Abstract
The mechanisms used by the immune system to discriminate between pathogenic and commensal bacteria have remained largely unclear. Recently, we have shown that virulence of Staphylococcus aureus depends on secretion of phenol-soluble modulin (PSM) peptides that disrupt neutrophils at micromolar concentrations. Moreover, all S. aureus PSMs stimulate and attract neutrophils at nanomolar concentrations via interaction with the formyl-peptide receptor 2 (FPR2). Here, we demonstrate that FPR2 allows neutrophils to adjust their responses in relation to the aggressiveness of staphylococcal species, which differ largely in their capacity to infect or colonize humans and animals. PSM-related peptides were detected in all human and animal pathogenic staphylococci, but were absent from most commensal species. Three PSMβ-like peptides produced by the serious human pathogen Staphylococcus lugdunensis were identified as the previously described S. lugdunensis-synergistic hemolysins (SLUSHs). SLUSHs attracted and stimulated human leukocytes in a FPR2-dependent manner, indicating that FPR2 is a general receptor for all PSM-like peptide toxins. Remarkably, the release of PSMs correlated closely with the apparent capacity of staphylococcal species to cause invasive infections and with their ability to activate FPR2. These findings suggest that the innate immune system may be able to respond in different ways to pathogenic or innocuous staphylococci by monitoring the presence of PSMs via FPR2.—Rautenberg, M., Joo, H. S., Otto, M., Peschel, A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence.
Keywords: pathogen-associated molecular patterns, coagulase-negative staphylococci, skin microbiota, neutrophil chemotaxis
The antimicrobial defense of the immune system involves complex interactions between host and infecting pathogen. The innate immune system can sense conserved microbial molecules, which are referred to as pathogen-associated molecular patterns (PAMPs). PAMPs are recognized by Toll-like and other receptors to initiate inflammation and host defense (1). Neutrophils are the first leukocytes that infiltrate affected tissues. They are recruited from the bloodstream to the site of infection by gradients of chemotactic factors. Such molecules may be released by microorganisms or produced by immune or other host cells in contact with infecting agents. Most microbial chemoattractants are sensed by the family of formyl peptide receptors (FPRs), which belong to the G protein-coupled 7-transmembrane receptors (GPCRs) (2). The human FPR family consists of FPR1, FPR2 (previously known as the FPR-like 1), and FPR3 (2–4). FPR1 and FPR2 are expressed on monocytes, macrophages, neutrophils, immature dendritic cells, and some other cell types, while FPR3 is only present on monocytes and macrophages (5). FPR1 is a high-affinity receptor for bacterial formylated peptides, such as the prototype fMLF peptide (4, 6). Because formylated peptides are released from bacteria, FPR1 is of crucial importance for the recognition of invading microbes by the innate immune system (7, 8). In contrast, FPR2 has only residual affinity for formylated peptides and is known to recognize a variety of virtually unrelated endogenous molecules, such as the lipid lipoxin A4, the host defense peptide LL37, and split products derived from host proteins, such as serum amyloid A, brain amyloid precursor, or annexin 1, which may act in a proinflammatory or anti-inflammatory fashion (5, 9–11).
We have recently demonstrated that FPR2 also senses a new class of Staphylococcus aureus peptide toxins named phenol-soluble modulins (PSMs) (12). Two classes of PSMs can be distinguished according to peptide mass (13, 14). The larger β-type PSMs have a length of ∼44 aa, while the α-type PSMs are shorter (20–26 aa) and, among others, include the peptides that are encoded in the S. aureus psmα operon. α-type PSMs also comprise δ-toxin, a well-known peptide toxin of S. aureus with homologues in other staphylococci. PSMs are secreted by an unknown mechanism in their formylated form but may also occur as unformylated peptides depending on the activity of staphylococcal peptide deformylase (15). Certain PSMs also exert antimicrobial activity in cooperation with host defense peptides (16). PSMs are also produced by the opportunistic pathogen Staphylococcus epidermidis (13, 17, 18), but it has remained unclear whether these peptides are also sensed by FPR2.
S. epidermidis and other coagulase-negative staphylococci (CoNS) are frequent causes of nosocomial and community-acquired infections such as implant-associated bacteremia, endocarditis, and sepsis (19–21). In contrast to the aggressive coagulase-positive S. aureus, which uses several effective strategies to counteract the immune system (22, 23), most CoNS have only small numbers of virulence genes and cause less severe infections (24). The CoNS species Staphylococcus lugdunensis was first described by Freney et al. (25) in 1988. It is a component of the normal human skin flora colonizing preferentially the perineal region (26) and, with lower frequency, the anterior nares or nasal cavities (27). S. lugdunensis can cause severe infections such as urinary tract infections, peritonitis, osteomyelitis, skin and soft tissue infections, and native or prosthetic valve endocarditis (28). Among the CoNS, S. lugdunensis is rather unusual, since virulence and severity of S. lugdunenesis infections are comparable to those of many S. aureus strains.
In 1997, Donvito et al. (29) observed a synergistic hemolytic activity in S. lugdunensis, which was phenotypically similar to that of S. aureus δ-toxin. This activity was mediated by three 43-aa peptides named S. lugdunensis synergistic hemolysin (SLUSH)-A, SLUSH-B, and SLUSH-C. The 3 peptides act synergistically with the S. aureus β-hemolysin, leading to zones of complete hemolysis inside the zone of incomplete hemolysis. The SLUSH peptides are encoded by the slush operon, which is controlled by agr activity (30). Notably, possible effects of SLUSH peptides on immune cells, such as neutrophils, have not been investigated.
