Abstract
Inhibition of angiotensin-converting enzyme (ACE) induces anemia in humans and mice, but it is unclear whether ACE is involved in other aspects of hematopoiesis. Here, we systemically evaluated ACE-knockout (KO) mice and found myelopoietic abnormalities characterized by increased bone marrow myeloblasts and myelocytes, as well as extramedullary myelopoiesis. Peritoneal macrophages from ACE-KO mice were deficient in the production of effector molecules, such as tumor necrosis factor-α, interleukin-12p40, and CD86 when stimulated with lipopolysaccharide and interferon-γ. ACE-KO mice were more susceptible to Staphylococcus aureus infection. Further studies using total or fractionated bone marrows revealed that ACE regulates myeloid proliferation, differentiation, and functional maturation via angiotensin II and substance P and through the angiotensin II receptor type 1 and substance P neurokinin 1 receptors. Angiotensin II was correlated with CCAAT-enhancer-binding protein-α up-regulation during myelopoiesis. Angiotensin II supplementation of ACE-KO mice rescued macrophage functional maturation. These results demonstrate a previous unrecognized significant role for ACE in myelopoiesis and imply new perspectives for manipulating myeloid cell expansion and maturation.—Lin, C., Datta, V., Okwan-Duodu, D., Chen, X., Fuchs, S., Alsabeh, R., Billet, S., Bernstein, K. E., Shen, X. Z. Angiotensin-converting enzyme is required for normal myelopoiesis.
Keywords: myeloid proliferation, myelodifferentiation, substance P
Hematopoiesis within the bone marrow (BM) microenvironment is regulated by the production or degradation of soluble factors, such as cytokines and bioactive peptides. Several lines of evidence indicate that a local BM renin-angiotensin system (RAS) contributes to hematopoietic regulation. For a review, see Park and Zambidis (1). In the RAS, angiotensin-converting enzyme (ACE) is a key component responsible for the final conversion of angiotensin (ANG) I to the principal effector ANG II. While most ACE is bound to cell membrane as an ectopeptidase, the shedding of ACE releases an active enzyme into the extracellular milieu, where it can participate in both local and systemic regulation of peptides (2). In addition to producing ANG II, ACE can also degrade a variety of peptides, such as substance P (SP) and bradykinin.
Among the ACE-regulated peptides, both ANG II and SP have been reported to facilitate the proliferation of hematopoietic progenitors (3, 4). ANG II signals through AT1 receptor, by which blood pressure can be elevated through vascular constriction (5); SP shows a higher affinity to the NK1 receptor than other neurokinin receptors, and NK1 receptor mediates most of SP's function in hematopoiesis (6). All the components of the RAS, as well as the NK1 receptor, are widely expressed in BM stromal cells and hematopoietic progenitors (7, 8). These studies, along with the recognition of local de novo generation of ANG II in a variety of tissues (9), suggest the existence of a potential BM autocrine/paracrine mechanism in which ACE affects hematopoiesis. Indeed, clinical studies (10, 11) have shown that high doses of ACE inhibitors induce anemia in humans, and our analysis of ACE-KO mice showed that the absence of ACE results in anemia (12). Evidence indicates that ANG II can influence erythropoiesis by increasing erythropoietin levels and by stimulating the growth of erythroid progenitors (13, 14). Mice with a constitutive activation of AT1 receptor present with increased red blood cell mass and an increased hematocrit (15). Recent studies (16, 17) also revealed that ACE marks primitive human embryonic hemangioblasts and hematopoietic stem cells (HSCs) in both fetal and adult hematopoietic tissues. Further, Aksu et al. (18) reported that surface ACE levels are positively correlated with the number of BM leukemic myeloid blast counts. These findings suggest a possible role of ACE in regulating aspects of hematopoiesis. However, while a variety of studies in humans and mice have focused on erythropoiesis, how ACE and RAS influence other hematopoietic lineages remains incomplete (19, 20).
In this study, we examined ACE-knockout (KO) mice and found myelopoietic abnormalities. Further studies show that, under normal physiological conditions, ACE regulates myeloid proliferation, differentiation, and final functional maturation. The effects appear to be mediated through the peptides ANG II and SP. These results highlight an important role for ACE in normal myelopoiesis.
MATERIALS AND METHODS
Mice and cells
ACE-KO mice have been described previously (21), and this line was backcrossed to C57BL/6 for >10 generations. Wild-type (WT) mice used in this research were either the littermates of ACE-KO mice or C57BL/6 mice (Jackson Laboratory Sacramento, CA, USA). For in vivo delivery of ANG II to ACE-KO mice, Alzet osmotic minipumps (Durect Corp., Cupertino, CA, USA) were implanted subcutaneously and delivered ANG II 0.3 mg/kg/d as previously reported (12). For mouse irradiation, C57BL/6 mice were irradiated with 9.5 Gy and then were immediately infused with 2 × 106 WT BM cells for life protection. All procedures and animal experiments were approved by the institutional animal care and use committee at Cedars-Sinai Medical Center. S17 cells were irradiated with 30 Gy immediately before coculturing. To generate BM-derived macrophages (MΦs), red blood cell-lysed BM cells were cultured in complete medium [RPMI 1640 supplemented with 10% FCS (low-endotoxin lots; Hyclone, South Logan, UT, USA), HEPES, antibiotics, and glutamine] with 4% L929-conditioned medium as a source of macrophage colony-stimulating factor (M-CSF) (22). On d 5 after being washed vigorously, MΦs were detached by incubating for 5 min in 10 mM EDTA and 4 mg/ml lidocaine. Thioglycollate-elicited MΦs were collected via peritoneal lavage 4 d after a 2 ml intraperitoneal injection of 3% aged thioglycollate broth (Difco, Surrey, UK). To collect thioglycollate-induced peritoneal MΦs from ACE-KO mice implanted with ANG II minipumps, thioglycollate broth was injected 7 d after implantation.
