Abstract
Parkinson's disease (PD) involves progressive loss of nigrostriatal dopamine (DA) neurons over an extended period of time. Mitochondrial damage may lead to PD, and neurotoxins affecting mitochondria are widely used to produce degeneration of the nigrostriatal circuitry. Deletion of the mitochondrial transcription factor A gene (Tfam) in C57BL6 mouse DA neurons leads to a slowly progressing parkinsonian phenotype in which motor impairment is first observed at ∼12 wk of age. l-DOPA treatment improves motor dysfunction in these “MitoPark” mice, but this declines when DA neuron loss is more complete. To investigate early neurobiological events potentially contributing to PD, we compared the neurochemical and electrophysiological properties of the nigrostriatal circuit in behaviorally asymptomatic 6- to 8-wk-old MitoPark mice and age-matched control littermates. Release, but not uptake of DA, was impaired in MitoPark mouse striatal brain slices, and nigral DA neurons lacked characteristic pacemaker activity compared with control mice. Also, hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channel function was reduced in MitoPark DA neurons, although HCN messenger RNA was unchanged. This study demonstrates altered nigrostriatal function that precedes behavioral parkinsonian symptoms in this genetic PD model. A full understanding of these presymptomatic cellular properties may lead to more effective early treatments of PD.—Good, C. H., Hoffman, A. F., Hoffer, B. J., Chefer, V. I., Shippenberg, T. S., Bäckman, C. M., Larsson, N.-G., Olson, L., Gellhaar, S., Galter, D., Lupica, C. R. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease.
Keywords: dopamine, patch clamp, striatum, substantia nigra pars compacta
Mitochondrial dysfunction and cellular energy failure are implicated in the degeneration of dopamine (DA) neurons in idiopathic Parkinson's disease (PD; ref. 1). In addition, many animal models of PD utilize neurotoxins that disrupt mitochondrial respiratory complex I function and impair midbrain DA neuron function (2–4). A “mitochondrial hypothesis” of PD is also supported by studies demonstrating higher levels of mitochondrial DNA (mtDNA) deletions in substantia nigra pars compacta (SNc) DA neurons in aging and PD patients (5, 6). Furthermore, several genes associated with PD (e.g., Parkin, Pink-1, DJ-1, and LRRK2) may be involved in regulating mitochondrial function (7), and studies with mice in which these genes have been silenced generally support the idea that mitochondrial impairment may lead to DA neuron degeneration and parkinsonism (8). However, the DA circuit changes in these models tend to be subtle, and many of the hallmark characteristics of PD are not observed (7, 8).
Recently, a novel genetic model was developed in which mitochondrial function was disrupted in DA neurons by elimination of the nuclear genome-encoded mitochondrial Tfam gene (9). The TFAM protein promotes mtDNA transcription and replication (10). Whereas mice homozygous for Tfam deletion are not viable, the selective deletion of Tfam in DA neurons using a Cre/loxP recombination strategy generates animals, known as “MitoPark” mice, that survive to adulthood and slowly develop a PD-like phenotype (9, 11). Furthermore, cellular changes similar to those seen in idiopathic PD are observed, such as intracellular dense bodies in DA neurons, the degeneration of DA pathways, and loss of striatal DA. Also similar to PD, the SNc DA neurons of MitoPark mice degenerate before those in the ventral tegmental area (VTA), and l-DOPA's normalizing effect on behavior weakens as the animals age. Therefore, as noted in recent reviews (12, 13), MitoPark mice possess several characteristics that are considered useful for a genetic animal model of PD.
Because most acute animal models of PD involve rapid neurotoxin-induced degeneration of the nigrostriatal pathway, it has been difficult to assess the extent and type of DA neuron dysfunction occurring before the onset of behavioral symptoms and how such prodromal changes relate to the eventual appearance of motor symptoms (14, 15). Given the prolonged asymptomatic phase in early stages of PD, knowledge of the progressive cellular events can inform the development of effective treatment strategies. In addition, there are potential confounds associated with nonspecific effects of neurotoxins in the acute models. While the prolonged time course of neurodegeneration and absence of neurotoxins represent strengths of the MitoPark model, it does not replicate other aspects of PD.
The present study describes the functional properties of SNc DA neurons and the dynamics of DA release and uptake in the Mitopark mouse striatum at a developmental time point where overt parkinsonian symptoms are absent. We find altered ion channel properties that may represent the earliest changes in DA neuron function and describe impairment in the release of functionally relevant pools of DA in these behaviorally asymptomatic mice.
MATERIALS AND METHODS
Animals
The breeding scheme for generating MitoPark mice has been described previously (9, 11). Briefly, MitoPark mice were bred on a C57BL6 background in which the DA transporter (DAT) promoter was used to drive cre recombinase expression, and these mice were crossed with mice containing a loxP-flanked Tfam gene. The male and female 6- to 8-wk-old MitoPark mice used in the experiments were heterozygous for DAT-cre expression (DAT/DATcre) and homozygous for the loxP-flanked Tfam gene (TfamloxP/TfamloxP). In nearly all experiments, age-matched littermates containing only heterozygous expression of DAT-cre and Tfam-LoxP (DAT/DATcre Tfam/TfamloxP) were used as controls. In some voltammetric recordings, mice lacking the DAT-cre construct (DAT/DAT Tfam/Tfamloxp or DAT/DAT Tfam/Tfam) were also used as controls.
A total of 23 MitoPark mice (12 male and 11 female) and 23 control mice (16 male and 7 female) were used for these experiments. The median ages for all animals was 7.8 wk (range 6.4–9.4 wk), and generally brain tissue from the same animal was used for both SNc DA neuron recordings and for fast-scan cyclic voltammetry (FSCV) in striatal brain slices. Genotyping was performed at the National Institute on Drug Abuse–Intramural Research Program (NIDA-IRP) facility. Animal protocols were approved by the NIDA-IRP Animal Care and Use Committee and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. In situ hybridization of hyperpolarization-activated cyclic nucleotide-gated (HCN) mRNA and protein localization studies were approved by the Stockholm Animal Ethics Committee.
Brain slice preparation
Mice were killed by cervical dislocation and decapitated. The brains were removed and placed into chilled (4°C) and aerated (95% O2-5% CO2) modified artificial cerebrospinal fluid (m-aCSF) consisting of the following (in mM): 30 NaCl, 4.5 KCl, 1 MgCl2, 1.2 NaH2PO4, 10 d-glucose, 194 sucrose, and 26 NaHCO3. Coronal slices containing the cerebral cortex and the striatum or the SNc were cut (250–300 μm) in chilled m-aCSF using a vibrating tissue slicer (VT1000S; Leica, Solms, Germany) and transferred to a holding chamber containing normal aCSF (in mM): 126 NaCl, 3.0 KCl, 1.5 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 26 NaHCO3; saturated with 95% O2-5% CO2. Slices were incubated at 35°C for 30 min, and the holding chamber was then removed from the water bath and allowed to equilibrate to room temperature (≥30 min). During electrophysiological and voltammetric recordings, slices were submerged in a low-volume (170 μl) recording chamber and continuously perfused with warm (30–32°C), oxygenated aCSF at a rate of 2 ml/min.
