were controlled with flow rates. The microfluidic device consisted of 6 parallel cell culture chambers, where the flow rates in each chamber varied by a factor of 4, and were tuned by specifying the geometry (i.e., length) of the flow-setting resistor channel at the culture chamber outlet (Fig. 1B and ref. 21). In this study, the flow rates were specified to correspond to an applied wall shear stress range of 0.016–16 dyn/cm2, which covers the typical shear stress magnitudes experienced by perfused stem cell cultures (6–8). We designed the chamber geometries such that the Peclet number (Pe) of a typical secreted cytokine (LIF) for all culture chambers were ≫1, which is indicative of a convection-dominated mass transport regime (12). Thus, variation in the soluble environment across the culture chambers is minimized from a device operation perspective. Figure 1C lists the theoretically calculated average flow velocity, wall shear stress, and Pe for LIF.
mESCs were simultaneously perfused at different flow rates for 72 h and assessed for colony growth (i.e., change in colony mass) and transcriptional expression of self-renewal and early differentiation markers (Fig. 2). Here, we note that flow-induced change is an obligatory but not a sole requirement to conclude that a phenotype is modulated by shear stress. Hence, the objective here is to identify possible phenotypic candidates that may be shear dependent for further investigation. We used 2 data analysis methods to ascertain whether any given phenotype exhibits flow-dependent behavior. First, we performed pairwise comparison of gene expression between individual flow magnitudes and the static control. A linear regression of a given phenotypic response across different flow magnitudes was also performed to determine the correlation strength.
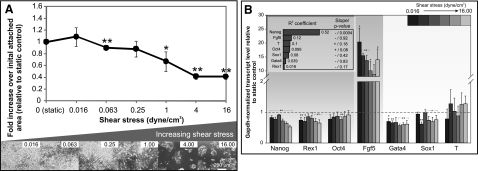
Figure 2.
Perfusion culture of mESCs at varying shear stresses. A) mESC colony growth after 72 h. Top: fold increase in cell area over initial cell attachment area relative to static culture. Bottom: phase images of mESCs at different shear stresses (indicated at top right). B) Gene expression of mESCs after 72 h perfusion. Inset: R2 and whether regression slope differs significantly from 0. Data are averages ± se of 3 independent experiments. *P < 0.1, **P < 0.05 vs. static cultures.
There was a significant decrease in the growth of the mESC colonies (indicated by cell attachment area) at flow rates corresponding to shear stress > 1 dyn/cm2 (P<0.1; Fig. 2A). This decrease in attachment area was observed as early as 24 h after the commencement of perfusion (Supplemental Fig. S1A). The decreased growth could be due to decreased proliferation, increased cell death, or physical removal of cells from the substrate, although at all shears, absolute cell area did increase over time (Supplemental Fig. S1), suggesting that wholesale physical shearing of cells from the substrate is not a primary cause. The negative correlation between mESC attachment area and flow magnitudes was significant (R2=0.64; P=0.004), suggesting that flow, and possibly shear stress, attenuate mESC colony growth in a graded manner.
We also examined the expression of a panel of markers representative of different developmental stages (22). These markers included the pluripotency markers Nanog, Rex1, and Oct4 (23, 24); the epiblast marker Fgf5 (22, 24); as well as early indicators of the respective germ layers endoderm (Gata4), ectoderm (Sox1), and mesoderm [Brachyury (T)] (23, 24). We observed an increase in Fgf5 expression across all perfused conditions, which was on average ∼10-fold higher than static controls (Fig. 2B). While up-regulation of Fgf5 expression was consistently detected at all the flow rates tested, the magnitude of this increase varied considerably (i.e., sd for independent experimental repeats at different flow rates ranged from 0.9 to 7). This may explain why Fgf5 expressions at certain flow rates were only significantly different from static controls at P < 0.1. Nevertheless, the high Fgf5 expression corroborated with the expression levels of other markers to indicate that the perfused mESCs, although still pluripotent, were primed for differentiation: differentiation markers (Gata4, Sox1, and T) were not significantly up-regulated, Oct4 was maintained, but Nanog and Rex1 were significantly down-regulated (Fig. 2B) (25, 26). When we assessed how the expression of the markers correlated to shear magnitudes, we observed that only Nanog had a significant negative correlation to increasing shear stress magnitudes (R2=0.52; P=0.00065; Fig. 2B, inset). These results suggest colony growth, Nanog, and Fgf5 as putative shear-responsive candidates, since their alterations by flow were the most pronounced out of the phenotypes assessed in this study.