Reagents and cytokines
Reagents used were purchased as indicated. Escherichia coli 055:B5 LPS, L-733060, ramipril, and ANG II: Sigma (St. Louis, MO, USA); recombinant murine (rm) IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), M-CSF, SCF, IL-3, IL-6, IL-11, and TPO: PeproTech (Rocky Hill, NJ, USA); rmEPO: R&D Systems (Minneapolis, MN, USA); lisinopril: Merck (Whitehouse Station, NJ, USA); losartan: LKT laboratories (St. Paul, MN, USA); SP-neutralizing antibody: Santa Cruz Biotechnology (Santa Cruz, CA, USA); and Pam3CSK4: InvivoGen (San Diego, CA, USA).
Antibodies, flow cytometry (FACS) analysis, and sorting
All the antibodies were purchased from eBioscience (San Diego, CA, USA), BioLegend (San Diego, CA, USA), or BD Pharmingen (San Jose, CA, USA). For staining lineage antigens, PE-conjugated antibodies specific for CD3 (145-2C11), B220 (RA3-6B2), Gr-1 (RB6-8C5), CD11b (M1/70), CD5 (53-7.3), and TER119 (TER119) were used. To delineate hematopoietic progenitors, FITC-conjugated anti-Sca-1 (D7), PE-Cy7-conjugated anti-c-Kit (2B8), APC-conjugated anti-IL-7Ra (A7R34), Pacific Blue-conjugated anti-CD34 (RAM34), and PerCP-Cy5.5-conjugated anti-CD16/CD32(93) were used. The progenitors were defined as common myeloid progenitor (CMP; Lin− Sca-1− c-kit+, IL-7Ra− CD34+ FcγRII/III−), granulocyte-macrophage progenitor (GMP; Lin− Sca-1− c-kit+, IL-7Ra− CD34+ FcγRII/III+), and megakaryocyte-erythroid progenitor (MEP; Lin− Sca-1− c-kit+, IL-7Ra− CD34− FcγRII/III−). For dissecting blood leukocyte populations, cells were classified as T cells (CD3+ B220− CD11b−), B cells (CD3− B220+ CD11b−), granulocytes (CD3− B220− CD11b+ Ly-6G+ F4/80−), and monocytes (CD3− B220− CD11b+ Ly-6G− F4/80+). Other antibodies used were anti-F4/80 (BM8), anti-CD80 (16–10A1), anti-CD86 (GL1), and anti-I-Ab (AF6–120.1). The PE-conjugated annexin V kit was from BD Pharmingen. A standard protocol was used to stain the surface markers. To stain intracellular CCAAT-enhancer-binding protein a (C/EBPa) and SP, we used rabbit polyclonal antibodies to C/EBPa (Cell Signaling Technology, Danvers, MA, USA) or SP (GeneTex, Irvine, CA, USA) followed by PE-conjugated goat anti-rabbit IgG (SouthernBiotech, Birmingham, AL, USA) with fixation and permeabilization buffers from eBioscience. The stained samples were analyzed on a Beckman Coulter CyAn ADP (Beckman Coulter, Fullerton, CA, USA), and data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR, USA). For sorting Lin− c-Kit+ (LK) cells, total BM cells were subjected to magnetic bead-powered lineage depletion (Miltenyi Biotec, Bergisch Gladbach, Germany) followed by PE-conjugated anti-c-Kit+ (eBioscience) sorting with a Beckman Coulter MoFlo.
Analysis of hematologic organs
BM, spleen, and blood cell counts were determined using a hemocytometer. Single-cell suspensions from femoral BM were cytospined and stained with Wright-Giemsa. Spleens were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin/eosin. The stained slides were examined using an Olympus BX-40 microscope (Olympus, Tokyo, Japan). BM and spleen and blood cells were also studied by FACS for subpopulation analysis.
Colony-forming and serial replating assays
Myeloid colony-forming assays were performed by plating 2 × 105 BM cells or 1 × 103 LSK cells in MethoCult M3234 medium (StemCell Technologies, Vancouver, BC, Canada) (containing 20% FCS) with growth factors at the following final concentrations: 20 ng/ml GM-CSF, 60 ng/ml G-CSF, or 10 ng/ml M-CSF. After 7 d, colonies were counted under a microscope, with a colony defined as >50 cells. For eythroid colony-forming unit (CFU-E) assay, 2 × 105 BM cells were plated with 3 U/ml EPO and examined on d 3, with the definition of >8 cells counted as a colony. For megakaryocyte CFU (CFU-Meg) assay, 1 × 105 BM cells were cultured in MegaCult-C medium (StemCell Technologies) with the presence of 50 ng/ml TPO, 10 ng/ml IL-3, 20 ng/ml IL-6, and 10 ng/ml IL-11. Colonies were stained on d 7 for acetylcholine esterase. The CFU-Meg colonies were defined as >3 megakaryocytes in a cluster. For serial replating assay, 2 × 105 BM cells were seeded into 35-mm plates in M3234 with 50 ng/ml SCF, 20 ng/ml IL-6, and 10 ng/ml IL-3. On d 7, 2 × 105 cells from the previous culture were replated into fresh medium. The replating process was repeated twice to produce tertiary CFC-GM colonies, and colonies were scored on each d 7. Three replicates were used in each experiment. In some experiments, the following reagents were included in the cell culture medium: lisinopril (1 μM), losartan (1–100 μM), L-733060 (1 μM), and ANG II (1–10 nM).
In vitro myeloid differentiation
LK cells (5 × 103) were seeded into each well of a 96-well plate with irradiated S17 cells as a feeder layer. RPMI 1640 medium with 10% FCS was used. Cells were stimulated with 12.5 ng/ml SCF, 2.5 ng/ml IL-3, and 5 ng/ml IL-6 or 2.5 ng/ml M-CSF. On d 3, 5, and 7, cells were stained for c-Kit, CD11b, FcγRII/III, Gr1, or F4/80 and then subjected to FACS analysis.