Whole-cell intracellular recordings
Visualization of neurons (×600 view) was performed with an upright microscope (Zeiss Axioscope; Carl Zeiss, Oberkochen, Germany), modified to provide a gradient contrast image utilizing infrared illumination. Whole-cell voltage clamp recordings were performed using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA, USA) and recording electrodes (3–5 MΩ) filled with a solution containing the following (in mM): 115 K-CH3-SO4, 20 KCl, 1 MgCl2, 10 HEPES, 1 EGTA, 2 ATP, 0.3 GTP, 10 phosphocreatine, and 0.15% biocytin; adjusted to pH 7.2 using KOH (270–274 mosmol). Whole-cell voltage steps were applied via the Strathclyde electrophysiology software package (WCP; courtesy of Dr. John Dempster, Strathclyde University, Glasgow, UK) using an A/D board (ITC-18; Instrutech, Bellmore, NY, USA) in a personal computer. Drugs were added to the flowing aCSF (2 ml/min) at 100× their final concentration at a rate of 20 μl/min using calibrated syringe pumps (Razel Scientific Instruments, Stamford, CT, USA).
Measurement of HCN ion channel currents (Ih)
HCN ion channels mediate a hyperpolarization-activated current Ih. This current activates slowly and displays virtually no time-dependent inactivation during hyperpolarizing voltage steps (16–18). In addition, in most neurons, Ih is not active at membrane potentials positive to approximately −55 to −75 mV and typically does not saturate at potentials as negative as −155 mV (17, 18). The slow activation kinetics of Ih results in a current that is larger at the end of a 2-s hyperpolarizing voltage step, at which point Ih is fully active (i.e., steady state, Iss), compared with the current observed near the beginning of a hyperpolarizing voltage step (i.e., the instantaneous current, Iins). Therefore, as described previously (19), Ih was generally measured by voltage clamping DA neurons at −40 mV, where it is inactive, and then stepping the membrane to −140 mV in successive −10-mV, 2-s, voltage steps. Iins was then subtracted from Iss at each voltage step to estimate Ih using the following formula:
Tail current analysis
In a subset of neurons, Ih tail current analyses were performed to more accurately estimate the voltage dependence of Ih activation and the hyperpolarization-activated whole cell conductance (Gh). For these experiments, a 2-s hyperpolarizing prepulse (−40 to −140 mV in 10-mV increments) was immediately followed by a 300-ms step to −120 mV. These values were then normalized to the maximal tail current measured at −140 mV, plotted, and fitted with the following Boltzmann equation:
where Vm is the membrane holding potential; V1/2 is the membrane voltage at which Ih is half of its maximal value measured at −140 mV; and k is the Boltzmann constant.
Immunohistochemical identification of SNc DA neurons
To confirm that each of the recorded neurons was DAergic, every cell was filled with biocytin (0.15%) during whole-cell recording and processed for DAT immunohistochemistry. After recording, each brain slice was transferred to a 4% paraformaldehyde PBS solution at 4°C for 20 h. Slices were then transferred into an 18% sucrose solution for 6 h. Slices were resectioned on a cryostat (20 μm), collected on slides, and washed in PBS. Sections were incubated in PBS blocking buffer containing 4% BSA and 0.3% Triton-X-100 for 1 h at room temperature and then incubated 24 h at 4°C with a DAT monoclonal antibody (Chemicon, Temecula, CA, USA; diluted 1:100 in blocking buffer). Sections were again washed in PBS and incubated in AlexaFluor-568-conjugated goat antibody to mouse IgG (Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. To detect biocytin, sections were washed twice in PBS and incubated for 1 h at room temperature in streptavidin AlexaFluor-488 conjugate (Invitrogen; diluted 1:800 in blocking buffer).
In situ hybridization of HCN1–4 transcripts
In situ hybridization autoradiography for HCN1–4 mRNAs followed previous protocols (20), with minor modifications. Control and MitoPark mice, as well as mice with muscle-specific mitochondrial dysfunction (21), were killed, and their hearts and brains dissected and frozen on dry ice. Cryostat sections (14 μm) were thawed onto slides (SuperFrost; VWR, Stockholm, Sweden) and kept at 20°C until use. Two different complementary oligonucleotides were selected for each of the HCN genes, and adjacent sections were hybridized with a tyrosine hydroxylase (TH) mRNA probe. The HCN-specific oligonucleotide sequences corresponded to HCN1: NM_010408.1, nt 731–682 and 1742–1693; HCN2: NM_008226.2, nt 1562–1513, 2077, and 2028; HCN3: NM_008227.1, nt 1603–1554 and 2040–1991; and HCN4: NM_001081192.1, nt 833–784, 979–942, 1079–1031, and 1308–1259. The oligonucleotide specific for TH has been described previously (9). Slides were air dried and hybridized overnight with 33P-end-labeled oligonucleotides in a hybridization cocktail. After being rinsed and dehydrated, radioactivity in the brain sections was mapped using phosphoimaging and X-ray film exposure (Kodak Biomax MR film; Eastman Kodak, Rochester, NY, USA). Slides were next dipped in photographic emulsion (NTB Kodak; VWR) and developed after 2–4 wk. The specificity of the signal was confirmed through comparison with published brain expression patterns: HCN1 probes showed a high signal in cerebellar Purkinje neurons as well as in hippocampus and cortex; HCN2 mRNA was detected at various levels in all areas of the brain and was ubiquitous in heart cells; HCN3 was expressed in restricted brain areas such as the olfactory bulb and hypothalamus and at low levels in cerebral cortex and the granular layer of cerebellum; and HCN4 mRNA hybridization was restricted to the olfactory bulb and some thalamic nuclei and absent in cerebral cortex, cerebellum, and heart.
FSCV recordings
Carbon fibers (7 μm diameter; Goodfellow Corp., Oakdale, PA, USA) were prepared as described previously (22, 23). Briefly, micropipettes containing the carbon fibers were backfilled with a 4 M potassium acetate/150 mM KCl solution and connected to a standard patch pipette holder/headstage assembly. A patch-clamp amplifier (EVA-8; HEKA Electronics, Bellmore, NY, USA) was used to change the carbon fiber electrode electrical potential and to measure current. Voltammetric scans and electrical stimulus timing protocols were performed using PCI-based analog to digital boards (National Instruments, Austin, TX, USA) and LabView-based software (courtesy of Dr. Mark Wightman, University of North Carolina, Chapel Hill, NC, USA). During electrochemical detection, the potential of the carbon fiber was driven from −0.4 to 1.0 V and back to −0.4 V, using a triangular waveform (400 V/s, 7 ms duration) applied every 100 ms. A 5-s (50-scan) control period was used to obtain a stable background current that was digitally subtracted from that obtained during the peak of the response following electrical tissue stimulation. Peak oxidation currents were converted to DA concentrations using a calibration performed for each electrode with a 1 μM DA standard solution. All signals used in the statistical analyses matched the expected voltammetric profile for DA (24).
Electrically evoked DA signals in brain slices
Under stereoscopic magnification, carbon fibers were lowered to a depth of ∼100 μm in the dorsolateral striatum. A bipolar stimulating electrode was positioned ∼75–100 μm from the carbon fiber and constant current pulses (10–1000 μA, 1 ms duration) were delivered between voltammetric scans to elicit DA release. Responses were obtained every 2 min, and all those used for analysis were stable throughout the duration of the recordings. For single-pulse experiments, DA uptake was assessed by fitting a single exponential function to the signal decay using a least-squares minimization algorithm (WinWCP; Dr. John Dempster, Strathclyde Institute for Biomedical Sciences, Glasgow, UK; http://spider.science.strath.ac.uk). A tau (τ) value was obtained for each recording site by averaging all time constants obtained from each DA signal generated during input-output curves (stimulus intensity vs. DA signal). The first-order rate constant (k or 1/τ) obtained using this approach provides an index of the efficiency (Vmax/Km) of DA clearance mediated by the DAT (22, 25).