Perfusion phenotypes are caused by soluble factor removal and shear stress application
Soluble factors secreted by cells can be freely diffusible or bound to extracellular matrices (ECMs). For instance, soluble factors, such as FGFs and WNTs, have been reported to bind to stem cell ECMs (27) and may not be effectively removed even at flow rates predicted to be in convective regimes. Hence, a simple theoretical estimation of Pe does not fully characterize the mass transport regime and is insufficient to ensure a uniform soluble environment at different flow rates. To understand whether the perfusion-mediated phenotypes observed in Fig. 2 were indeed modulated by shear, there is a need to experimentally decouple the 2 perfusion-mediated environmental effects, namely soluble factor removal and shear stress application. We perfused the mESCs with mESC-CM to saturate the soluble environment, allowing us to isolate shear stress as the only perfusion-mediated environmental cue that varies with flow rate.
CM perfusion was able to rescue the decrease in mESC colony growth previously observed at flow rates corresponding to shear stress > 1 dyn/cm2. There was no significant difference in the extent of mESC colony growth between perfused and static cultures (Fig. 3A) and little correlation between colony growth and flow magnitudes (R2=0.11; P=0.65). Similarly, Nanog expression was restored to a level seen in static control in the presence of CM. (Fig. 3B). The negative correlation of Nanog with increasing flow magnitudes observed in unsupplemented perfusion was also abolished (Fig. 3B, inset). The expression profiles of Rex1, Oct4, Gata4, Sox1, and T in CM perfusion were similar to that of unsupplemented perfusion (Fig. 3B). These markers also exhibited little correlation to shear stress magnitudes (Fig. 3B, inset), which is consistent with our initial assessment that they were independent of perfusion-mediated effects. Supplementing soluble factors with CM, however, did not completely attenuate the up-regulation of Fgf5 in all perfused conditions; Fgf5 expression in CM-perfused mESCs was maintained at 7- to 10-fold increase over static control (Fig. 3B) while displaying a weak correlation with shear stress magnitudes (Fig. 3B, inset). We ascertained that this up-regulation in Fgf5 in CM perfusion was not due to soluble factors present in CM since Fgf5 expression level in mESCs cultured statically in CM was not significantly different from SC medium (Supplemental Fig. S2).
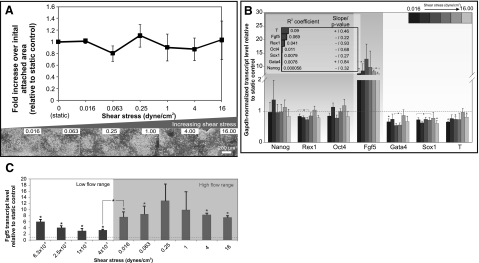
Figure 3.
Perfusion culture of mESCs in mESC-CM at varying shear stresses. A) mESC colony growth after 72 h. Top: fold increase in cell area over initial cell attachment area relative to static culture. Bottom: phase images of mESCs at different shear stresses (indicated at top right). B) Gene expression of mESCs after 72 h perfusion. Inset: R2 and whether regression slope differs significantly from 0. C) Fgf5 expression of mESCs perfused in CM over a lower range of shear stresses. Data are averages ± se of 3 independent experiments. *P < 0.05 vs. static cultures or between selected flow rates, as indicated.