MΦ functional assays
For testing oxidative burst, cells were first incubated for 5 min with 1 μg/ml of dihydrorhodamine 123 (Invitrogen, Carlsbad, CA, USA) at 37°C in HBSS buffer plus 0.1% BSA. Then, 1 μM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) was added to stimulate the cells for 30 min at 37°C. The reaction was stopped by moving the cells onto ice; cells were then washed and subjected to FACS analysis. The background superoxide generation was measured using the cells without PMA stimulation. For testing CD86, CD80, and I-Ab expression, MΦs were cultured in complete medium plus 50 μM 2-mercaptoethanol with either 100 ng/ml LPS or 100 U/ml IFN-γ. After 20 h, cell were stained and evaluated by FACS analysis. For cytokine detection, MΦs were cultured for 20 h with either 100 ng/ml LPS or 100 ng/ml Pam3CSK4. Supernatant TNF-α and IL-12p40 were tested by enzyme-linked immunosorbent assay (eBioscience).
Methicillin-resistant Staphylococcus aureus (MRSA) infection
MRSA strain USA 300 SF4300 was obtained from Dr. George Liu (Cedars-Sinai Medical Center). Mice were infected intraperitoneally with 1 × 108 CFU MRSA in 200 μl saline. Mice were killed 24 h after infection. The peritoneal lavage and blood bacterial titers were ascertained by serial dilution in agar-supplemented Todd-Hewitt broth (Difco).
Statistics
All P values were calculated using an unpaired 2-tailed Student's t test with equal or unequal variance as appropriate.
RESULTS
Perturbed myeloproliferation in ACE-KO mice
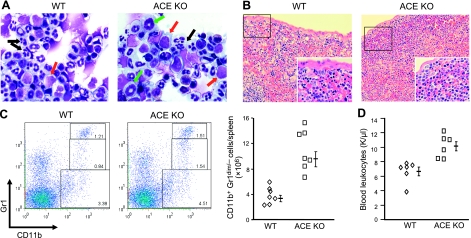
To investigate the role of ACE in myelopoiesis, we examined myeloid lineage cells within BM, spleen, and peripheral blood of ACE-KO and WT littermates at 7–10 wk of age. Histological examination of whole bone mounts demonstrated an intact architecture of the trabecular bone with a normal total BM cellularity in ACE-KO mice. However, when BM cell populations were quantitated by microscopic evaluation of Wright-Giemsa-stained cytospins, significant abnormalities in the ACE-KO mice were noted (Fig. 1A). In WT mice, the predominant cells were neutrophils (band and segmented cell) and erythroid lineage cells. However, these two populations were reduced in number in the ACE-KO BM (Table 1). There were more myeloid precursors, such as myeloblasts, myelocytes, and metamyelocytes, in ACE-KO BM. Specifically, ACE-KO BM had a 2.2-fold increase in myeloblasts and a 2.5-fold increase in myelocytes accompanying a 37% decrease of segmented neutrophils. Among other myeloid cells, the eosinophils expanded by 2.3-folds in ACE-KO BM, while the quantity of monocyte/macrophages was equivalent to levels in WT mice. In the erythroid lineage, nucleated erythroid precursors were mildly decreased to 77% of control values. We also enumerated BM early progenitors using FACS (Table 2). Most remarkable was that in ACE-KO BM, there was a 2-fold expansion of Lin− Sca-1+ c-kit+ LSK cells, a fraction enriched for HSCs. Neither CMP nor GMP frequency was altered in ACE-KO mice, whereas MEPs were mildly but significantly reduced.
Figure 1.
Elevated BM myeloid precursors and extramedullary myelopoiesis in ACE-KO mice. A) Wright-Giemsa-stained BM cytospins show increased myeloblasts (red arrows) and myelocytes (green arrows) but decreased segmented neutrophils (black arrows) in ACE-KO BM as compared with WT BM. Pictures, taken with a ×100 objective, represent 4 pairs of mice. B) Spleen hematoxylin/eosin staining shows increased extracellular hematopoiesis in the subcapsular regions of ACE-KO spleen, as compared with WT spleen. Pictures (low power: ×20; insets: ×40) represent 6 pairs of mice. C) Left panel: dot plots show the representative profiles of CD11b+ Gr1bright, CD11b+ Gr1dim, and CD11b+ Gr1− populations of splenocytes. Right panel: quantity of splenic CD11b+/Gr1dim/− cells. P < 0.001; n = 7. D) Blood leukocyte counts. P < 0.005; n = 6.
Table 1.
Wright-Giemsa-stained BM cytospin analysis
| Genotype | Myeloblast | Myelocyte | Meta-myelocyte | Band cell | Segmented cell | Eosinophil | Monocyte/MΦ | Erythroblast | Other erythroid |
|---|---|---|---|---|---|---|---|---|---|
| WT | 1.7 ± 0.8 | 3.3 ± 0.3 | 12.0 ± 2.1 | 19.8 ± 4.5 | 23.8 ± 1.2 | 3.8 ± 0.7 | 2.4 ± 0.5 | 2.3 ± 0.3 | 31.5 ± 3.5 |
| ACE KO | 3.8 ± 0.8* | 8.3 ± 1.1** | 17.9 ± 3.8 | 17.8 ± 3.6 | 14.9 ± 1.8** | 8.8 ± 0.4** | 2.0 ± 0.4 | 2.3 ± 0.7 | 24.1 ± 2.8* |
Data are presented as the percentage ± se of total cells and were obtained by counting 400 cells (n=4).
P < 0.05;
P < 0.01.
Table 2.
FACS analysis of BM progenitors
| Genotype | LSK | CMP | GMP | MEP |
|---|---|---|---|---|
| WT | 0.47 ± 0.07 | 0.51 ± 0.03 | 1.38 ± 0.25 | 1.67 ± 0.17 |
| ACE KO | 0.93 ± 0.16** | 0.47 ± 0.04 | 1.39 ± 0.26 | 1.20 ± 0.13* |
Data are presented as the percentage ± se of total cells (n=7).
P < 0.05;
P < 0.01.