In the present study, we analyzed 17 staphylococcal species for production of PSM-like peptides and corresponding stimulation of FPR2. Interestingly, we found that all human and animal pathogens secrete such peptides while most commensal species lack PSM production. The masses of PSM-related peptides in the culture supernatant of S. lugdunensis corresponded exactly to those of the 3 SLUSH peptides, which shared profound similarity with β-PSMs. We demonstrate that SLUSHs are chemotactic for human neutrophils and are efficient ligands of FPR2. Moreover, staphylococcal species that secreted PSM-like peptides elicited profound FPR2-mediated responses in leukocytes.
MATERIALS AND METHODS
Synthetic peptides
SLUSH-A, SLUSH-B, and SLUSH-C were synthesized by EMC Microcollections (Tübingen, Germany) and by Bachem (Weil am Rhein, Germany), respectively, according to the published sequences (29). The FPR2 inhibitor WRW4 (WRWWWW; ref. 31) was synthesized by EMC Microcollections. Peptides were high-pressure liquid chromatography (HPLC) purified to yield final purities ≥80%.
Detection and analysis of PSM peptides in bacterial culture supernatants
Bacterial culture supernatants were grown for 17 h in tryptic soy broth (TSB) and were analyzed by reversed-phase HPLC mass spectrometry (RP-HPLC-MS) on an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA) essentially as described previously (18), with a slightly shortened gradient [injection at buffer A: 0.1% TFA/water; wash with 10% buffer B (0.1% TFA/acetonitrile) for 2 min; elution with 50% B, 2 min; 50–100% B, 5 min, 100% B, 4 min; reequilibration 0% B, 3 min; everything at 0.5 ml/min]. Peptide masses were calculated from multiply charged ions obtained by the coupled electrospray ionization-equipped LC/MSD Trap SL mass spectrometer using Agilent LC/MSD 5.2 software.
Protein sequence analysis and alignment
SLUSHs were aligned with other PSMβ-like peptides using DNASIS MAX 2.05 software (Hitachi Software Engineering, Tokyo, Japan). Helical wheel computation of SLUSH-A was performed using an online application (http://cti.itc.virginia.edu/%7Ecmg/Demo/wheel/wheelApp.html).
Bacterial strains, culture supernatants, and cell lines
Most of the bacterial strains used were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). S. lugdunensis IVK28 is a human nasal isolate. Bacterial culture supernatants were obtained by centrifugation of overnight cultures grown in TSB and filtered through 0.2-μm-pore filters. No neutrophil-stimulatory activity was detected in noninoculated medium at relevant concentrations. HL60 cells stably transfected with human FPR1, FPR2, and FPR3 have been described recently (32, 33). These cell lines were grown in RPMI medium (Biochrom, Cambridge, UK) supplemented with 10% FCS (Sigma-Aldrich, St. Louis, MO, USA), 20 mM HEPES (Biochrom), penicillin (100 U/ml), streptomycin (100 μg/ml; Gibco, Rockville, MD, USA), and 1× Glutamax (Gibco). Transfected cells were cultivated in the presence of G418 (Biochrom) at a final concentration of 1 mg/ml.
Cell lysis
Lysis of neutrophils by SLUSH peptides was measured by release of cytoplasmic lactate dehydrogenase (Cytotoxicity Detection Kit; Roche, Mannheim, Germany), as described recently (14).
Neutrophil chemotaxis
Human neutrophils were isolated from freshly obtained blood by standard Ficoll/Histopaque gradient centrifugation. Chemotaxis of neutrophils toward synthetic SLUSH peptides was determined using fluorescence-labeled neutrophils that migrated through a 3-μm-pore polycarbonate transwell filter, as described recently (8). The fluorescence measured was corrected for the buffer control (only buffer added to the lower compartment).
Measurement of calcium ion flux in human neutrophils and HL60 cells
Calcium flux was measured by stimulating cells labeled with Fluo-3-AM (Molecular Probes, Eugene, OR, USA). Fluorescence was monitored with a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), as described previously (34). To measure the influence of the chemotaxis-inhibitory protein of S. aureus (CHIPS), a 14.1-kDa bacteriophage-encoded protein found in a majority of S. aureus isolates and WRW4, 1 × 106 cells/ml were preincubated with CHIPS or WRW4 at a final concentration of 1.4 μg/ml or 10 μM, respectively, for 20 min under slight agitation at room temperature. To stimulate neutrophils and HL60 cells, synthetic SLUSH peptides were used at concentrations of 0.25, 0.5, 1, and 2 μM. Culture supernatants were used at dilutions ranging from 0.125–1%. Measurement of 2000 events was performed. Calcium flux was expressed as relative fluorescence.
Statistical analyses
Statistical analyses were performed with the Prism 4.0 package (GraphPad Software, San Diego, Ca, USA), and the between-group differences were analyzed for significance with the 2-tailed Student's t test unless otherwise noted.