These results suggest that the perfusion-mediated decreases in mESC colony growth and Nanog expression were due to the removal of soluble factors, since replenishing these factors with CM restored colony growth and Nanog to a level comparable to static culture. The graded response of mESC colony growth and Nanog expression to soluble factor removal implies that in addition to shear stress, the soluble environment also varied with perfusion flow rates. Thus, all subsequent perfusion experiments were conducted with CM to isolate shear stress effects. Of the 3 mESC phenotypic markers selected as shear stress-dependent candidates, only Fgf5 was not completely restored to the static control baseline with CM perfusion. Since Fgf5 expression remained high in CM perfusion but exhibited no correlation to increasing shear magnitudes, we wanted to investigate whether this was a saturated response to shear application. We assessed Fgf5 expression in CM over a lower range of flow rates, corresponding to shear magnitudes ranging from 6.3 × 10−5 to 4 × 10−3 dyn/cm2 (Fig. 3C). We observed a smaller extent of up-regulation (∼4× vs. ∼7–10× at higher shears) except at the lowest flow rate investigated (corresponding to 6.3×10−5 dyn/cm2 shear). This was the minimum flow rate that mESCs could be cultured in the device, possibly due to mass transport limitations. Since the increase in gene expression at the minimum flow rate was also observed for markers other than Fgf5 (data not shown), it was unlikely that nutritional limitation directly resulted in an epiblast-like state (i.e., increased Fgf5). Taken together, these results suggest either that Fgf5 expression is extremely sensitive to shear stress or that culture of mESCs in a closed perfused microenvironment itself increases Fgf5 expression. Importantly, the substantial increase (P=0.04) of Fgf5 expression observed at shear magnitudes > 0.016 dyn/cm2 over the baseline up-regulation (at low shears) suggests that mESCs are shear responsive. Therefore, the application of shear stress in perfusion culture may mediate the transition of mESCs into an epiblast-like state as indicated by Fgf5 expression.
HSPGs mediate Fgf5 up-regulation in perfusion
The up-regulation of Fgf5 is associated with an epiblast-like state, where cells retain their pluripotency potential but are biased toward lineage commitment instead of self-renewal (28). This state is transient during development in vivo, but cells have been isolated from postimplantation blastocysts that resemble epiblast and can be stably propagated in culture as epiblast stem cells (EpiSCs) (22, 29). The in vitro initiation of differentiation in mESCs via an epiblast-like state has been found to be dependent on the Fgf-signaling cascade (29). Besides Fgf ligands such as FGF4, HSPGs, which form a major component of the mESC ECM, are also involved in the transition of mESCs into epiblast-like cells (18). Our results demonstrate that shear stress is another environmental factor that can also trigger mESC differentiation into epiblast-like cells. It follows that shear stress experienced by the mESCs must be mechanically transduced by cell surface components to activate intracellular signaling pathways (30). Thus, we attempted to identify the cell surface component on mESCs that is involved in shear stress sensing. Studies (17, 31) with endothelial cells proposed that HSPGs are involved in shear mechanosensing. Because HSPGs are also abundant in the stem cell ECM and they are implicated in epiblast transition (18), we hypothesized that HSPGs are involved in shear stress sensing in this case, causing the up-regulation of Fgf5 in perfused mESCs.
We first examined whether disrupting HSPG function will abrogate the up-regulation of Fgf5 in perfused mESCs. HSPG function can be disrupted by interfering with the normal sulfation of the heparan sulfate side chains via NaClO3 treatment (Fig. 4A). NaClO3 has been shown to reversibly inhibit ATP-sulfurylase activity without detrimental effects on cell viability (18, 32). We perfused mESCs in the presence of 20 mM NaClO3 and did not observe changes in mESC viability and colony growth across the tested flow rates (Supplemental Fig. S3A). When we compared perfused, NaClO3-treated mESCs with untreated static controls, we observed that the extent of Fgf5 up-regulation was attenuated (Supplemental Fig. S3B). Since the addition of NaClO3 in static mESC cultures caused a more compact colony morphology and an increase in the expression of Nanog and Oct4 (Supplemental Fig. S3C, D), consistent with observations by Lanner et al. (18), we wanted to control for the possibility that the decrease in Fgf5 was due to the non-shear-specific effects of NaClO3. Thus, we compared gene expression of both perfused and static mESC in the presence of NaClO3 to isolate flow-specific effects. By blocking normal sulfation of HSPGs with NaClO3, we observed that the Fgf5 up-regulation previously seen in perfusion was attenuated (Fig. 4B). At shear stresses of up to 0.25 dyn/cm2, there were no significant differences in Fgf5 expression between static and perfusion cultures. We only observed a >2-fold increase in Fgf5 expression at shear stresses of >4 dyn/cm2. While the overall extent of perfusion-mediated Fgf5 up-regulation was attenuated in the presence of NaClO3, there was now a significant positive correlation (R2=0.69; P=0.002) between Fgf5 expression and shear stress magnitudes (Fig. 4B, inset). Nanog, Rex1, and Oct4 expressions in perfused mESCs were ∼2- to 4-fold higher than static culture, possibly due to the reversion of the epiblast-like population (Ffg5high population) back to a more self-renewing state (Fig. 4B).