The expansion of immature myeloid cells in ACE-KO mice was also associated with splenomegaly due to increased extramedullary hematopoiesis. The spleens of ACE-KO mice were 1.6-fold bigger than those of WT mice, as measured by spleen:body weight ratio (n=8; P<0.001). Not surprisingly, there was also an increase in the total splenic cellularity in ACE-KO mice (13.0± 0.75×107) compared with WT littermates (8.1±0.94× 107; n=6; P<0.01). Histological examination of the spleen showed that, in the WT samples, hematopoietic sites were mainly restricted around trabeculae projecting from the capsule. In ACE-KO spleens, while the gross architecture is reserved, there was extensive extramedullary hematopoiesis in subcapsular regions (Fig. 1B). This was composed predominantly of nonlymphoid nonerythroid immature cells with scarce cytoplasm, large nuclei, and fine nuclear chromatin. These may represent immature myeloid cells, which would be consistent with our finding of a 3-fold expansion of CD11b+ Gr1dim/− cells in ACE-KO spleens (Fig. 1C).
Previous analyses demonstrated anemia in ACE-KO mice (12), which was confirmed in our study of peripheral blood (Supplemental Table S1). ACE-KO mice had unchanged platelet counts. However, the ACE-KO blood showed mild leukocytosis (Fig. 1D), but the percentages of granulocytes, monocytes, and T and B cells were equivalent to WT. To examine whether the expanded immature myeloid cells present in the ACE-KO BM and spleen were found in the periphery, blood smear examination and myeloid colony-forming assay were performed. These showed no difference between ACE-KO and WT mice (data not shown). Therefore, for mice in the age range we evaluated, the elevation of myeloid cell precursors appear restricted to BM and spleen.
In the absence of ACE activity, SP mediates myeloproliferation
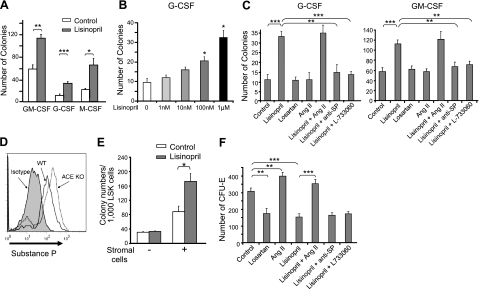
To investigate the role of ACE in myelopoiesis, we first studied WT BM using myeloid colony-forming assays. An intrinsic difficulty in studying the role of ACE in vitro is that ACE activity is present in virtually all sources of serum used for cell culture (42). To create culture conditions where there is no ACE activity, we used WT BM but eliminated ACE activity by adding the specific ACE inhibitor lisinopril. When WT BM was cultured with GM-CSF, M-CSF, or G-CSF, ACE inhibition consistently led to significantly more colonies (Fig. 2A). The effect of lisinopril is dose dependent (Fig. 2B), Similar results were observed using another ACE inhibitor, ramipril, which has a distinctive structure from lisinopril (data not shown). The increased CFUs seen in this assay were not due to reduced apoptosis, because culture of BM in GM-CSF with lisinopril for 5 d led to similar frequency of annexin V+ cells (5.5 vs. 4.9% without lisinopril). Thus, these experiments indicate that inhibiting ACE activity increases myeloid colony formation and that this is not dependent on which of the myeloid cytokines is the predominant factor.
Figure 2.
ACE regulates myeloproliferation through SP. A–C) WT BM cells were cultured in methylcellulose medium with indicated CSFs, and colonies were scored on d 7 (n≥6 mice) with or without lisinopril (A), with different doses of lisinopril (B), and in the presence of the indicated reagents (C). D) Intracellular staining total BM cell for SP. Histogram represents 4 pairs of mice. E) Sorted WT LSK cells were cultured with GM-CSF. Cells were cocultured with or without S17 stromal cells, and colonies were scored on d 7 (n=4 mice). F) WT BM cells were cultured with erythropoietin in methylcellulose medium with the indicated reagents. CFU-E numbers were scored on d 3 of culture (n=6 mice). Values are means ± se. *P < 0.01; **P < 0.005; ***P < 0.001.
Among ACE-regulated peptides, ANG II and SP have been implicated in myeloproliferation (4, 6). Physiologically, ACE produces ANG II but degrades SP (2, 23) (Supplemental Fig. S1). To measure the effects of ANG II, WT BM was cultured for 7 d with G-CSF or GM-CSF (Fig. 2C). To some cultures, we added ANG II daily or treated the cultures with losartan, an AT1 antagonist. Finally, some cultures received both lisinopril and ANG II. To investigate the role of SP, we treated BM cultures with lisinopril and also added either a SP-neutralizing antibody or the antagonist L-733060, which inhibits the major SP receptor NK1. When the number of colonies was analyzed, neither losartan nor ANG II had any effect. However, the ability of lisinopril to increase the colony number was totally reversed either by the SP neutralizing antibody or by L-733060.
To investigate whether SP is increased in ACE-KO mice, we permeabilized and stained WT and ACE-KO BM with a polyclonal anti-SP antibody and analyzed SP levels by FACS analysis (24). This showed significantly more SP present in ACE-KO total BM cells (Fig. 2D), which is consistent with the idea that elevated SP could be the cause of myeloproliferation in ACE-KO mice.
Since both hematopoietic cells and BM stromal cells express ACE and the NK1 receptor, we next investigated whether the effect of ACE inhibition in myeloproliferation is hematopoietic cell intrinsic or stromal cell dependent. For this, we sorted WT LSK cells and cultured them with or without the stromal cell line S17. Only in the presence of the S17 cells did ACE inhibition with lisinopril increase myeloid colony number (Fig. 2E). These data are consistent with the previous reports that SP could induce BM stromal cells to secrete a panel of myeloid growth factors (3, 6) and indicate an important role of stromal cells in facilitating the effect of ACE on myeloproliferation.