RESULTS
S. lugdunensis and many other pathogenic staphylococcal species release PSM-like peptides
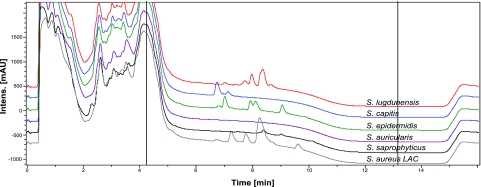
In previous work, we reported that human FPR2 senses S. aureus PSM peptides (12) and that PSM production determines to a large extent the virulence of S. aureus (14, 35). Since PSM peptides are known to be also released by S. epidermidis (13, 17, 18), we investigated a larger variety of the CoNS species for release of PSM-like peptides. The amphipathic and α-helical nature of PSMs leads to a characteristic elution behavior in RP-HPLC that allows identification of the peptides in culture supernatants of overnight cultures (ref. 18 and Fig. 1). Masses of detected peaks were determined by mass spectrometry and compared to those of known S. aureus and S. epidermidis PSMs. We detected peptides with masses similar to those of PSMβ peptides (4.3–4.7 kDa) in culture supernatants from most human and animal pathogens except for the two tested Staphylococcus warneri, one of two tested Staphylococcus capitis, and two of three tested Staphylococcus saprophyticus strains (Table 1). Similarly, peptides with smaller masses in the range of α-type PSMs (2.2–3.0 kDa) were produced by all species that regularly cause infections in humans or animals. Of note, most staphylococcal species with commensal life style did not produce PSM-related peptides, with Staphylococcus cohnii being the only exception. To analyze possible variations in PSM production by a given CoNS species, we analyzed two or three different strains of some species and found considerable differences in the production of PSM-related peptides, which may be due to major intraspecies alterations in the abundance and expression of relevant genes. This notion is in accordance with our recent data on variation of PSM expression levels in S. aureus (14), which is likely to result, at least in part, from mutations in the agr regulatory system that has been shown to control PSM production in S. aureus (35) and S. epidermidis (17). In several strains, peptide pairs with mass differences of 28 kDa were detected, which were regarded as putative N-formylated and N-deformylated variants of a particular PSM peptide (Table 1).
Figure 1.
HPLC spectra of selected staphylococcal culture supernatants. Peaks between ∼6 and 10 min correspond to PSM peptides, which are known to elute at these positions under the experimental conditions.
Table 1.
Detection of peptides with PSM-related chromatographic behavior in culture supernatants of Staphylococcus species
| Species and strain | Detected peptide mass (kDa) |
||
|---|---|---|---|
| β-PSM range, 4.3–4.7 | α-PSM range, 2.2–3.0 | Other peptides | |
| Aggressive human pathogens | |||
| S. aureus USA300 | 4.53 (PSM-β1)a | 2.26 (PSM-α1)a | ND |
| 4.49 (PSM-β2)a | 2.28 (PSM-α2)a | ||
| 2.61 (PSM-α3)a | |||
| 2.17 (PSM-α4)a | |||
| 2.98 (δ-toxin)a | |||
| S. lugdunensis IVK28 | 4.43 (SLUSH-A)a | 2.75 (OrfX)a | ND |
| 4.32 (SLUSH-B) | 3.05 | ||
| 4.53 (SLUSH-C)a | |||
| S. lugdunensis ATCC43809 | 4.57 | 2.55 | ND |
| Opportunistic human pathogens | |||
| S. capitis ATCC27840 | 4.56 | 2.45 | 1.27 |
| 4.60 | 2.55 | 3.63 | |
| 4.64 | 2.61a | 3.87 | |
| 2.65 | |||
| 2.94 | |||
| S. capitis ATCC49324 | ND | 2.62a | 3.71 |
| 2.91a | |||
| 2.62 | |||
| 2.63 | |||
| S. epidermidis Tü3298 | 4.64 (PSM-β1)a | 2.33 (PSM-α)a | ND |
| 4.64 (PSM-β2)a | 2.62 (PSM-δ)a | ||
| 2.77 (PSM-ε)a | |||
| 2.82 (δ-toxin)a | |||
| S. haemolyticus ATCC29970 | 4.58 | 2.64 | ND |
| 4.73 | 2.66 | ||
| 4.75 | 2.93 | ||
| S. saprophyticus NT219 | 4.57 | 2.89 | ND |
| 2.71 | |||
| S. saprophyticus ATCC35552 | ND | 2.71 | ND |
| S. saprophyticus ATCC15305 | ND | ND | ND |
| S. warneri ATCC17917 | ND | 2.34 | ND |
| 2.56a | |||
| 2.76a | |||
| 2.87a | |||
| S. warneri ATCC49518 | ND | 2.64 | ND |
| 2.98a | |||
| Animal pathogens | |||
| S. intermedius ATCC49052 | 4.54 | 2.72a | ND |
| 2.77 | |||
| S. schleiferi ATCC43808 | 4.73 | 2.58 | ND |
| 2.61a | |||
| 2.67 | |||
| 2.66 | |||
| 2.90a | |||
| 2.98 | |||
| S. simulans ATCC31432 | ND | 2.45 | ND |
| 2.74 | |||
| 2.88a | |||
| 2.93 | |||
| S. simulans ATCC700576 | 4.67 | 2.25 | ND |
| 2.45 | |||
| 2.49 | |||
| 2.65 | |||
| 2.66 | |||
| 2.80 | |||
| 2.82a | |||
| S. simulans ATCC27848 | ND | 2.45 | ND |
| 2.71a | |||
| 2.87a | |||
| Commensals rarely causing infections | |||
| S. auricularis ATCC337535 | ND | ND | ND |
| S. caprae ATCC51548 | ND | ND | ND |
| S. cohnii ATCC49328 | 4.46 | ND | ND |
| S. cohnii ATCC29972 | 4.46 | 2.01 | ND |
| 4.48 | 2.41 | ||
| 2.69 | |||
| S. hominis ATCC27844 | ND | ND | ND |
| S. hominis ATCC25615 | ND | ND | ND |
| S. pulveri ATCC51698 | ND | ND | ND |
| S. xylosus ATCC49148 | ND | ND | ND |
| S. xylosus ATCC29966 | ND | ND | ND |
| S. saccharolyticus ATCC14953 | ND | ND | ND |
ND, no peptides detectable.