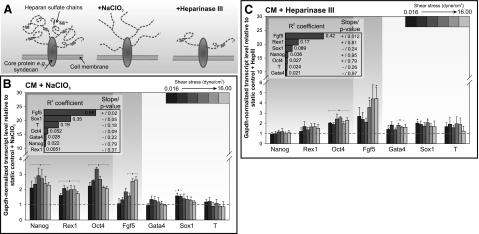
Figure 4.
Disruption of HSPGs in mESCs. A) Two schemes were used for disrupting HSPG function: NaClO3 blocks sulfation of the heparan sulfate chains, while heparinase III cleaves heparan sulfate chains from the core glycoproteins. B, C) Gene expression of mESCs after 72 h perfusion at varying shear stresses with mESC-CM + 20 mM NaClO3 (B) or mESC-CM + 15 mU/ml heparinase III (C). Inset: R2 and whether regression slope differs significantly from 0. Data are averages ± se of 3 independent experiments. *P < 0.05 vs. static cultures,.
To knock down HSPG function more specifically, we treated mESCs with heparinase III, which cleaves heparan sulfate chains from the core glycoproteins (Fig. 4A and ref. 17). Indeed, treatment with 15 mU/ml of heparinase III was able to reduce the quantity of heparan sulfate chains without affecting growth of the mESC colonies (Supplemental Fig. S4A, B). Owning to a more specific mode of HSPG functional interference as compared with NaClO3, heparinase III treatment did not significantly change mESC gene expression (Supplemental Fig. S4C). The extent of Fgf5 up-regulation in perfused heparinase III-treated cells over untreated (Supplemental Fig. S4D) or similarly heparinase III-treated (Fig. 4C) static controls was attenuated when compared with perfused, untreated cells (Fig. 3B), illustrating that the mESC response to heparinase III was shear dependent. As with NaClO3-treated mESCs, this attenuation in Fgf5 up-regulation was accompanied by a significant positive correlation with shear stress magnitudes (R2=0.42; P=0.07; Fig. 4C, inset). There were no significant changes in Nanog and Rex1 expression, although we observed a small but significant increase in Oct4 expression for all flow rates when compared with static controls (Fig. 4C). Our results demonstrate that HSPGs are involved in mediating the up-regulation of Fgf5 in perfused mESCs, since 2 modes of blocking normal HSPG function (i.e., preventing normal sulfation or cleaving of heparan sulfate chains) suppressed perfusion-mediated Fgf5 up-regulation.
HSPGs are involved in mechanosensing in mESCs
To further test whether HSPGs modulate Fgf5 expression by mechanically sensing shear, we needed to distinguish between the dual roles that HSPGs serve. In addition to being a mechanotransducer, HSPGs act as a binding partner for FGF ligands and their receptors (27). Thus, the attenuation of Fgf5 up-regulation on HSPGs inhibition can be due to their inability to sense shear stress or the inability for FGF ligands to bind effectively to their receptors. A number of studies (18, 33) have previously shown that the ligand-receptor binding function, but not the shear-sensing function, of disrupted HSPGs can be rescued by exogenous heparin. Thus, we added heparin to NaClO3-treated mESCs to distinguish whether perfusion-mediated Fgf5 up-regulation was due to mechanosensing or ligand-receptor binding function of HSPGs. We determined that heparin concentrations of ≥100 ng/ml were able to restore Fgf5 expression in NaClO3-treated mESCs to that of untreated cells since expression levels at higher concentrations were not significantly different (ANOVA; P=0.2; Supplemental Fig. S5A). For subsequent experiments, we added 1 μg/ml heparin to NaClO3-treated mESCs in static culture and observed that colony morphology (Fig. 5A), as well as most gene expressions (Supplemental Fig. S5B), was reverted to that of untreated cells. These results ascertained that exogenous heparin can replace the ligand-receptor binding function of HSPGs disrupted by NaClO3.