In contrast to myeloid colony formation, the number of CFU-Es was dependent on ACE production of ANG II (Fig. 2F). Either blocking AT1 receptor or inhibiting ACE activity reduced CFU-Es, while the addition of ANG II increased CFU-Es. In this assay, the addition of SP neutralizing antibody or the NK1 blocker had little effect. We also investigated the effects of ACE and ANG II on megakaryocyte colony formation (Supplemental Fig. S2). Neither inhibiting ACE nor adding ANG II could affect the number of CFU-Megs. Thus, ACE has distinctive effects on the proliferation of different lineages, regulating myeloid and erythroid lineages through SP and ANG II, respectively.
Both angiotensin II and SP facilitate myeloid differentiation
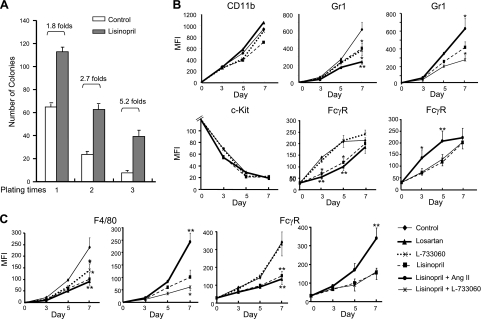
Analysis of ACE-KO mice showed differences in both the number of myeloid cells and myeloid differentiation. The studies above have not explained the observation that the increased myeloid populations in ACE-KO mice are relatively immature. To study the role of ACE in myeloid differentiation, we first tested the replating ability of cells in colony formation, since it is only immature cells that maintain the capacity for self-renewal and colony formation. In this assay, we used a cytokine cocktail composed of SCF, IL-3, and IL-6, which can drive the formation of granulocyte-macrophage CFU (CFU-GM). BM cells (2 × 105) were cultured, and 7 d later the colony number was counted. Cells (2 × 105) harvested from the previous culture were replated into fresh medium. The replating process was repeated twice to produce tertiary colonies. The assay was performed with or without ACE inhibitor lisinopril. Similar to what was described in Fig. 2A, the initial yield of colonies was 1.8-fold greater in the presence of lisinopril (Fig. 3A). With secondary and tertiary replating, the colony-forming capacity diminished in both groups. However, the number of colonies diminished far more rapidly in the control population, such that by the tertiary plating, the lisinopril-treated population showed 5.2-fold more colonies. These data suggest that, in the absence of ACE activity, myeloid cell precursors are relatively retarded in their maturation.
Figure 3.
ACE regulates myeloid differentiation through ANG II and SP. A) Serial replating assays were performed on WT BM cells cultured with SCF, IL-3, and IL-6 with or without lisinopril (n=6 mice). B) WT BM LK cells were cultured with SCF, IL-3, and IL-6 in the presence of S17 cells. Cell surface levels of CD11b, c-Kit, Gr1, and FcγR were monitored by FACS on d 0, 3, 5, and 7 when cocultured with indicated reagents. Data are presented as mean fluorescence intensity (MFI; n=6 mice). C) Similar to B, WT BM LK cells were cultured with M-CSF and cocultured with indicated reagents. Surface expression of F4/80 and FcγR was measured by FACS (n=6 mice). Bars = se. *P < 0.02; **P < 0.01.
To directly assess whether ACE and its related peptides can regulate myeloid differentiation, we studied in vitro differentiation using sorted LK cells, a cell fraction containing a variety of myeloid progenitors. The WT LK cells were cultured with S17 stromal cells and with the myeloid cytokine cocktail SCF, IL-3, and IL-6. Populations of cells were also treated with lisinopril, losartan, or L-733060. Myeloid cell maturation was assessed by surface down-regulation of the progenitor marker c-Kit and up-regulation of the early maturation markers CD11b and FcγR II/III. We also measured the up-regulation of the late lineage marker Gr1. All cell groups showed a similar temporal CD11b acquisition and c-Kit loss in the 7-d culture (Fig. 3B). However, the kinetics of Gr1 and FcγR up-regulation were different among the groups, with treatment by losartan and lisinopril slowing the up-regulation of both markers. L-733060-treated cells showed only slowed Gr1 elevation but unaffected FcγR elevation. This suggests that FcγR expression is dependent on ANG II but that Gr1 expression might be affected by both ANG II and SP. To investigate this further, we repeated the assay but supplemented the lisinopril-treated cells with either ANG II or L-733060. ANG II restored the normal expression patterns of Gr1 and FcγR while L-733060 further suppressed Gr1 expression in lisinopril-treated cells.
One caveat to the above experiment is that the SCF, IL-3, and IL-6 cocktail may be insufficient to facilitate lineage-specific differentiation. To test this, we cultured cells with an instructive growth factor (M-CSF) and analyzed LK cell differentiation to MΦs by monitoring the up-regulation of CD11b, FcγR, and the late lineage marker F4/80 (Fig. 3C and Supplemental Fig. S3). Again, there was no difference in CD11b expression patterns between the cell groups treated with losartan, lisinopril, or L-733060 (data not shown). However, both lisinopril and losartan suppressed expression of F4/80 and FcγR. L-733060 had no effect on FcγR up-regulation and a substantially smaller effect on F4/80 than either the inhibitor of ACE or the AT1 receptor. Moreover, ANG II supplementation corrected the effects of lisinopril treatment, and coblocking ACE and NK1 receptor further slowed F4/80 up-regulation. These data suggest a prominent role for ANG II in myeloid differentiation. Although SP does affect the expression of late lineage markers (Gr1, F4/80), this peptide appears less important than ANG II.