N-deformylated identified in addition to N-formylated peptides.
The masses of β-type PSMs found in culture supernatants from S. lugdunensis corresponded exactly to those of the three previously described SLUSH peptides. Nonformylated variants of SLUSH A and C were also detected. In addition, two smaller peptides with the masses of 2.75 kDa and 3.05 kDa were identified. The former corresponded exactly to the mass of formylated OrfX peptide, which is also encoded in the SLUSH operon (30), while the gene encoding the latter peptide remains unknown.
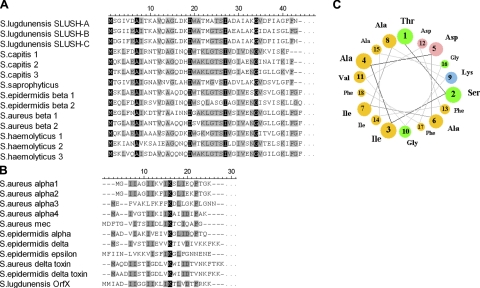
When the amino acid sequences of S. lugdunensis SLUSH peptides were compared with those of S. aureus and S. epidermidis β-PSMs, we noted strong similarities of 35.9 to 45.2% (Fig. 2A), indicating that SLUSH peptides belong to the β-type PSM family. We also analyzed the available staphylococcal genomes for the presence of β-psm-related peptide genes and identified closely related peptides in S. capitis, Staphylococcus haemolyticus, S. saprophyticus, and S. warneri (Fig. 2A). The Staphylococcus hominis genome lacks such peptide genes, which is in agreement with the absence of peptides in the size range of 4.3–4.7 kDa in supernatants of this species (Table 1). The homologues of PSMβ peptides encoded in the S. haemolyticus genome were the previously described peptides with gonococcal growth inhibitory activity (36). However, the published masses did not match exactly with the masses that we detected in S. haemolyticus culture supernatants, which may be due to strain-specific sequence differences. The previously described α-type PSMs of S. aureus (PSMα1, PSMα2, PSMα3, PSMα4, δ-toxin, and PSM-mec), S. epidermidis (PSMα, PSMδ, PSMε, and δ-toxin), and the S. lugdunensis OrfX peptide shared some sequence similarity (Fig. 2B), although to a much lower degree compared to members of the β-type PSM class. This limited conservation precluded the identification of further α-type PSM genes in the available staphylococcal genomes. Collectively, these data indicate that the production of PSM peptides is a frequent trait of the genus Staphylococcus and appears to be associated with the capacity of staphylococcal species to cause infections.
Figure 2.
Similarities and secondary structure prediction of SLUSH peptides. Alignment of SLUSH peptides with other staphylococcal PSM-β peptides (A) and staphylococcal PSM-α peptides (B). Partially and completely conserved positions are boxed in gray and black, respectively. A) The following proteins were compared: (SwissProt accession numbers are given in parentheses): S. lugdunensis SLUSH-A (P95769), S. lugdunensis SLUSH-B (P95770), S. lugdunensis SLUSH-C (P95771), S. capitis 1 (B9CTG4), S. capitis 2 (B9CTG7), S. capitis 3 (B9CTG8), S. saprophyticus (Q4A0W3), S. epidermidis PSMβ1 (Q5HQ19), S. epidermidis PSMβ2 (Q5HQ20), S. aureus PSMβ1 (Q2FHR4), S. aureus PSMβ2 (Q2FHR3), S. haemolyticus 1 (P11697), S. haemolyticus 2 (P11698), S. haemolyticus 3 (P116999), S. warneri (C4W8T1). B) S. aureus PSMα1 (A9JX05), S. aureus PSMα2 (A9JX06), S. aureus PSMα3 (A9JX07), S. aureus PSMα4 (A9JX08), S. aureus PSM-mec (D2EBB2), S. epidermidis PSMα (Q5HRV9), S. epidermidis PSMδ (no SwissProt accession number available), S. epidermidis PSMε (no SwissProt accession number available), S. aureus δ-toxin (D1GPU5), S. epidermidis δ-toxin (Q79MA7), S. lugdunensis OrfX (no SwissProt accession number available). C) Helical wheel computation of positions 25 to 42 of SLUSH-A. Colors represent amino acid charges as follows: yellow, nonpolar; green, polar and uncharged; pink, acidic; blue, basic.