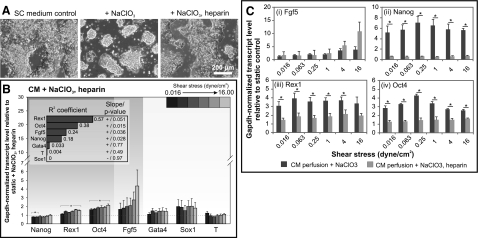
Figure 5.
Exogenous heparin rescues ligand-receptor binding function of HSPGs disrupted by NaClO3 treatment. A) Phase images illustrating that 1 μg/ml heparin was able to revert colony morphology of mESCs treated with NaClO3 for 72 h to that of control cells cultured in SC medium. B) Gene expression of mESCs after 72 h perfusion at varying shear stresses with mESC-CM + 20 mM NaClO3 and 1 μg/ml heparin. Inset: R2 and whether regression slope differs significantly from 0. C) Comparison between expression of Fgf5 (i), Nanog (ii), Rex1 (iii), and Oct4 (iv) in mESC-CM perfusion cultures with NaClO3 alone or NaClO3 + heparin. Data are averages ± se of 3 independent experiments. *P < 0.05.
When we perfused mESCs with NaClO3 and heparin, we observed that Fgf5 expression was similar to static culture similarly treated with NaClO3 and heparin at shear stresses < 4 dyn/cm2 (Fig. 5B). At the highest shear stress tested (i.e., 16 dyn/cm2), Fgf5 expression in perfused culture was 5-fold higher than static culture, which was still lower than the ∼10-fold increase observed in CM perfusion alone (Fig. 3B). This implies that Fgf5 remained relatively insensitive to perfusion-mediated effects even when heparin was added to restore the ligand-receptor binding function of HSPGs. To more specifically determine whether exogenous heparin can rescue gene expression changes as a result of attenuated HSPG function induced by NaClO3, we compared gene expression in NaClO3-treated perfusion cultures with and without heparin. There was no significant change in Fgf5 expression (Fig. 5Ci). In contrast, the addition of heparin was able to suppress the increase in pluripotency markers expression induced by NaClO3. Nanog expression was completely reverted to the baseline level (Fig. 5Cii) observed in CM perfusion alone (Fig. 3B). Rex1 and Oct4 expression was also significantly reduced with the addition of heparin (Fig. 5Ciii, iv), although still slightly elevated when compared with the levels expressed in CM perfusion. These results confirmed that the attenuation of Fgf5 up-regulation on HSPG inhibition was due to the inability to mechanically sense shear stress. On the other hand, the up-regulation of Nanog, Rex1 and Oct4 on HSPG inhibition by NaClO3 was due to disruption in FGF ligand-receptor binding, which is consistent with our previous findings (Fig. 3) that pluripotency markers were primarily mediated by soluble factors (in this instance, bound to HSPGs).
DISCUSSION
Self-renewing stem cells are typically subjected to shear stresses when cultured in bioreactors designed for stem cell expansion or in vivo in their niche. Since the in vivo equivalent of mESCs (i.e., the inner cell mass) does not experience shear, there is no in vivo equivalent state to guide the choice of relevant shear stresses. Thus, we designed our multiplex microfluidic array to apply shear stresses spanning those reported in mESCs bioreactor culture systems, which ranged from ∼0.5 (8) to 10 dyn/cm2 (6, 7, 34), in our initial screen for shear-responsive stem cell phenotypes. In the bioreactor studies, mESCs cultured either on microcarriers or as aggregates were all reported to maintain their pluripotency markers, such as Oct4, SSEA-1, Nanog, and Rex 1, at similar or higher levels than static suspension cultures at varying shear magnitudes (6–8, 34). The growth and proliferation of mESCs in these bioreactors, however, exhibited a negative relationship with higher shear stresses, where at shears > 4.5 dyn/cm2, mESC growth and proliferation were lower than in static suspension cultures (6, 7). When cultured at a lower shear stress of 0.5 dyn/cm2, mESCs grew at a similar rate as in static cultures (8). Our mESC colony growth data from the microfluidic array were in concordance with the bioreactor studies (Fig. 2A). However, we observed that mESCs subjected to microfluidic perfusion adopted an epiblast-like state, where cells were pluripotent (maintenance of Oct4; no increase of Gata4, Sox1, and T) but were biased toward differentiation (decreased Nanog, Rex1; increased Fgf5; Fig. 2B). Although the sheared mESCs exhibited some features of epiblast-like cells, it is unclear whether these cells were in a similar state as established epiblast-like pluripotent stem cells, such as EpiSCs and early primitive ectoderm-like (EPL) cells (35). Fgf5 expression was high in both the sheared mESCs and established epiblast-like pluripotent stem cell lines, whereas Rex1 expression was merely down-regulated in the sheared mESCs instead of being absent (28, 35). To fully assess the state of the sheared mESCs so as to determine whether any shear-induced changes are desirable or not for downstream applications will necessitate both molecular and functional characterizations that include genetic and epigenetic markers as well as in vivo differentiation potential (35).