ACE and angiotensin II are required for functional maturation of macrophages
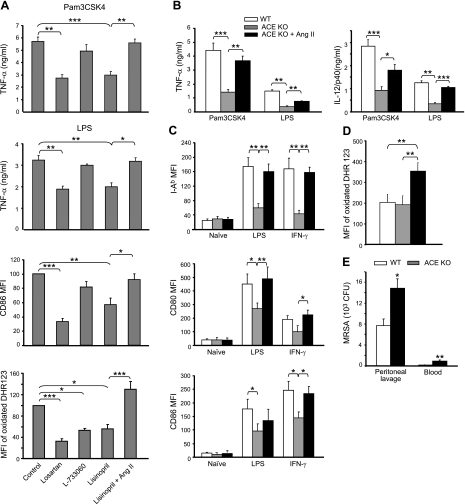
Despite the difference in MΦ differentiation profiles, we still observed the generation of CD11bhigh F4/80high cells when ACE activity or the AT1 or NK1 receptors were blocked. As these markers are phenotypically associated with mature MΦs, we studied whether there was any downstream sequela in these “mature” cells following ACE or receptor blockade. WT BM was cultured with M-CSF under conditions that allow MΦ selection by adherence to Petri dish (22). During the differentiation process, some BM cells were treated with either lisinopril, losartan, or L-733060. After 5 d, attached cells were harvested using EDTA. Virtually all such cells showed a mature MΦ appearance, with ruffled borders and 1 round to oval nucleus having fine chromatin surrounded by vacuolous cytoplasm. There was no difference in morphology between the individual groups. Cells were then evaluated for production of the effector molecules TNF-α and CD86 and the oxidative species (Fig. 4A). Before each of these measures, cells were washed and any inhibitor treatment was discontinued. Significant decreases in TNF-a production and CD86 up-regulation were observed in MΦs derived from BM treated with lisinopril or losartan but not from BM treated with L-377060. When stimulated with PMA, MΦs derived from losartan-, lisinopril-, or L-733060-treated BM produced significantly less reactive oxygen. In all these measures, the phenotype induced by lisinopril reverted back to near normal levels by supplementing the BM cells with ANG II.
Figure 4.
MΦ functional maturation requires ACE and ANG II. A) WT BM was cultured with M-CSF and with indicated reagents for 5 d. MΦs were then purified by adhesion, washed free of the pretreated reagents, and then stimulated for testing effector molecules. After 20 h incubation with PAM3CSK4 or LPS, supernatant TNF-α levels were determined by ELISA (n=6 mice); after 20 h incubation with IFN-γ, cell surface CD86 expression was evaluated by FACS (n=mice); and oxidative burst was measured by oxidation of dihydrorhodamine 123 after 30 min activation with 1 μM PMA (n=5 mice). For CD86 and oxidative burst, data are presented as the MFI of all cells with a signal above background and are normalized as a percentage of control values. B–D) TPMs were harvested from WT mice, ACE-KO mice, and ACE-KO mice infused with ANG II. Cells were then subjected to a variety of stimuli for testing effector molecules. WT, n = 6; ACE KO, n = 10; ACE KO with ANG II minipump, n = 4. B) After 20 h incubation with PAM3CSK4 or LPS, supernatant TNF-α and IL-12p40 levels were measured by ELISA. C) After 20 h incubation with IFN-γ or LPS, cell surface expression of I-Ab, CD80, and CD86 were determined by FACS. D) MΦ oxidative burst was similarly measured as in A. E) WT and ACE-KO mice were intraperitoneally infected with 1 × 108 CFU MRSA. After 24 h, the mice were killed, and the bacteria titers in their peritoneal lavage and blood were determined (n=6 mice). Values are means ± se. *P < 0.05; **P < 0.01; ***P < 0.005.
The ANG II level is 10-fold lower in ACE-KO mice compared with WT mice (12). To understand the role of ACE and ANG II in developing functionally normal MΦs in vivo, we first tested the expression of effector molecules by thioglycollate-elicited peritoneal MΦs (TPMs) collected from WT mice, ACE-KO mice, and ACE-KO mice having an osmotic minipump infusing ANG II. To verify that TPMs are the cells freshly developed from BM but not the existent monocytes/MΦs recruited from other tissues, we lethally irradiated WT mice. The mice were challenged the next day with thioglycollate broth and peritoneal cells were collected 4 d later. Although the irradiated mice were given 2 × 106 fresh BM for life protection, they yielded dramatically low number of peritoneal cells (1.67±0.63×106 vs. 35.4±4.3×106 from nonirradiated mice), which indicates BM incapability. Thus, most TPMs are freshly derived from BM.
TPMs were stimulated ex vivo, and we then measured the levels of the secreted proinflammatory cytokines TNF-α and IL-12/p40 (Fig. 4B), surface major histocompatability complex class II and costimulatory factors CD80 and CD86 (Fig. 4C), and reactive oxygen production (Fig. 4D). In all these measures, excepting the oxidative burst, MΦs derived from ACE-KO mice showed a suppressed response. In all instances, the pretreatment of the ACE-KO mice with ANG II partially or completely reverted the phenotype to that of cells obtained from WT mice. As for reactive oxygen production, the infusion of ANG II actually induced ACE-KO cells to produce higher levels than WT cells.
Next, we tested the response of ACE-KO mice to acute bacteria infection. WT and ACE-KO mice were challenged with intraperitoneal injection of MRSA. Twenty-four hours later, the ACE-KO mice showed significantly less efficiency in bacteria clearance in the peritoneal cavity (Fig. 4E). Moreover, there was 5-fold increase of bacteria dissemination in the blood of ACE-KO mice. Collectively, the results from MΦ functional assays and in vivo MRSA infection strongly implicate ACE as important in the functional maturation of myeloid cells.
ACE and ANG II are required for normal C/EBPα up-regulation during myeloid development
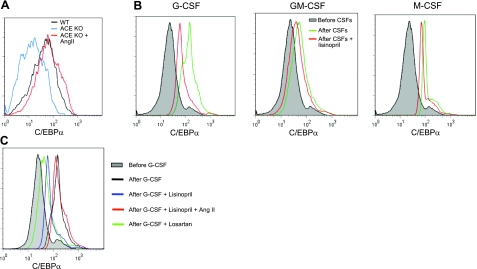
C/EBPα is a central transcription factor in myelopoiesis (25), and C/EBPα up-regulation is associated with myeloid differentiation from HSCs (26). In addition, newborn C/EBPα-deficient mice have defective myeloid cell development with significantly increased myeloid precursors in the peripheral (27, 28). Therefore, we asked whether ACE or ANG II correlated with C/EBPα expression in myeloid differentiation. To test this, we first studied the expression of C/EBPα in TPMs collected from WT mice, ACE-KO mice, and ACE-KO mice infused with ANG II, since TPMs are freshly developed from BM myeloid precursors. ACE-KO MΦs expressed significantly lower C/EBPα levels as compared with WT cells (Fig. 5A and Supplemental Fig. S4). In contrast, ANG II infusion into ACE-KO mice resulted in MΦs with normal C/EBPα levels.