SLUSH peptides are chemoattractants for human neutrophils stimulating the FPR2 receptor
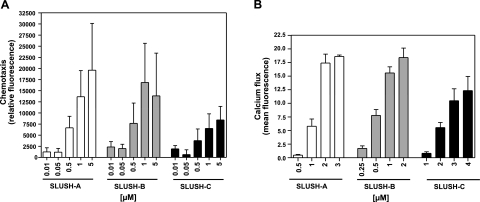
We found that S. lugdunensis SLUSH peptides have similar lengths (43–44 aa), anionic net charges (−1 to −3), and α-helical, amphiphatic properties as the S. aureus or S. epidermidis PSMβ peptides (Fig. 2), suggesting that all these peptides may share relevant biological activities. To study whether SLUSH peptides have neutrophil-stimulating or damaging activity, we incubated synthetic N-formylated SLUSH peptides A, B, and C with primary human neutrophils. The 3 peptides exhibited only weak cytotoxic activity at concentrations above 4 μM (SLUSH C) or 6 μM (SLUSH A and B) (data not shown). This finding is in agreement with previous studies, which found only minor cytotoxic activities in β-type PSMs (14, 37) or detected considerable cytotoxic activities of SLUSH peptides only in synergy with staphylococcal β-toxin (30). However, the 3 SLUSH peptides induced chemotactic migration and chemotaxis-associated calcium ion fluxes in human neutrophils in a dose-dependent manner at concentrations below 1 μM (Fig. 3), which demonstrates that SLUSH peptides represent neutrophil attractants and elicit similar responses in neutrophils as S. aureus PSMβ peptides.
Figure 3.
Chemotactic and stimulatory activities of SLUSH peptides toward human neutrophils. A) SLUSH peptides induce chemotaxis in human neutrophils. B) SLUSH peptides induce chemotaxis-associated calcium fluxes in human neutrophils. Data represent means ± se of ≥ independent experiments. Buffer controls were subtracted from all values.
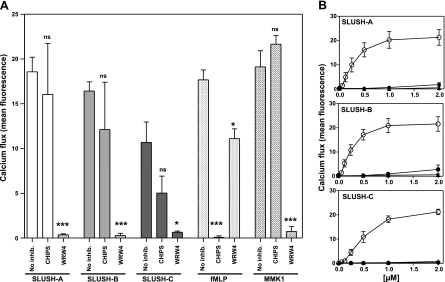
Bacterial formylated peptides and PSM peptides have been shown to stimulate neutrophils via the human FPR1 and FPR2 receptors, respectively (8, 38). As SLUSH peptides bear N-terminal formylated methionine residues and are related to PSMs, we analyzed which member of the FPR receptor family may be responsible for the proinflammatory SLUSH activities. A specific inhibitor of human FPR1, CHIPS (34), had no effect on the ability of SLUSH-A and SLUSH-B or only a minor effect on the ability of SLUSH-C to induce calcium fluxes in neutrophils (Fig. 4A), indicating that FPR1 does not play a major role in neutrophil stimulation by SLUSH peptides. However, the FPR2 inhibitor WRW4 (31) blocked neutrophil responses to all three SLUSH peptides completely indicating that neutrophils sense SLUSH peptides mainly via FPR2 (Fig. 4A).
Figure 4.
The FPR2 receptor is responsible for stimulation of neutrophils by SLUSH peptides. A) Neutrophil stimulation with or without the FPR1- or FPR2-specific inhibitors CHIPS or WRW4, respectively. fMLP and MMK1 are synthetic control ligands of FPR1 and FPR2, respectively. Data represent means ± se of ≥3 independent experiments. *P < 0.05; ***P < 0.001 vs. no inhibition. B) Stimulation of FPR1, FPR2, and FPR3-transfected HL60 cells by SLUSH peptides. Untransfected HL60 cells exhibited no response (mean fluorescence values <1, data not shown). Open circles denote FPR2-transfected HL60 cells; solid circles denote FPR1-transfected HL60 cells; triangles denote FPR3-transfected HL60 cells. Data represent means ± se of ≥3 independent experiments.
To substantiate these results, we stimulated human HL60 cell lines stably transfected with FPR1, FPR2, or FPR3. While untransfected (data not shown) or FPR3-transfected HL60 were almost unresponsive and FPR1-transfected cells exhibited only very weak responses, FPR2-transfected cells exhibited strong calcium ion fluxes in response to the 3 SLUSH peptides at concentrations of 0.2 μM or higher (Fig. 4B), confirming that the human FPR2 receptor detects S. lugdunensis SLUSH peptides very efficiently.
The ability to activate FPR2 correlates with the release of PSM-related peptides and with the pathogenicity of staphylococcal species
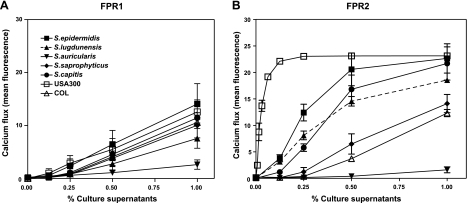
The widespread expression of PSM-like peptides in CoNS that we observed suggests that FPR2 may respond to all these species in relation to PSM release. To evaluate this hypothesis, we selected representative aggressive staphylococcal species (S. aureus, S. lugdunensis), opportunistic pathogens (S. epidermidis, S. capitis, S. saprophyticus), and commensals (Staphylococcus auricularis) and studied their capacity to stimulate leukocytes via FPR2. Culture supernatants of the various species were incubated with receptor-transfected HL60 cells and FPR1-, FPR2-, or FPR3-dependent induction of calcium ion fluxes was monitored.