We further identified the respective flow-induced environmental cues that were responsible for the altered mESC phenotypes in self-renewing conditions. We determined that the decreases in mESC colony growth and Nanog expression were mediated by convective transport of soluble factors, while Fgf5 up-regulation was at least partially in response to shear application (Fig. 3). Our results highlight that perfusion culture can alter multiple environmental cues and one should be mindful when attributing flow-induced stem cell phenotypes to shear stress. Flow-induced mass transport of autocrine signaling factors has also been previously implicated in the hemodynamic response of endothelial cells, although shear stress appears to be the dominant environmental modulator (30). In endothelial cells, the 2 mechanisms are distinguished primarily by measurements of specific shear-responsive functions, for example, nitric oxide and prostaglandin production, in the presence or absence of exogenous agonists (17, 36). Due to the lack of known shear-responsive functions or elements and the pervasiveness of autocrine signaling in self-renewing ESCs (29, 37), the flow-dependent environmental modulator of cell fate cannot be determined retrospectively. Hence, more rigorous experimental assessment is required when determining whether a particular stem cell phenotype is being modulated by shear stress. In this study, we only ascertained that Fgf5 is shear-responsive after isolating shear stress as the experimental variable both during the design and operation of the microfluidic perfusion array (i.e., ensuring convective mass transport (Pe≫1) and using CM). The fact that theoretical mass transport consideration (based on Pe calculation) alone cannot fully account for soluble signaling effects during perfusion points to the role of ECM-bound autocrine signaling molecules in modulating mESC phenotype. This is further supported by the observation that Nanog, Rex1, and Oct4 were partly modulated by signaling molecules bound to HSPGs (Fig. 5C).
Besides contributing to the development of scalable perfusion stem cell cultures, a systematic quantitative microfluidic-based approach can also help us gain insight into the mechanisms involved in stem cell fate specification by shear stress. Currently, most shear stress studies are performed in the context of differentiation, focusing on changes in lineage-specific markers, e.g., Flk-1, αSMA in response to shear (1, 2, 11). Researchers are beginning to identify the signaling pathways e.g., VEGF signaling activated by shear stress during ESC differentiation into endothelial cells (11). However, information regarding shear-activated signaling pathways during ESC self-renewal or differentiation into other lineages as well as mechanotransduction mechanisms is scarce (11). In this study, we demonstrated that shear stress led to Fgf5 up-regulation in self-renewing mESCs. By targeting the signaling mechanisms known to regulate Fgf5 in mESCs, we identified HSPG as a molecular player involved in sensing shear stress (Figs. 4 and 5). It is still not clear what the role of HSPGs in ESC shear mechanosensing is. HSPGs may be directly transmitting shear stress via their transmembrane component, e.g., syndecans, to the cytoskeletal network or associated signaling proteins (31). Alternatively, shear stress has been shown to activate the receptor tyrosine kinase (RTK) Flk-1 in endothelial cells (38); thus, HSPGs may function as accessory proteins to modulate the activity of FGF receptor, a RTK in ESCs known to bind to HSPGs. The large body of literature on shear mechanotransduction in endothelial cells (30) provides useful references in elucidating these mechanisms in ESCs, although it is unclear whether the mechanisms in these 2 cell types will be equivalent because self-renewing or nonendothelial differentiating ESCs may not express the same signaling proteins, e.g., Flk-1 is not expressed in self-renewing ESCs (1, 2). Hence, further experimental investigation is still required to fully test and characterize the shear stress responses (i.e., mechanosensors, signaling pathways, gene expression, and resulting phenotypes) of self-renewing ESCs.