Figure 5.
Normal C/EBPα up-regulation during myeloid development requires ACE and ANG II. A) FACS analysis of TPMs for C/EBPα intracellular staining. TPMs were collected from either WT mice, ACE-KO mice, or ACE-KO mice infused with ANG II. B) WT BM Lin− cells were cultured with the indicated CSFs and treated with or without lisinopril for 24 h, and then their intracellular C/EBPα levels were evaluated. C) Similar to B, WT BM Lin− cells were cultured with G-CSF and treated with the indicated reagents for 24 h. and their C/EBPα expression was evaluated. Data represent 3 independent experiments.
To have a better picture of the relationship between C/EBPα up-regulation and ACE in myelopoiesis, we treated WT Lin− BM cells with either G-CSF, GM-CSF, or M-CSF overnight. Parallel cultures were coincubated with the ACE inhibitor lisinopril. Each of the CSFs induced marked C/EBPα expression (Fig. 5B). In all instances, lisinopril reduced C/EBPα expression. Here, the defect in C/EBPα up-regulation induced by lisinopril could be completely corrected by ANG II supplementation (Fig. 5C). Further, this experiment showed that the AT1 receptor blocker losartan also acted to suppress C/EBPα up-regulation. These data suggest a correlation between ACE expression and C/EBPα induction during myeloid differentiation and strongly suggest that ACE may have at least part of its effects on myeloid differentiation through effects on C/EBPα expression. Further, this effect appears mediated by ANG II.
DISCUSSION
ACE is traditionally viewed in the context of hemodynamic homeostasis, since it is the enzyme that produces ANG II. However, there is now a plethora of data defining the important roles of ACE in metabolizing a variety of peptides and influencing physiological processes far beyond blood pressure control (2, 29). Using ACE-KO mice, we now show that ACE plays roles in aspects of hematopoiesis. The influence of ACE is seen at different stages (HSCs and myelocytes) and on different lineages (myeloid and erythroid). In particular, ACE is important for normal myelopoiesis. In the absence of ACE, there are myelopoiesis abnormalities characterized by increased bone marrow myeloblasts and myelocytes, as well as extramedullary myelopoiesis. By inhibiting ACE activity when culturing WT BM, we recapitulated the myelopoiesis perturbation observed in ACE-KO mice. Here, we show that SP accumulation, in the absence of ACE activity, facilitates myeloproliferation, and it is the lack of ACE-mediated ANG II formation that is predominantly responsible for the abnormal myeloid differentiation. Interestingly, ANG II could not affect the expression of an early myeloid marker CD11b, but it profoundly delayed the expression of late lineage markers (Gr1 and F4/80). These data echo the findings that there are increased BM myelocytes and splenic CD11b+ Gr1dim/− cells in the ACE-KO mice, which indicates a blockage at the post-GMP stage of myeloid differentiation. One of the intriguing recent observations in this field is that ACE is expressed and can be used as a marker for human embryonic hemangioblasts and HSCs (16, 17). Although mouse HSCs have not been systemically tested for the expression of ACE or other components of the RAS, we found a 2-fold expansion of LSK cells in the ACE-KO BM, which indicates a role of ACE in HSC homeostasis. Identifying the ACE substrates and their receptors involved in HSC homeostasis will be an interesting prospect in this field.
Notably, for the first time, we unveil the importance of ANG II in C/EBPα up-regulation under myelopoiesis and in molding the normal function of MΦs. The RAS works locally, as well as systemically; the local production of ANG II can be of great importance in normal and abnormal physiological process. Previous work (4, 30) has suggested that ANG II could accelerate the proliferation of CFU-GMs and may act as a growth factor in acute myeloid leukemia. Here we present data that ANG II is an important factor in facilitating myeloid maturation but may not act as a myeloid mitogen. We also find that AT1 receptor signaling mediates the effects of ANG II in myeloid differentiation. This echoes a published report (31) showing that AT1 receptor is important in monocyte formation in vitro. However, Tsubakimoto et al. (31) reported that AT1−/− hematopoietic cells had reduced MΦ colony-forming activity and slowed expression of the differentiation marker CD11b. These effects were not seen in WT cells treated with the AT1 antagonist losartan. At present, we have no firm explanation for these inconsistencies, although it seems possible that WT cells treated with short-term losartan and AT1−/− cells may not be identical.
SP is a neuropeptide belonging to the tachykinin family. SP is released from BM myeloid cells (32), as well as by peptidergic nerve endings. Most hematopoietic cells and BM stroma cells are equipped with the NK1 receptor (3, 33). Rameshwar et al. (3, 6) have revealed that SP is a growth factor for myeloid progenitors with many of its effects mediated by increased secretion of myeloid growth factors, such as SCF and IL-1, from stromal cells. Thus, it is not surprising that, in our system, ACE affects myeloproliferation through its effects on SP levels and the SP NK1 receptor can affect myeloid differentiation. However, in the ACE inhibition environment having high SP levels, the skewed differentiation profile of myeloid progenitors appears due to deficient ANG II production. This may represent a higher sensitivity of BM to ANG II insufficiency than to SP increase.