To assess the capacity of axon terminals to release DA during burst stimulation, 3 voltammetric signals were obtained at each recording site using a single pulse and 2, 5, and 10 pulses delivered at 25 Hz. After 3 signals at each site were averaged, the difference between the peak DA signal obtained immediately after burst stimulation or single pulses was determined as DAnp − DA1p, where DAnp is the amplitude of the voltammetric signal for n pulses, and DA1p is the amplitude of the voltammetric signal obtained following a single electrical pulse. Data were obtained for each slice before and during drug treatment. The data were fit to a linear regression model (y=mx+b; Prism 5.02; GraphPad, San Diego, CA, USA), where the slope m represents the relative change in DA concentration per pulse.
In vivo microdialysis
Mice were anesthetized with ketamine-xylazine (80–18 mg/kg i.p.) and placed in a stereotactic frame (David Kopf, Topanga, CA, USA). A small hole was drilled into the skull to enable implantation of a microdialysis guide cannula (CMA/7; CMA Microdialysis, North Chelmsford, MA, USA) over the right striatum using stereotactic coordinates (anteroposterior: +0.2–0.4, lateral: −2.0, ventral: −2.4 mm; ref. 26). The cannula was secured to the skull with dental acrylic cement (Geristore Dual Cure; DenMat, Santa Maria, CA, USA). After surgery, animals were housed individually in the colony room for 4–5 d before commencement of microdialysis experiments.
At 12 to 16 h before the start of the microdialysis experiments, microdialysis probes (CMA/7; 1×0.24 mm) were connected to an assembly (see below) and flushed with aCSF (composition in mM: 145 NaCl, 2.8 KCl, 1.2 MgCl2, 1.2 CaCl2, 0.25 ascorbic acid, and 5.4 d-glucose, adjusted to pH 7.2 using NaOH or H3PO4). The mouse was gently restrained, and the probe was slowly inserted into the guide cannula. The dialysis assembly consisted of fluorinated ethylene propylene tubing (CMA Microdialysis) that connected the probe to a 1-ml gastight syringe (Hamilton Co., Reno, NV, USA) mounted on a microdialysis pump (CMA/102) through a quartz-lined, low-resistance swivel (375/D/22QM; Instech, Plymouth Meeting, PA, USA). The mouse was then placed in the microdialysis chamber with food and water freely available, and the probe was perfused overnight with aCSF at a flow rate of 0.3 μl/min. At 1 h before the experiments, the syringes were filled with fresh aCSF, and the probes were allowed to equilibrate for 1 h at the 1.0 μl/min flow rate used for no-net-flux experiments. The probes were perfused in random order with solutions of 0, 2, 5, 10, and 20 nM DA in aCSF. Two consecutive 10-min samples were collected from each probe for each DA concentration following a 30-min equilibration period. The steady-state DA concentration in the probe effluent was plotted as the difference between the DA concentration inside and outside the probe as a function of the DA concentration inside the probe, i.e., Cin − Cout vs. Cin. The slope of the resulting plot was obtained by linear regression and is equal to Ed, an indirect measure of DA uptake (27, 28). The point when no DA is gained or lost by the probe (x intercept, Cin−Cout=0) represents an unbiased estimate of the extracellular DA concentration.
Following the completion of no-net-flux experiments, aCSF was perfused through the probe for 40 min to allow equilibration of basal DA levels. Three consecutive 10-min dialysis samples were then collected to determine basal dialysate DA levels. The aCSF was then changed to aCSF containing high-K+ (in mM: 87.8 NaCl, 60.0 KCl, 1.2 CaCl2, 1.2 MgCl2, 0.25 ascorbic acid, and 5.4 d-glucose, adjusted to pH 7.4 with 0.5 M NaOH), and following a 30-min equilibration period, 3 consecutive 10-min samples were collected. The perfusate was then changed to fresh aCSF, and 3 more consecutive 10-min samples were collected following a 30-min equilibration period.
All microdialysis samples were frozen on dry ice and analyzed for DA content within 24 h after collection. DA was determined by HPLC coupled to electrochemical detection. The chromatographic system consisted of a CMA/200 refrigerated microinjector, a BAS PM-80 pump, and a BAS LC-4C amperometric detector (BAS, West Lafayette, IN, USA). The mobile phase (analytical grade of 0.15 M sodium phosphate, 2.24 mM sodium octanesulfonic acid, 0.94 mM EDTA, and 13% methanol v/v, adjusted to pH 5.0) was passed through a 0.22-mm nylon filter and a BAS online degasser at a flow rate of 0.47 ml/min. DA was separated on a BAS C18 column (100 mm×2.0 mm×3 μm) and detected on a glassy carbon working electrode at an oxidation potential of +700 mV vs. Ag/AgCl. Dialysate DA levels were quantified by external standard curve calibration, using peak heights for quantification. Under these conditions, the retention time for DA was ∼2.5 min, and the limit of detection was <0.25 nM.
After the completion of each experiment, animals were deeply anesthetized with pentobarbital and killed by decapitation. The brains were removed and frozen, and the site of probe placement was verified histologically via coronal sections (20 μm thick) obtained using a microtome (model 5030; Hacker Instrument Inc., Fairfield, NJ, USA).
Statistical analyses
Data in text and figures are represented as means ± se. Statistical analyses were performed using either a 2-tailed Student's t test or ANOVA followed by post hoc analyses for multiple comparisons (GraphPad Prism 5.02; GraphPad Scientific, San Diego, CA, USA).
RESULTS
In the original report of the MitoPark mice, mitochondrial function was assessed in DA neurons beginning at 6 wk of age (9). At this time point, intracellular inclusions are seen, and mitochondrial DNA-encoded cytochrome c oxidase mRNA expression is significantly reduced. However, despite these alterations in DA neuron mitochondrial markers, no behavioral or neurochemical deficits are observed until ∼12 wk of age in these mice (9, 11). Therefore, to assess nigrostriatal function before the onset of behavioral deficits, we utilized 7- to 8-wk-old MitoPark mice and age-matched control littermates throughout our study.
Reduced HCN channel function in SNc DA neurons of MitoPark mice
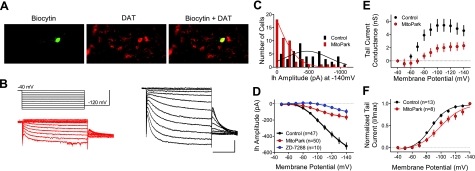
Whole-cell patch-clamp recordings were performed in SNc DA neurons in brain slices obtained from 7- to 8-wk-old MitoPark mice (n=50 neurons) and controls (DAT/DATcre Tfam/TfamloxP; n=47 neurons). The DAergic phenotype of all recorded neurons was confirmed using intracellular biocytin labeling and immunohistochemical detection of the DAT (Fig. 1A).
Figure 1.
SNc DA neurons from behaviorally asymptomatic MitoPark mice exhibit impaired HCN channel function. A) Neuron from a 7-wk-old MitoPark mouse filled with biocytin during whole-cell electrophysiological recording and counterstained for the DAT. B) Ih tail current analysis. Top left: voltage step protocol. Bottom left: series of currents recorded from a MitoPark DA neuron (red). Right: currents obtained from a SNc DA neuron from a control (DAT/DATcre Tfam/TfamloxP) 7-wk-old mouse (black) Scale bars = 100 ms; 500 pA. C) Frequency histogram showing Ih amplitude at −140 mV for all DA neurons. Note the high number of SNc DA neurons from the MitoPark mice demonstrating little to no Ih. D) Mean current-voltage relationship for Ih in SNc DA neurons obtained from 7- to 8-wk-old control and MitoPark littermates. Also shown is the blockade of Ih by ZD7288 (50 μM) in control DA neurons. E) Mean tail current conductance mediated by HCN channels on hyperpolarization (Gh). Note that Gh saturates at more depolarized membrane potentials and is much smaller in MitoPark DA neurons. F) Mean current-voltage relationship for normalized Ih tail currents in SNc DA neurons from control and MitoPark mice. Note the significant rightward shift in the voltage dependence of Ih in the MitoPark neurons. Type I, II, and III DA neurons were pooled for these analyses.