CONCLUSIONS
We have employed a multiplex logarithmic microfluidic array to investigate perfusion-mediated effects, in particular shear stress, on self-renewing mESCs in a quantitative, graded manner. We show that perfusion elicits phenotypic changes, which collectively indicate for an epiblast-like state, and delineated the environmental factors (i.e., soluble factor removal or shear stress) underpinning each phenotype. The determination of a specific shear-responsive phenotype in mESCs allows us to target related signaling elements and identify HSPGs to be involved in stem cell mechanosensing. This is the first instance that demonstrates that self-renewing mESCs possess molecular machinery for responding to shear stress and identifies HSPGs to be one of the molecular components involved in shear mechanosensing. This study provides a foundation for further elucidation on shear mechanotransduction mechanisms in stem cells.
Supplementary Material
Acknowledgments
The authors thank Katarina Blagović for technical assistance and Lily Y. Kim for developing the initial concept and first version of a multi-flowrate device. Y.-C.T. contributed to conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. J.V. contributed to conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
This work was supported by U.S. National Institutes of Health grant EB007278 and an A*STAR International Fellowship.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Adamo L., Naveiras O., Wenzel P. L., McKinney-Freeman S., Mack P. J., Gracia-Sancho J., Suchy-Dicey A., Yoshimoto M., Lensch M. W., Yoder M. C., Garcia-Cardena G., Daley G. Q. (2009) Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamamoto K., Sokabe T., Watabe T., Miyazono K., Yamashita J. K., Obi S., Ohura N., Matsushita A., Kamiya A., Ando J. (2005) Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Heart Circ. Physiol. 288, H1915–H1924 [DOI] [PubMed] [Google Scholar]
- 3. King J. A., Miller W. M. (2007) Bioreactor development for stem cell expansion and controlled differentiation. Curr. Opin. Chem. Biol. 11, 394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kehoe D. E., Jing D., Lock L. T., Tzanakakis E. S. (2010) Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng. A 16, 405–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sikavitsas V. I., Bancroft G. N., Holtorf H. L., Jansen J. A., Mikos A. G. (2003) Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl. Acad. Sci. U. S. A. 100, 14683–14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cormier J. T., Nieden N. I. Z., Rancourt D. E., Kallos M. S. (2006) Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 12, 3233–3245 [DOI] [PubMed] [Google Scholar]
- 7. Fok E. Y. L., Zandstra P. W. (2005) Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells 23, 1333–1342 [DOI] [PubMed] [Google Scholar]
- 8. Abranches E., Bekman E., Henrique D., Cabral J., M. S. (2007) Expansion of mouse embryonic stem cells on microcarriers. Biotechnol. Bioeng. 96, 1211–1221 [DOI] [PubMed] [Google Scholar]
- 9. Lock L. T., Tzanakakis E. S. (2009) Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng. A 15, 2051–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oh S. K. W., Chen A. K., Mok Y., Chen X., Lim U. M., Chin A., Choo A. B. H., Reuveny S. (2009) Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2, 219–230 [DOI] [PubMed] [Google Scholar]
- 11. Stolberg S., McCloskey K.E. (2009) Can shear stress direct stem cell fate? Biotechnol. Prog. 25, 10–19 [DOI] [PubMed] [Google Scholar]
- 12. Walker G. M., Zeringueb H. C., Beebe D. J. (2004) Microenvironment design considerations for cellular scale studies. Lab Chip 4, 91–97 [DOI] [PubMed] [Google Scholar]
- 13. Schaff U. Y., Xing M. M. Q., Lin K. K., Pan N., Jeon N. L., Simon S. I. (2007) Vascular mimetics based on microfluidics for imaging the leukocyte-endothelial inflammatory response. Lab Chip 7, 448–456 [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez E., Petrich B. G., Shattil S. J., Ginsberg M. H., Groisman A., Kasirer-Friede A. (2008) Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip 8, 1486–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song J. W., Gu W., Futai N., Warner K. A., Nor J. E., Takayama S. (2005) Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 77, 3993–3999 [DOI] [PubMed] [Google Scholar]