All tested strains induced moderate FPR1-dependent responses at similar levels (Fig. 5), which is in agreement with the release of peptides that retain N-terminal formyl groups and meet the substrate specificity of FPR1 (8). None of the strains led to considerable activation of FPR3-transfected cells (data not shown). However, all strains that were positive in the HPLC-based screening for PSM-like peptides (Table 1) exhibited activity toward FPR2-transfected cells (Fig. 5). The responses varied strongly between the bacterial strains, and, by far, the strongest activity was noted for the highly pathogenic strain S. aureus USA300, a community-associated methicillin-resistant S. aureus (39). The low-pathogenicity, hospital-acquired MRSA clone COL exhibited only weak FPR2-dependent-stimulating activity. S. lugdunensis and other pathogenic species such as S. epidermidis, S. saprophyticus, and S. capitis were rather strong inducers of FPR2-dependent responses, albeit at a lower level than USA300. The commensal S. auricularis, which is only very infrequently associated with infections, exhibited only marginal activity, which is in agreement with the absence of PSM-related peptides in culture supernatants of that species. Taken together, our data indicate that FPR2 can sense many staphylococcal species and induces proinflammatory responses that correlate with the capacity of the strains to release PSM-like peptides and to cause invasive infections.
Figure 5.
Stimulation of receptor-transfected HL60 by staphylococcal culture supernatants. Activation of FPR1-transfected (left panel) or FPR2-transfected cells (right panel) by culture supernatants of the indicated staphylococcal strains was measured. Supernatants were taken from S. lugdunensis IVK28, S. epidermidis Tü3298, S. auricularis ATCC337535, S. saprophyticus NT219, S. capitis ATCC27840, S. aureus USA300, and S. aureus COL. Data represent means ± se of ≥3 independent experiments.
DISCUSSION
There is an ongoing debate about the question of whether microbial molecular “patterns” that are sensed by the innate immune system should be referred to as “pathogen-associated molecular patterns” (PAMPs) or “microbe-associated molecular patterns” (MAMPs) (40), as most known microbial patterns are shared by pathogens and commensals and thus do not allow the immune system to assess the virulence potential of microbes. In our recent study, we showed that by sensing the PSM class of peptide toxins, FPR2 responds specifically to major staphylococcal virulence factors (12), suggesting that FPR2 may represent a sensu strictu PAMP receptor. The data presented herein suggest that FPR2 may equip human neutrophils to sense the pathogenic potential of members of the genus Staphylococcus, thereby enabling the innate immune system to adjust appropriate immune responses.
The specific detection of peptide toxins distinguishes FPR2 from FPR1, which responds to commensals and pathogens with similar efficiency, suggesting that FPR1 might better be referred to as a MAMP receptor. The activation of leukocytes via FPR-like receptors has several proinflammatory consequences, including leukocyte influx, cytokine release, and induction of oxidative burst, which altogether contribute to the clearance of bacterial infection (3). It remains to be investigated whether FPR2 also detects molecules from bacterial pathogens other than staphylococci and may have a general role in antimicrobial host defense in addition to its involvement in endogenous inflammatory processes. Along this line, FPR2 has previously been shown to respond to a peptide ligand from Helicobacter pylori (41) and to a lipid from Leishmania major (42).
In the present study, we demonstrate that the capacity of staphylococcal species to cause infections in humans or animals is strongly correlated with the secretion of PSM-like peptides. While the innocuous commensal S. auricularis elicited hardly any response, there was a gradual increase in FPR2 activation by opportunistically pathogenic staphylococci with by far the strongest response to highly aggressive S. aureus USA300. Our study indicates that PSMs are produced by a major number of staphylococcal species and are thus much more frequent among CoNS than other staphylococcal toxins. PSMs are active as cytolysins and chemoattractants for leukocytes of distantly related hosts, such as man and mouse and are not strictly species specific (12, 14, 43), which distinguishes PSMs from other staphylococcal toxins, such as α-toxin or Panton-Valentine leukocidin and may explain why all tested staphylococcal animal pathogens produced PSMs.
Our discoveries that production of PSM-like peptides and strong activation of FPR2 correspond to the virulence potential of staphylococcal strains further confirm the key role of PSMs for staphylococcal virulence. Accordingly, measuring FPR2 responses may become of importance for assessing the virulence of new clonal lineages of staphylococci, such as the emerging community-associated methicillin-resistant S. aureus strains. S. cohnii produced α and β PSMs, which represents a contrast to the rarely documented occurrence of human S. cohnii infections (19) and suggests that S. cohnii may be more important in infections than currently known. Notably, our knowledge on the frequency of infections caused by specific CoNS species is still limited because most of the widely used diagnostic procedures do not permit a species-specific diagnosis of CoNS infections. Furthermore, note that PSM production and corresponding FPR2 responses vary widely between different strains of a given staphylococcal species, indicating that adaptation to a specific niche of a specific host organism may require an appropriately adjusted level of PSM production. PSM expression has been shown to depend on the global virulence regulator agr in S. aureus and S. epidermidis (17, 35). Mutation of the agr system leading to inactive agr or altered agr activation levels has repeatedly been described (44, 45) and may explain the interstrain differences in PSM secretion and FPR2 activation.