The hyperpolarization-activated cation current Ih is mediated by HCN ion channels, and is a reliable electrophysiological marker used to identify DA neurons in vitro (18, 29, 30). Consistent with previous literature, SNc DA neurons in slices from control mice exhibited robust Ih on hyperpolarization of the cell membrane from a holding potential of −40 mV (Fig. 1C, D). In control neurons, peak currents > 500 pA (conductance, Gh=5.78±0.45 nS; n=47) were routinely observed at the most hyperpolarized membrane potentials (−140 mV; Fig. 1 and Table 1). In control SNc DA neurons, Ih was blocked by ZD7288 (50 μM; n=10; Fig. 1B), confirming HCN ion channel mediation of this current (31). In contrast to control DA neurons, those from MitoPark mice demonstrated dramatically smaller Ih amplitudes across the entire range of applied membrane voltages (Fig. 1B–D and Table 1). The reduction in Ih was identical in both male and female MitoPark mice at the −140-mV voltage step (male=−185±40 pA, female=−205±57 pA; P=0.7673, unpaired t test). Analysis of tail currents revealed a significant reduction in the maximum hyperpolarization-activated cation conductance Gh (Fig. 1E and Table 1) and a rightward shift in the half-maximal membrane voltage V1/2 for activation of Ih (Fig. 1F). This suggests that fewer functional HCN channels were available for activation in the SNc DA neurons of MitoPark mice and that there was a shift in the voltage dependence of the remaining pool of functional HCN channels (Fig. 1E, F).
Table 1.
Membrane properties of control (DAT/DATcre Tfam/TfamloxP) and MitoPark (DAT/DATcre TfamloxP/TfamloxP) SNc DA neurons
| Neuron type | Rin (MΩ) | Resting membrane potential (mV) | Action potential frequency (Hz) | Gh (nS) |
|---|---|---|---|---|
| Control, n = 47 | 528.8 ± 38.3 | −49.2 ± 0.9 | 1.9 ± 0.2 | 5.78 ± 0.45 |
| MitoPark, n =32 | ||||
| Type I (7/32, 22%) | 666.5 ± 147.1 | −43.2 ± 1.8 | 2.6 ± 0.6 | 4.55 ± 0.99 |
| Type II (13/32, 41%) | 1244 ± 140.3# | −61.2 ± 1.4# | 0.05 ± 0.02* | 1.12 ± 0.37# |
| Type III (12/32, 37%) | 1135 ± 238.7# | −52.1 ± 1.2 | 12.2 ± 1.6# | 2.06 ± 0.60# |
Data are means ± se. DA neurons recorded in brain slices from MitoPark mice were classified according to electrophysiological properties. Type I MitoPark neurons were not statistically different from control DA neurons, whereas type II and type III neurons differed from control neurons with regard to several physiological parameters. Thus, type II and type III DA neurons demonstrated significantly increased resting whole-cell membrane resistance and significantly decreased maximal hyperpolarization-activated whole-cell conductance Gh (measured from a voltage step to −140 mV), mediated by HCN ion channels (P<0.001 by ANOVA). Type II neurons also exhibited significantly elevated resting membrane potential compared with control DA neurons.
P < 0.01,
P < 0.0001 vs. control; ANOVA and Bonferroni post hoc analysis.
Transcriptional activity of HCN genes in mouse DA neurons
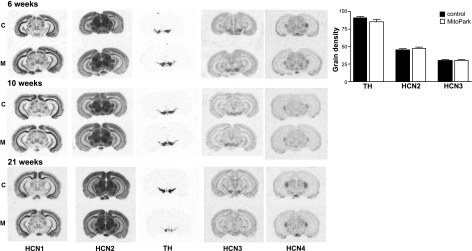
To determine whether the decrease in Ih observed in DA neurons of MitoPark mice resulted from reduced transcription of HCN channels, in situ hybridization was performed in which mRNA for each of the 4 known HCN genes was measured. The brains of MitoPark and control mouse brains were compared at 6, 10, and 21 wk of age. For each HCN gene, 2 different oligonucleotide probes generated similar signal patterns, and all 4 HCN genes appeared similarly transcribed in control and MitoPark brain areas. Compared with HCN1 and HCN4, HCN2 and HCN3 mRNA levels showed strong expression in the SNc and VTA. Therefore, these transcripts were measured and compared with TH mRNA levels in the same area in 10-wk-old MitoPark and control mice. As shown in Fig. 2, there were no detectable differences in either TH or HCN2 and HCN3 mRNA levels at this age. However, at 21 wk of age, brains from MitoPark mice demonstrated a significant reduction in TH but not HCN transcripts (Fig. 2).
Figure 2.
HCN channel transcripts are unaltered in 6- to 10-wk-old MitoPark mice. Left panel: in situ hybridization of mRNA encoding HCN1–4 and TH in coronal sections were performed in control (c) and MitoPark (M) mice at 6, 10, and 21 wk of age. Note the decrease in TH mRNA at the level of the SNc/VTA at the 21 wk time point, although HCN levels were unaltered at any time point. Right panel: quantification of mRNA levels at 10 wk of age (n=4 pairs of control and MitoPark mice). TH, HCN2, and HCN3 mRNA levels were not significantly affected at this time point.
Altered DA neuron activity in asymptomatic MitoPark mice
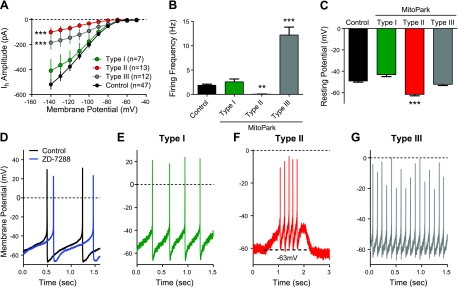
Current clamp recordings of SNc DA neurons were also performed to assess baseline electrophysiological properties. Recording from a large group of SNc DA neurons (n=32) revealed that the physiological properties of these cells in the MitoPark mice were more heterogeneous than that of control mice (Table 1) and that the neurons could be classified into 3 distinct groups. Thus, 1 group of SNc DA neurons, classified as type I neurons, exhibited spontaneous pacemaker-like firing, whole-cell input resistances (Rin), Gh values (measured at −140 mV), and resting membrane potentials that did not differ significantly from control mice (Table 1). These type I neurons represented a minority of the SNc DA neurons in the MitoPark SNc (n=7/32, 21.9%). Furthermore, these neurons did not significantly differ from control DA neurons with regard to mean firing frequency (type I: 2.5±0.6 Hz vs. control: 1.9±0.2 Hz; P>0.05, ANOVA), and their resting membrane potentials were not statistically different from those observed in control DA neurons (type I: −43.2±1.8 mV vs. WT: −49.2±0.9 mV; P>0.05, ANOVA). Based on their similarity to control SNc DA neurons, we speculate that type I MitoPark DA neurons likely represent a population of relatively healthy cells, not yet physiologically compromised at this age by the disruption of mitochondrial function.