- 16. Chau L., Doran M., Cooper-White J. (2009) A novel multishear microdevice for studying cell mechanics. Lab Chip 9, 1897–1902 [DOI] [PubMed] [Google Scholar]
- 17. Florian J. A., Kosky J. R., Ainslie K., Pang Z., Dull R. O., Tarbell J. M. (2003) Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 93, e136–142 [DOI] [PubMed] [Google Scholar]
- 18. Lanner F., Lee K. L., Sohl M., Holmborn K., Yang H., Wilbertz J., Poellinger L., Rossant J., Farnebo F. (2010) Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. Stem Cells 28, 191–200 [DOI] [PubMed] [Google Scholar]
- 19. Blagović K., Kim L. Y., Skelley A. M., Voldman J. (2008) Microfluidic control of stem cell diffusible signaling. Proc. Micro Total Anal. Syst. 2008 Conf. 1, 677–679 [Google Scholar]
- 20. Langer R., Linhardt R. J., Hoffberg S., Larsen A. K., Cooney C. L., Tapper D., Klein M. (1982) An enzymatic system for removing heparin in extracorporeal therapy. Science 217, 261–263 [DOI] [PubMed] [Google Scholar]
- 21. Kim L., Vahey M. D., Lee H. Y., Voldman J. (2006) Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip 6, 394–406 [DOI] [PubMed] [Google Scholar]
- 22. Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., McKay R. D. G. (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 [DOI] [PubMed] [Google Scholar]
- 23. Ying Q.-L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. (2008) The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mansergh F., Daly C., Hurley A., Wride M., Hunter S., Evans M. (2009) Gene expression profiles during early differentiation of mouse embryonic stem cells. BMC Dev. Biol. 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450, 1230–1234 [DOI] [PubMed] [Google Scholar]
- 26. Toyooka Y., Shimosato D., Murakami K., Takahashi K., Niwa H. (2008) Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135, 909–918 [DOI] [PubMed] [Google Scholar]
- 27. Cool S. M., Nurcombe V. (2006) Heparan sulfate regulation of progenitor cell fate. J. Cell. Biochem. 99, 1040–1051 [DOI] [PubMed] [Google Scholar]
- 28. Graf T., Stadtfeld M. (2008) Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 3, 480–483 [DOI] [PubMed] [Google Scholar]
- 29. Kunath T., Saba-El-Leil M. K., Almousailleakh M., Wray J., Meloche S., Smith A. (2007) FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895–2902 [DOI] [PubMed] [Google Scholar]
- 30. Davies P. F. (1995) Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75, 519–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tarbell J. M., Pahakis M. Y. (2006) Mechanotransduction and the glycocalyx. J. Intern. Med. 259, 339–350 [DOI] [PubMed] [Google Scholar]
- 32. Humphries D. E., Silbert J. E. (1988) Chlorate: a reversible inhibitor of proteoglycan sulfation. Biochem. Biophys. Res. Commun. 154, 365–371 [DOI] [PubMed] [Google Scholar]
- 33. Ornitz D. M., Yayon A., Flanagan J. G., Svahn C. M., Levi E., Leder P. (1992) Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol. Cell. Biol. 12, 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. zur Nieden N. I., Cormier J. T., Rancourt D. E., Kallos M. S. (2007) Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J. Biotechnol. 129, 421–432 [DOI] [PubMed] [Google Scholar]
- 35. Pera M. F., Tam P. P. L. (2010) Extrinsic regulation of pluripotent stem cells. Nature 465, 713–720 [DOI] [PubMed] [Google Scholar]
- 36. Mochizuki S., Vink H., Hiramatsu O., Kajita T., Shigeto F., Spaan J. A. E., Kajiya F. (2003) Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am. J. Physiol. Heart Circ. Physiol. 285, H722–H726 [DOI] [PubMed] [Google Scholar]
- 37. Davey R. E., Zandstra P. W. (2006) Spatial organization of embryonic stem cell responsiveness to autocrine gp130 ligands reveals an autoregulatory stem cell niche. Stem Cells 24, 2538–2548 [DOI] [PubMed] [Google Scholar]
- 38. Chen K.-D., Li Y.-S., Kim M., Li S., Yuan S., Chien S., Shyy J. Y. J. (1999) Mechanotransduction in response to shear stress. J. Biol. Chem. 274, 18393–18400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.