PSMβ peptides have only weak leukocidic properties and exhibit lower chemotactic activity toward neutrophils than most α-type PSM peptides (12, 14). Nevertheless, most staphylococcal strains that were found to produce PSM-like peptides and activated FPR2 produced both α- and β-type PSMs, suggesting that the two types have crucial, maybe distinct, roles in staphylococcal physiology and infection. In accord with this assumption, the relative amounts of α- and β-type PSMs have recently been shown to be different in S. aureus and S. epidermidis and have been correlated with characteristic differences in infections caused by these two species (37). Thus, the biological roles of β PSMs remain poorly defined and need to be elucidated in future studies.
Acknowledgments
The authors thank Nele Nikola for excellent technical help, Jos van Strijp (Medical Microbiology, University Medical Center Utrecht, Utrecht, The Netherlands) for CHIPS, Francois Boulay (Université Joseph Fourier, Grenoble, France) for FPR1 and FPR2-transfected cell lines, and Bernhard Krismer (Interfaculty Institute of Microbiology and Infection Medicine, University of Tuebingen, Tuebingen, Germany) for staphylococcal strains.
This research is supported by grants from the German Research Foundation (SFB685, GRK685, TR34), the German Ministry of Education and Research (SkinStaph Menage), and the IZKF program of the Medical Faculty, University of Tübingen, to A. P. and from the Intramural Program of the National Institutes of Allergy and Infectious Diseases, U.S. National Institutes of Health, to M.O.
REFERENCES
- 1. Medzhitov R. (2007) Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 [DOI] [PubMed] [Google Scholar]
- 2. Fu H., Karlsson J., Bylund J., Movitz C., Karlsson A., Dahlgren C. (2006) Ligand recognition and activation of formyl peptide receptors in neutrophils. J. Leukoc. Biol. 79, 247–256 [DOI] [PubMed] [Google Scholar]
- 3. Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Y., Oppenheim J. J., Wang J. M. (2001) Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 12, 91–105 [DOI] [PubMed] [Google Scholar]
- 5. Migeotte I., Communi D., Parmentier M. (2006) Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 17, 501–519 [DOI] [PubMed] [Google Scholar]
- 6. Boulay F., Tardif M., Brouchon L., Vignais P. (1990) Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem. Biophys. Res. Commun. 168, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 7. Gao J. L., Lee E. J., Murphy P. M. (1999) Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durr M. C., Kristian S. A., Otto M., Matteoli G., Margolis P. S., Trias J., van Kessel K. P., van Strijp J. A., Bohn E., Landmann R., Peschel A. (2006) Neutrophil chemotaxis by pathogen-associated molecular patterns–formylated peptides are crucial but not the sole neutrophil attractants produced by Staphylococcus aureus. Cell. Microbiol. 8, 207–217 [DOI] [PubMed] [Google Scholar]
- 9. Perretti M., Chiang N., La M., Fierro I. M., Marullo S., Getting S. J., Solito E., Serhan C. N. (2002) Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 8, 1296–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fiore S., Maddox J. F., Perez H. D., Serhan C. N. (1994) Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 180, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tiffany H. L., Lavigne M. C., Cui Y. H., Wang J. M., Leto T. L., Gao J. L., Murphy P. M. (2001) Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J. Biol. Chem. 276, 23645–23652 [DOI] [PubMed] [Google Scholar]
- 12. Kretschmer D., Gleske A. K., Rautenberg M., Wang R., Koberle M., Bohn E., Schoneberg T., Rabiet M. J., Boulay F., Klebanoff S. J., van Kessel K. A., van Strijp J. A., Otto M., Peschel A. (2010) Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7, 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehlin C., Headley C. M., Klebanoff S. J. (1999) An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J. Exp. Med. 189, 907–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang R., Braughton K. R., Kretschmer D., Bach T. H., Queck S. Y., Li M., Kennedy A. D., Dorward D. W., Klebanoff S. J., Peschel A., DeLeo F. R., Otto M. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514 [DOI] [PubMed] [Google Scholar]
- 15. Somerville G. A., Cockayne A., Durr M., Peschel A., Otto M., Musser J. M. (2003) Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J. Bacteriol. 185, 6686–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cogen A. L., Yamasaki K., Sanchez K. M., Dorschner R. A., Lai Y., MacLeod D. T., Torpey J. W., Otto M., Nizet V., Kim J. E., Gallo R. L. (2010) Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Invest. Dermatol. 130, 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vuong C., Durr M., Carmody A. B., Peschel A., Klebanoff S. J., Otto M. (2004) Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell. Microbiol. 6, 753–759 [DOI] [PubMed] [Google Scholar]
- 18. Yao Y., Sturdevant D. E., Otto M. (2005) Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J. Infect. Dis. 191, 289–298 [DOI] [PubMed] [Google Scholar]
- 19. Queck S. Y., Otto M. (2008) Staphylococcus epidermidis and other coagulase-negative staphylococci. In Staphylococcus: Molecular Genetics (Lindsay J. ed) pp. 227–255, Caister Academic, Norfolk, UK [Google Scholar]
- 20. Von Eiff C., Peters G., Heilmann C. (2002) Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2, 677–685 [DOI] [PubMed] [Google Scholar]