A second population of SNc DA neurons, termed type II, were more prevalent than type I cells (13/32=41%). These cells demonstrated significantly smaller Gh values, compared with controls (control: 5.78±0.45 nS vs. type II cells: 1.12±0.37 nS; P<0.001, ANOVA; Table 1), which is consistent with diminished HCN channel function. Type II neurons also exhibited significantly hyperpolarized resting membrane potentials (control: −49.2±0.9 vs. type II: −61.6±1.4 mV; P<0.001, ANOVA; Table 1), and were not spontaneously active (0.05±0.02 Hz; P<0.001, ANOVA; Table 1). However, although type II DA neurons were not active at rest, they could be driven to fire in a normal pacemaker fashion by injection of positive current through the patch pipette (Fig. 3F). Therefore, it appears that the hyperpolarized membrane potential of type II SNc DA neurons from MitoPark mice resulted in the absence of spontaneous pacemaker firing.
Figure 3.
SNc DA neurons from asymptomatic MitoPark mice show altered electrophysiological properties. Confirmed DAT+ neurons in the SNc of MitoPark mice were classified according to their firing frequencies. A) Mean current-voltage relationship for Ih among type I (n=7), type II (n=13), and type 3 (n=12) cells. Note that type I cells were most similar to DA neurons (n=47) in control mice. B) Mean firing frequencies of SNc DA neurons. Note that both type II and type III neurons show both reduced Ih and significant differences in baseline firing frequencies. No significant differences were observed between type I and cells from control mice (P>0.05, ANOVA). C) Mean resting membrane potentials of classified cell types. Type II cells were significantly more hyperpolarized and showed no spontaneous firing. D–G) Representative current clamp traces from control mice (D) and type I (E), type II (F), and type III (G) MitoPark mice. In F, a depolarizing current was applied during the time indicated by the dashed line in order to promote cell firing.
The final group of MitoPark SNc DA neurons that could be differentiated (type III neurons, 12/32=37.5%) also exhibited a significantly smaller Gh values compared with neurons from control mice (type III: 2.06±0.60; P<0.001, ANOVA; Table 1). However, in contrast to the silent type II neurons, and all of the groups of control or MitoPark DA neurons, type III cells demonstrated significantly elevated spontaneous firing frequencies (12.2±1.6 Hz; P<0.001, ANOVA; Table 1). Examples of the properties of type I, II, and III MitoPark DA neurons are shown in Fig. 3. These results suggest that the passive and active membrane properties of SNc DA neurons in 6- to 8-wk-old asymptomatic MitoPark mice are more heterogeneous than observed in age-matched control mice. It is possible that these patterns may represent varying states of cellular impairment at this particular developmental time point.
Neurochemical assessment of DA function in asymptomatic MitoPark mice
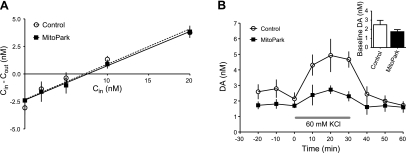
A previous study (9) with MitoPark mice at a similar developmental time point demonstrated no overt alterations in biochemical measures of DA metabolism. However, the differences in SNc DA neuron activity observed between MitoPark and control mice suggested that the dynamics of striatal DA release might be altered as a result of these physiological changes. Therefore, we performed in vivo microdialysis in the dorsal striatum of unanesthetized control (n=6; DAT/DATcre Tfam/TfamloxP) and MitoPark mice (n=5). No-net-flux assays were used to examine basal extracellular DA concentrations and DAT-mediated uptake (32, 33). As shown in Fig. 4, neither the extraction fraction (Ed; control: 0.32±0.04, MitoPark: 0.31±0.01; P=0.75, unpaired t test) nor the basal extracellular concentration of DA (control: 6.63±1.24 nM, MitoPark: 7.31±1.71 nM; P=0.75, unpaired t test), differed significantly between control and MitoPark mice.
Figure 4.
Microdialysis experiments in 8-wk-old MitoPark mice reveal differences in DA release. A) No-net-flux experiments, plotting the net gain or loss of dialysate DA concentration (Cin − Cout) as a function of varying DA concentration in the perfusate Cin in control (DAT/DATcre Tfam/TfamloxP; open circles; n=6) and MitoPark (solid squares; n=5) mice. Neither the slope (extraction fraction Ed), nor x intercept (basal extracellular DA) values differed significantly between the groups. B) Conventional microdialysis experiment comparing the effects of potassium (60 mM KCl) perfusion on DA release in the dorsal striatum of control and MitoPark mice. KCl-evoked overflow was significantly reduced in MitoPark mice, relative to controls (P<0.05; 2-way repeated-measures ANOVA). Inset: average basal DA levels during the 30-min period preceding KCl application. No differences were observed between groups (P>0.05; unpaired t test).
Although no-net-flux microdialysis provides an unbiased estimate of extracellular DA levels, it may be less sensitive to changes in basal DA levels than conventional microdialysis, even following substantial destruction of DA terminals (27, 28). Therefore, basal and potassium-evoked DA overflow were also measured using microdialysis. Basal concentrations of DA were slightly lower in MitoPark mice, although this did not achieve statistical significance (control: 2.503±0.477 nM, n=6; MitoPark: 1.741±0.224 nM, n=5; P=0.2099, unpaired t test; Fig. 4B). However, DA overflow elicited by perfusion of 60 mM KCl through the dialysis probe was significantly reduced in MitoPark animals [genotype× time, F(8,72)=2.78, P=0.0098, 2-way repeated-measures ANOVA; Fig. 4B]. These data suggest that the dynamics of presynaptic DA function are impaired in the dorsal striatum of asymptomatic MitoPark mice, despite the observation that biochemical measures demonstrate normal tissue DA concentrations at this age (9, 11).
To more fully explore these changes in the dynamics of DA release and uptake in asymptomatic 6- to 8-wk-old MitoPark mice, we used FSCV and mild focal electrical stimulation of striatal tissue in brain slices obtained from MitoPark and control mice. However, since lines of DAT-cre transgenic mice, such as those used to generate MitoPark mice, may exhibit reduced DAT activity (34), and this can influence DA release and uptake (35), we included an additional control group of mice that lacked the DAT-cre transgene (control 1), as well as mice heterozygous for DAT-cre and Tfam-loxp (control 2), and the MitoPark mice (DAT/DATcre Tfamloxp/Tfamloxp).
Striatal DA release, elicited by single-pulse stimulation over a range of stimulus intensities, was significantly reduced in striatal brain slices from MitoPark mice, relative to both groups of control mice [Fig. 5; genotype×stimulus intensity, F(2,56)=9.95, P=0.0002, 2-way repeated-measures ANOVA]. DA release was reduced to a similar extent in both male and female MitoPark mice [gender×stimulus intensity, F(1,28)=1.31, P=0.2630, 2-way repeated-measures ANOVA]. Furthermore, DA release did not differ between the 2 control groups, suggesting that at this age, mice heterozygous for the DATcre construct did not differ from mice lacking the DAT-cre transgene. We also assessed DAT-mediated uptake among these groups by comparing decay time constants of the electrically evoked signals (22). No significant differences in uptake were observed among the groups [F(2,56)=2.907, P=0.06, ANOVA].
Figure 5.
Reduced dopamine release in behaviorally asymptomatic, 8-wk-old MitoPark mice. A) Representative voltammetric signals recorded in the dorsal striatum in brain slices obtained from an 8-wk-old control (DAT/DATcre Tfam/Tfamloxp) and a MitoPark (DAT/DATcre Tfamloxp/Tfamloxp) mouse. DA release was elicited by a single pulse, indicated by the arrowhead (300 μA, 1 ms). Insets: cyclic voltammogram obtained at the peak of the current is consistent with DA in each case. B) Summary of input-output curves for single-pulse release elicited in slices obtained from control and MitoPark mice (30 slices, 8 mice). Control 1 (18 slices, 5 mice) lacks the DATcre construct (DAT/DAT Tfam/Tfamloxp or DAT/DAT Tfam/Tfam), whereas control 2 mice contain the construct (11 slices, 3 mice; DAT/DATcre Tfam/Tfamloxp). Signals were significantly reduced in the MitoPark mice relative to both control groups. ***P < 0.001; 2-way repeated-measures ANOVA. C) Summary of decay time constants (tau) for DA signals. A 1-way ANOVA revealed no significant differences (P=0.06) among the groups.
The reduction in DA release observed with single-pulse stimulation and FSCV is consistent with the lower levels of evoked DA release in MitoPark mice observed in the microdialysis studies described above. However, DA release dynamics differ according to tonic (∼4 Hz) or phasic (∼25 Hz) firing patterns of midbrain DA neurons, and these patterns of activity are important for motivated behavior (36–38). Therefore, we also evaluated DA release by comparing peak concentrations elicited by single and multiple stimuli, delivered at 25 Hz (see Materials and Methods). There was a linear increase in DA concentration as a function of pulse number (Fig. 6). The dependence of this relationship on vesicular DA content was also demonstrated in control (DAT/DATcre Tfam/TfamloxP) mice by inhibiting the vesicular monoamine transporter 2 (VMAT-2) with tetrabenazine (TBZ; 3 μM). TBZ significantly reduced the amount of DA released per pulse in control striata, as demonstrated by the change in the slope of the curve relating DA concentration to pulse number (Fig. 6B; control slope: 46±8 nM/pulse, n=14; TBZ: 4±1 nM/pulse, n=11; F(2,108)=15.92, 1-way ANOVA; P<0.01, Tukey's post hoc). In the absence of TBZ, MitoPark striatal slices demonstrated a significant reduction in DA concentration per pulse, relative to control mice (MitoPark slope: 16±2 nM/pulse, n=13; P<0.01, Tukey's post hoc), and this was unaffected by TBZ (Fig. 6B; P>0.05, Tukey's post hoc). TBZ produced a concentration-dependent inhibition of the voltammetric response that did not significantly differ between control and MitoPark mice [Fig. 6D; F(1,15)= 0.05, P=0.8233, 2-way ANOVA].
Figure 6.
Burst-induced DA release impairment in 8-wk-old MitoPark mice. A1) Representative voltammetric signals obtained in a control (DAT/DATcre Tfam/Tfamloxp) mouse by delivering either a single pulse (1p, solid line) or 10 pulses at 25 Hz (10p, shaded line). Traces represent an average of 3 signals obtained predrug (control), following nomifensine (5 μM, 10 min), and following TBZ (3 μM, 30 min). Note that the difference between signals is increased by the presence of nomifensine and that this is reversed by depletion of vesicles by TBZ. A2) Voltammetric signals from a MitoPark mouse before (control) and after nomifensine application. B) Summary of the relationship between the observed change in DA concentration per pulse in slices from control (DAT/DATcre Tfam/Tfamloxp, 14 slices, 4 mice), MitoPark (13 slices, 3 mice), and control slices treated with TBZ (11 slices, 4 mice). Slopes of the regression lines from MitoPark and control TBZ-treated slices were significantly reduced, relative to controls (P<0.05; 1-way ANOVA, Tukey's post hoc). C) In the presence of nomifensine, the slope of the regression line was significantly increased only in slices from control mice. Slopes from both MitoPark and control TBZ-treated slices (n=12 slices, 6 mice) did not differ significantly from each other (P>0.05; 1-way ANOVA, Tukey's post hoc). D) Concentration-dependent inhibition of DA signals by TBZ. Data are means ± se of the response 40 min following TBZ application at the indicated concentrations, normalized to the control (predrug) response. No significant differences were observed between slices from control and MitoPark mice at either concentration (number of slices given in parentheses; P>0.05, 2-way ANOVA).
To determine the role of uptake in the regulation of frequency-dependent DA release in control and MitoPark mice, the DAT inhibitor nomifensine was used. Nomifensine (5 μM) greatly increased the frequency-dependent DA signal in the striata of control mice (133±20 nM/pulse; compare Fig. 6B, C), and this was reversed by TBZ [14±4 nM/pulse; F(2,103)=33.71, 1-way ANOVA; P<0.001, Tukey's post hoc]. However, nomifensine did not significantly affect the frequency-dependent DA signal in striatal slices from MitoPark mice (Fig. 6C; 31±3 nM/pulse, P>0.05, Tukey's post hoc). These data demonstrate that the DAT greatly limits the size of the electrically released DA signal in striatal slices from control mice and reveals the extent of the decrease in DA release in 6- to 8-wk-old MitoPark mice. Thus, vesicular DA release is selectively impaired in the MitoPark mice before the onset of locomotor deficits.
DISCUSSION
The present study describes functional changes in SNc DA neurons that occur before the appearance of overt behavioral symptoms in a novel animal model of PD. These changes in DA neuron function are associated with a selective impairment of DA release in the dorsal striatum in the absence of alterations in DAT activity. Thus, genetic deletion of Tfam and the deficit in mitochondrial function initiated aberrant physiological processes in DA neurons and selectively reduced DA release from axon terminals in the striatum. This suggests that subtle changes in nigrostriatal function can be observed before more extensive alterations in this circuit that inevitably progress in the MitoPark transgenic model.
It is well known that relatively normal motor function is maintained despite large nigrostriatal DA depletions in human PD and in animal models of the disease. This has led to the hypothesis that compensatory changes that offset the loss of DA might occur in the nigrostriatal circuitry (39–41). The adaptations that are proposed, or experimentally supported include reduced DAT-mediated uptake of DA, increased release of DA by surviving axon terminals, and increased pacemaker and burst firing in the surviving SNc DA neurons (41–43). Whereas our study demonstrated clear alterations in firing patterns of SNc DA neurons, we found no evidence for either reduced DAT function or increased DA release at an early stage of nigrostriatal neurodegeneration in the MitoPark mice.
Although earlier studies (44, 45) suggested that DA release was enhanced following partial 6-hydroxydopamine lesions, a more recent voltammetric study found that DA release and uptake decreased proportionally with the extent of the lesion (46). Consistent with this latter result, we observed that DA overflow elicited by potassium during in vivo microdialysis, or by local electrical stimulation in vitro, was significantly reduced in young MitoPark mice. This is despite the finding that whole-tissue striatal DA content, measured using HPLC, is unchanged at 6–8 wk of age in these mice (11). Diminished DA release was observed in MitoPark striata under both tonic (single-pulse) and phasic (25 Hz) stimulation conditions at this early time point. Furthermore, the extent of this deficit was made more apparent by inhibition of DA uptake by nomifensine, which caused large increases in FSCV signals only in control mice. Thus, nomifensine blocked the strong DAT-mediated uptake in the striatum and revealed the true extent of the impairment of DA release in the MitoPark mice. This, and the similar decay kinetics of single-pulse evoked DA release in control and MitoPark mice, suggests that uptake was not affected and that DA release was selectively impaired. In addition, since TBZ in control slices resulted in a DA release profile similar to that seen in untreated MitoPark slices, we conclude that the reduction in DA release in the MitoPark mice likely reflects a deficit in vesicular DA content. Interestingly, inhibition of mitochondrial complex I by the insecticide rotenone also disrupts VMAT-2 function and depletes vesicular DA (47). Thus, a decrease in vesicular DA content may represent one of the initial responses to mitochondrial impairment.