- 21. Huebner J., Goldmann D. A. (1999) Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50, 223–236 [DOI] [PubMed] [Google Scholar]
- 22. Foster T. J. (2005) Immune evasion by staphylococci. Nat. Rev. Microbiol. 3, 948–958 [DOI] [PubMed] [Google Scholar]
- 23. Rooijakkers S. H., van Kessel K. P., van Strijp J. A. (2005) Staphylococcal innate immune evasion. Trends Microbiol. 13, 596–601 [DOI] [PubMed] [Google Scholar]
- 24. Otto M. (2009) Staphylococcus epidermidis–the ‘accidental’ pathogen. Nat. Rev. Microbiol. 7, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freney J., Brun Y., Des M., Meugnier H., Grimont F., Grimont P. A. D., Nervi C., Fleurette J. (1988) Staphylococcus lugdunensis sp. nov., and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int. J. Syst. Bacteriol. 38, 168–172 [Google Scholar]
- 26. Van der Mee-Marquet N., Achard A., Mereghetti L., Danton A., Minier M., Quentin R. (2003) Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J. Clin. Microbiol. 41, 1404–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen T. T., Kirkeby L. P., Poulsen K., Reinholdt J., Kilian M. (2000) Resident aerobic microbiota of the adult human nasal cavity. APMIS 108, 663–675 [DOI] [PubMed] [Google Scholar]
- 28. Frank K. L., Del Pozo J. L., Patel R. (2008) From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin. Microbiol. Rev. 21, 111–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donvito B., Etienne J., Greenland T., Mouren C., Delorme V., Vandenesch F. (1997) Distribution of the synergistic haemolysin genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol. Lett. 151, 139–144 [DOI] [PubMed] [Google Scholar]
- 30. Donvito B., Etienne J., Denoroy L., Greenland T., Benito Y., Vandenesch F. (1997) Synergistic hemolytic activity of Staphylococcus lugdunensis is mediated by three peptides encoded by a non-agr genetic locus. Infect. Immun. 65, 95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin E. H., Lee H. Y., Kim S. D., Jo S. H., Kim M. K., Park K. S., Lee H., Bae Y. S. (2006) Trp-Arg-Trp-Trp-Trp-Trp antagonizes formyl peptide receptor like 2-mediated signaling. Biochem. Biophys. Res. Commun. 341, 1317–1322 [DOI] [PubMed] [Google Scholar]
- 32. Christophe T., Karlsson A., Dugave C., Rabiet M. J., Boulay F., Dahlgren C. (2001) The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J. Biol. Chem. 276, 21585–21593 [DOI] [PubMed] [Google Scholar]
- 33. Dahlgren C., Christophe T., Boulay F., Madianos P. N., Rabiet M. J., Karlsson A. (2000) The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-DMet activates neutrophils preferentially through the lipoxin A(4) receptor. Blood 95, 1810–1818 [PubMed] [Google Scholar]
- 34. De Haas C. J., Veldkamp K. E., Peschel A., Weerkamp F., Van Wamel W. J., Heezius E. C., Poppelier M. J., Van Kessel K. P., van Strijp J. A. (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199, 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Queck S. Y., Khan B. A., Wang R., Bach T. H., Kretschmer D., Chen L., Kreiswirth B. N., Peschel A., Deleo F. R., Otto M. (2009) Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 5, e1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watson D. C., Yaguchi M., Bisaillon J. G., Beaudet R., Morosoli R. (1988) The amino acid sequence of a gonococcal growth inhibitor from Staphylococcus haemolyticus. Biochem. J. 252, 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheung G. Y. C., Rigby K., Wang R., Queck S. Y., Braughton K. R., Whitney A. R., Teintze M., DeLeo F. R., Otto M. (2010) Staphylococcuss epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 6, e1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mader D., Rabiet M. J., Boulay F., Peschel A. (2010) Formyl peptide receptor-mediated proinflammatory consequences of peptide deformylase inhibition in Staphylococcus aureus. Microbes Infect. 12, 415–419 [DOI] [PubMed] [Google Scholar]
- 39. Deleo F. R., Otto M., Kreiswirth B. N., Chambers H. F. (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375, 1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boller T., Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant. Biol. 60, 379–406 [DOI] [PubMed] [Google Scholar]
- 41. Betten A., Bylund J., Christophe T., Boulay F., Romero A., Hellstrand K., Dahlgren C. (2001) A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J. Clin. Invest. 108, 1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wenzel A., Van Zandbergen G. (2009) Lipoxin A4 receptor-dependent leishmania infection. Autoimmunity 42, 331–333 [DOI] [PubMed] [Google Scholar]
- 43. Loffler B., Hussain M., Grundmeier M., Bruck M., Holzinger D., Varga G., Roth J., Kahl B. C., Proctor R. A., Peters G. (2010) Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6, e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Somerville G. A., Beres S. B., Fitzgerald J. R., DeLeo F. R., Cole R. L., Hoff J. S., Musser J. M. (2002) In vitro serial passage of Staphylococcus aureus: changes in physiology, virulence factor production, and agr nucleotide sequence. J. Bacteriol. 184, 1430–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shopsin B., Drlica-Wagner A., Mathema B., Adhikari R. P., Kreiswirth B. N., Novick R. P. (2008) Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J. Infect. Dis. 198, 1171–1174 [DOI] [PubMed] [Google Scholar]