Discrete changes in the physiological properties of SNc DA neurons were also observed in 6- to 8-wk-old MitoPark mice. These alterations are similar to those of prior studies in which cellular energy was acutely compromised by hypoxic insult (48–50). These studies demonstrated that brief periods of hypoxia led to a pronounced DA neuron hyperpolarization mediated by the activation of ATP-sensitive potassium (KATP) channels, the absence of spontaneous action potential discharge, and a reduction in Ih amplitude. In contrast, prolonged hypoxia hyperpolarized SNc DA neurons, followed by an irreversible depolarization (50). The electrophysiological characteristics of type II and type III SNc DA neurons described in the present study were qualitatively similar to those described in this previous study. The hyperpolarization and silencing of action potentials in type II cells might represent a mechanism to protect DA neurons, whereby less ATP is needed to maintain cellular functions. On the other hand, type III neurons likely represent a subset of MitoPark DA neurons that were physiologically compromised to a greater degree than type I or II neurons. Based on these data, we hypothesize that type I-III neurons may represent a continuum of physiologically impaired DA neurons sampled at this 6- to 8-wk developmental stage.
Although a causal relationship between the observed electrophysiological changes in DA neurons and axonal DA release in MitoPark mice remains to be established, previous studies (51, 52) have shown that the pacemaker firing activity of DA neurons likely supports tonic release of DA in terminal fields. However, significant differences in basal DA release using in vivo no-net-flux or microdialysis measurement of DA overflow were not observed. This is not surprising given the range of spontaneous firing rates of SNc DA neurons detected in the MitoPark mice and the fact that microdialysis sampling of basal DA overflow reflects net contributions of release and uptake. Since uptake was unaffected at this age, we hypothesize that the opposing activity patterns of type II and type III neurons limited our ability to observe changes in basal DA dynamics. In contrast, reliable depolarization of synaptic terminals using KCl (microdialysis) or electrical stimulation (FSCV) overcomes any contribution of somatic activity to DA release and provides an estimate of functionally relevant releasable pools of DA.
A consistent observation from our electrophysiological studies was the large reduction in Ih in DA neurons from the MitoPark mice. Ih is mediated by HCN ion channels and is ubiquitous in neurons and cardiac cells (53–55). The presence of Ih is also extensively used to identify midbrain DA neurons (29, 56). Although various functions have been ascribed to HCN channels, the fact that Ih is an inward current that is activated by membrane hyperpolarization suggests that it may be involved in resetting the membrane potential following strong hyperpolarizing stimuli. This characteristic could permit Ih to participate in regulating the pacemaker activity of DA neurons, although its precise role in this regard remains to be determined (17, 53–55, 57). Alternatively, since Ih makes such a large contribution to cellular conductance at hyperpolarized membrane potentials, the reduction in this current also increased the input resistance of MitoPark DA neurons, and this would tend to make these neurons more responsive to all synaptic inputs. As a result, for example, a strong inhibitory signal might have a larger effect in neurons with compromised Ih, and the neurons would less effectively recover from this inhibition.
Although HCN channel function was greatly reduced in MitoPark DA neurons, mRNA levels for the isoforms most prominently expressed in DA-rich midbrain areas (HCN-3 and HCN-1) were similar to those of the control mice. This suggests that Ih channel transcription was not compromised in MitoPark DA neurons but rather that channel function was post-translationally affected by Tfam gene deletion. In general, the absence of Ih in the SNc DA neurons appears to be one of the earliest and most prominent physiological changes observed in the MitoPark mouse, and this functional change occurs before the appearance of parkinsonian symptoms.
In summary, the present study demonstrates that disruption of mitochondrial function by selective deletion of the Tfam gene in midbrain DA neurons results in physiological changes in the nigrostriatal circuitry that occur before the onset of locomotor impairments. We hypothesize that the changes observed with this animal model are similar to those occurring in the nigrostriatal circuitry of humans afflicted with idiopathic PD, and we anticipate that a more complete understanding of these early functional changes will aid in the design of therapeutic interventions designed to slow the progress of the disease.
Acknowledgments
This work supported by the U.S. National Institutes of Health, the National Institute on Drug Abuse Intramural Research Program, the Swedish Research Council, the Swedish Brain Foundation, Hållsten's Foundation, the Swedish Parkinson's Foundation, Swedish Brain Power, the Michael J. Fox Foundation, and the Karolinska Institute. N.-G.L. and L.O. are co-owners of a company owning commercial rights to the MitoPark mice.
REFERENCES
- 1. Dauer W., Przedborski S. (2003) Parkinson's disease: mechanisms and models. Neuron 39, 889–909 [DOI] [PubMed] [Google Scholar]
- 2. Mizuno Y., Sone N., Saitoh T. (1987) Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J. Neurochem. 48, 1787–1793 [DOI] [PubMed] [Google Scholar]
- 3. Sherer T. B., Betarbet R., Testa C. M., Seo B. B., Richardson J. R., Kim J. H., Miller G. W., Yagi T., Matsuno-Yagi A., Greenamyre J. T. (2003) Mechanism of toxicity in rotenone models of parkinson's disease. J. Neurosci. 23, 10756–10764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smeyne R. J., Jackson-Lewis V. (2005) The MPTP model of Parkinson's disease. Mol. Brain Res. 134, 57–66 [DOI] [PubMed] [Google Scholar]
- 5. Bender A., Krishnan K. J., Morris C. M., Taylor G. A., Reeve A. K., Perry R. H., Jaros E., Hersheson J. S., Betts J., Klopstock T., Taylor R. W., Turnbull D. M. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 38, 515–517 [DOI] [PubMed] [Google Scholar]
- 6. Kraytsberg Y., Kudryavtseva E., McKee A. C., Geula C., Kowall N. W., Khrapko K. (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 38, 518–520 [DOI] [PubMed] [Google Scholar]
- 7. Westerlund M., Hoffer B., Olson L. (2010) Parkinson's disease: Exit toxins, enter genetics. Prog. Neurobiol. 90, 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terzioglu M., Galter D. (2008) Parkinson's disease: genetic versus toxin-induced rodent models. FEBS J. 275, 1384–1391 [DOI] [PubMed] [Google Scholar]
- 9. Ekstrand M. I., Terzioglu M., Galter D., Zhu S., Hofstetter C., Lindqvist E., Thams S., Bergstrand A., Hansson F. S., Trifunovic A., Hoffer B., Cullheim S., Mohammed A. H., Olson L., Larsson N. G. (2007) Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. U. S. A. 104, 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsson N. G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G. S., Clayton D. A. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 [DOI] [PubMed] [Google Scholar]
- 11. Galter D., Pernold K., Yoshitake T., Lindqvist E., Hoffer B., Kehr J., Larsson N. G., Olson L. (2010) MitoPark mice mirror the slow progression of key symptoms and L-DOPA response in Parkinson's disease. Genes Brain Behav. 9, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson T. M., Ko H. S., Dawson V. L. (2010) Genetic animal models of Parkinson's disease. Neuron 66, 646–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beal M. F. (2010) Parkinson's disease: a model dilemma. Nature 466, S8–S10 [DOI] [PubMed] [Google Scholar]
- 14. Leenders K. L., Salmon E. P., Tyrrell P., Perani D., Brooks D. J., Sager H., Jones T., Marsden C. D., Frackowiak R. S. (1990) The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson's disease. Arch. Neurol. 47, 1290–1298 [DOI] [PubMed] [Google Scholar]
- 15. Agid Y. (1991) Parkinson's disease: pathophysiology. Lancet 337, 1321–1324 [DOI] [PubMed] [Google Scholar]
- 16. Mayer M. L., Westbrook G. L. (1983) A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J. Physiol. 340, 19–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maccaferri G., McBain C. J. (1996) The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J. Physiol. 497, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watts A. E., Williams J. T., Henderson G. (1996) Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J. Neurophysiol. 76, 2262–2270 [DOI] [PubMed] [Google Scholar]
- 19. Svoboda K. R., Lupica C. R. (1998) Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J. Neurosci. 18, 7084–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dagerlind A., Friberg K., Bean A. J., Hokfelt T. (1992) Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry 98, 39–49 [DOI] [PubMed] [Google Scholar]
- 21. Wang J., Wilhelmsson H., Graff C., Li H., Oldfors A., Rustin P., Bruning J. C., Kahn C. R., Clayton D. A., Barsh G. S., Thoren P., Larsson N. G. (1999) Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 21, 133–137 [DOI] [PubMed] [Google Scholar]
- 22. Chen Y. H., Harvey B. K., Hoffman A. F., Wang Y., Chiang Y. H., Lupica C. R. (2008) MPTP-induced deficits in striatal synaptic plasticity are prevented by glial cell line-derived neurotrophic factor expressed via an adeno-associated viral vector. FASEB J. 22, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X., Hoffman A. F., Peng X. Q., Lupica C. R., Gardner E. L., Xi Z. X. (2009) Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology 204, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawagoe K. T., Zimmerman J. B., Wightman R. M. (1993) Principles of voltammetry and microelectrode surface states. J. Neurosci. Methods 48, 225–240 [DOI] [PubMed] [Google Scholar]
- 25. Sabeti J., Adams C. E., Burmeister J., Gerhardt G. A., Zahniser N. R. (2002) Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J. Neurosci. Methods 121, 41–52 [DOI] [PubMed] [Google Scholar]
- 26. Franklin K. B. J, Paxinos G. (1997) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA, USA [Google Scholar]
- 27. Parsons L. H., Smith A. D., Justice J. B., Jr. (1991) The in vivo microdialysis recovery of dopamine is altered independently of basal level by 6-hydroxydopamine lesions to the nucleus accumbens. J. Neurosci. Methods 40, 139–147 [DOI] [PubMed] [Google Scholar]
- 28. Justice J. B., Jr. (1993) Quantitative microdialysis of neurotransmitters. J. Neurosci. Methods 48, 263–276 [DOI] [PubMed] [Google Scholar]
- 29. Jiang Z. G., Pessia M., North R. A. (1993) Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J. Physiol. 462, 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonci A., Grillner P., Mercuri N. B., Bernardi G. (1998) L-type calcium channels mediate a slow excitatory synaptic transmission in rat midbrain dopaminergic neurons. J. Neurosci. 18, 6693–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris N. C., Constanti A. (1995) Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J. Neurophysiol. 74, 2366–2378 [DOI] [PubMed] [Google Scholar]
- 32. Smith A. D., Justice J. B. (1994) The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J. Neurosci. Methods 54, 75–82 [DOI] [PubMed] [Google Scholar]
- 33. Chefer V. I., Zapata A., Shippenberg T. S., Bungay P. M. (2006) Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J. Neurosci. Methods 155, 187–193 [DOI] [PubMed] [Google Scholar]
- 34. Backman C. M., Malik N., Zhang Y., Shan L., Grinberg A., Hoffer B. J., Westphal H., Tomac A. C. (2006) Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44, 383–390 [DOI] [PubMed] [Google Scholar]
- 35. Jones S. R., Gainetdinov R. R., Jaber M., Giros B., Wightman R. M., Caron M. G. (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. U. S. A. 95, 4029–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grace A. A., Bunney B. S. (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-01. Identification and characterization. Neuroscience 10, 301–315 [DOI] [PubMed] [Google Scholar]
- 37. Hyland B. I., Reynolds J. N., Hay J., Perk C. G., Miller R. (2002) Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114, 475–492 [DOI] [PubMed] [Google Scholar]
- 38. Schultz W. (2007) Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 [DOI] [PubMed] [Google Scholar]
- 39. Zigmond M. J., Stricker E. M. (1973) Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science 182, 717–720 [DOI] [PubMed] [Google Scholar]
- 40. Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neuro. Sci. 20, 415–455 [DOI] [PubMed] [Google Scholar]
- 41. Zigmond M. J., Abercrombie E. D., Berger T. W., Grace A. A., Stricker E. M. (1990) Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 13, 290–296 [DOI] [PubMed] [Google Scholar]
- 42. Zigmond M. J., Berger T. W., Grace A. A., Stricker E. M. (1989) Compensatory responses to nigrostriatal bundle injury. Studies with 6-hydroxydopamine in an animal model of parkinsonism. Mol. Chem. Neuropathol. 10, 185–200 [DOI] [PubMed] [Google Scholar]
- 43. Hollerman J. R., Grace A. A. (1990) The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 533, 203–212 [DOI] [PubMed] [Google Scholar]
- 44. Stachowiak M. K., Keller R. W., Jr., Stricker E. M., Zigmond M. J. (1987) Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J Neurosci. 7, 1648–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castaneda E., Whishaw I. Q., Robinson T. E. (1990) Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J. Neurosci. 10, 1847–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garris P. A., Walker Q. D., Wightman R. M. (1997) Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 753, 225–234 [DOI] [PubMed] [Google Scholar]
- 47. Watabe M., Nakaki T. (2008) mitochondrial complex i inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol. Pharmacol. 74, 933–940 [DOI] [PubMed] [Google Scholar]
- 48. Guatteo E., Marinelli S., Geracitano R., Tozzi A., Federici M., Bernardi G., Mercuri N. B. (2005) Dopamine-containing neurons are silenced by energy deprivation: a defensive response or beginning of cell death? Neurotoxicology 26, 857–868 [DOI] [PubMed] [Google Scholar]
- 49. Mercuri N. B., Bonci A., Calabresi P., Stratta F., Bernardi G. (1994) Responses of rat mesencephalic dopaminergic neurons to a prolonged period of oxygen deprivation. Neuroscience 63, 757–764 [DOI] [PubMed] [Google Scholar]
- 50. Mercuri N. B., Bonci A., Johnson S. W., Stratta F., Calabresi P., Bernardi G. (1994) Effects of anoxia on rat midbrain dopamine neurons. J. Neurophysiol. 71, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 51. Heien M. L., Khan A. S., Ariansen J. L., Cheer J. F., Phillips P. E., Wassum K. M., Wightman R. M. (2005) Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. U. S. A. 102, 10023–10028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sombers L. A., Beyene M., Carelli R. M., Wightman R. M. (2009) Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J. Neurosci. 29, 1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Biel M., Schneider A., Wahl C. (2002) Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc. Med. 12, 206–213 [DOI] [PubMed] [Google Scholar]
- 54. Robinson R. B., Siegelbaum S. A. (2003) Hyperoplarization-activated cation currents: from molecules to physiological function. Ann. Rev. Physiol. 65, 453–480 [DOI] [PubMed] [Google Scholar]
- 55. Wahl-Schott C., Biel M. (2009) HCN channels: structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 66, 470–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mercuri N. B., Bonci A., Calabresi P., Stefani A., Bernardi G. (1995) Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur. J. Neurosci. 7, 462–469 [DOI] [PubMed] [Google Scholar]
- 57. McCormick D. A., Pape H. C. (1990) Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 431, 291–318 [DOI] [PMC free article] [PubMed] [Google Scholar]