AT1 signaling can directly lead to activation of the NF-κB pathway, and ANG II can work as a proinflammatory cytokine to prime mature MΦs (34, 35). Here, we show that ANG II, through the AT1 receptor, also acts to boost myeloid differentiation and functional maturation. Markers of acute injury, such as Toll-like receptor ligands (36), IL-1 (37), and TNF-α (38) often act to mobilize BM myelopoiesis, in addition to their direct roles in triggering immunity. Undoubtedly the rapid replenishment of the innate immune system after acute injury is biologically advantageous. While today ANG II is often linked to chronic diseases such as hypertension, heart failure and atherosclerosis, previously, humans often saw elevated ANG II levels as a consequence of acute injury and blood loss. Thus a role of ANG II in promoting erythropoiesis and myelopoiesis may also function as an advantageous compensatory mechanism.
Although our results showed that ANG II and SP contribute to the normal myelopoiesis, the early emergence of ACE in phylogeny (39) and its broad substrate specificity indicate that it may have a more complicated peptide network in regulating aspects of hematopoiesis (40, 41). In conclusion, this study identifies ACE as a myelopoietic regulator. In particular, these data highlight the critical roles of ACE in balancing the production/destruction of different peptides that influence myelopoiesis.
Supplementary Material
Acknowledgments
The authors thank Dr. Kenneth Dorshkind (University of California, Los Angeles, CA, USA) for the S17 cell line, Ellan Bernstein for animal husbandry, and Patricia Lin for cell sorting.
This work was supported by U.S. National Institutes of Health grants R01 DK-039777 and R01 DK-051445 (to K. E. Bernstein). The authors have no financial conflicts of interest.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Park T. S., Zambidis E. T. (2009) A role for the renin-angiotensin system in hematopoiesis. Haematologica 94, 745–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corvol P., Williams T. A., Soubrier F. (1995) Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 248, 283–305 [DOI] [PubMed] [Google Scholar]
- 3. Rameshwar P., Ganea D., Gascon P. (1993) In vitro stimulatory effect of substance P on hematopoiesis. Blood 81, 391–398 [PubMed] [Google Scholar]
- 4. Rodgers K. E., Xiong S., Steer R., diZerega G. S. (2000) Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells 18, 287–294 [DOI] [PubMed] [Google Scholar]
- 5. Sugaya T., Nishimatsu S., Tanimoto K., Takimoto E., Yamagishi T., Imamura K., Goto S., Imaizumi K., Hisada Y., Otsuka A., Uchida H., Sugiura M., Fukuta K., Fukamizu A., Murakami K. (1995) Angiotensin II type 1a receptor-deficient mice with hypotension and hyperreninemia. J. Biol. Chem. 270, 18719–18722 [DOI] [PubMed] [Google Scholar]
- 6. Rameshwar P., Gascon P. (1995) Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 86, 482–490 [PubMed] [Google Scholar]
- 7. Strawn W. B., Richmond R. S., Ann Tallant E., Gallagher P. E., Ferrario C. M. (2004) Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br. J. Haematol. 126, 120–126 [DOI] [PubMed] [Google Scholar]
- 8. Nowicki M., Ostalska-Nowicka D., Kondraciuk B., Miskowiak B. (2007) The significance of substance P in physiological and malignant haematopoiesis. J. Clin. Pathol. 60, 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Re R. N. (2001) The clinical implication of tissue renin angiotensin systems. Curr. Opin. Cardiol. 16, 317–327 [DOI] [PubMed] [Google Scholar]
- 10. Griffing G. T., Melby J. C. (1982) Enalapril (MK-421) and the white cell count and haematocrit. Lancet 1, 1361. [DOI] [PubMed] [Google Scholar]
- 11. Pratt M. C., Lewis-Barned N. J., Walker R. J., Bailey R. R., Shand B. I., Livesey J. (1992) Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br. J. Clin. Pharmacol. 34, 363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole J., Ertoy D., Lin H., Sutliff R. L., Ezan E., Guyene T. T., Capecchi M., Corvol P., Bernstein K. E. (2000) Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J. Clin. Invest. 106, 1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato H., Ishida J., Imagawa S., Saito T., Suzuki N., Matsuoka T., Sugaya T., Tanimoto K., Yokoo T., Ohneda O., Sugiyama F., Yagami K., Fujita T., Yamamoto M., Nangaku M., Fukamizu A. (2005) Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J. 19, 2023–2025 [DOI] [PubMed] [Google Scholar]
- 14. Mrug M., Stopka T., Julian B. A., Prchal J. F., Prchal J. T. (1997) Angiotensin II stimulates proliferation of normal early erythroid progenitors. J. Clin. Invest. 100, 2310–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Billet S., Bardin S., Verp S., Baudrie V., Michaud A., Conchon S., Muffat-Joly M., Escoubet B., Souil E., Hamard G., Bernstein K. E., Gasc J. M., Elghozi J. L., Corvol P., Clauser E. (2007) Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J. Clin. Invest. 117, 1914–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zambidis E. T., Park T. S., Yu W., Tam A., Levine M., Yuan X., Pryzhkova M., Peault B. (2008) Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood 112, 3601–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jokubaitis V. J., Sinka L., Driessen R., Whitty G., Haylock D. N., Bertoncello I., Smith I., Peault B., Tavian M., Simmons P. J. (2008) Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal, and adult hematopoietic tissues. Blood 111, 4055–4063 [DOI] [PubMed] [Google Scholar]
- 18. Aksu S., Beyazit Y., Haznedaroglu I. C., Canpinar H., Kekilli M., Uner A., Sayinalp N., Buyukasik Y., Goker H., Ozcebe O. I. (2006) Over-expression of angiotensin-converting enzyme (CD 143) on leukemic blasts as a clue for the activated local bone marrow RAS in AML. Leuk. Lymphoma 47, 891–896 [DOI] [PubMed] [Google Scholar]
- 19. Rousseau-Plasse A., Wdzieczak-Bakala J., Lenfant M., Ezan E., Genet R., Robinson S., Briscoe T., Melville J., Riches A. (1998) Lisinopril, an angiotensin I-converting enzyme inhibitor, prevents entry of murine hematopoietic stem cells into the cell cycle after irradiation in vivo. Exp. Hematol. 26, 1074–1079 [PubMed] [Google Scholar]
- 20. Charrier S., Michaud A., Badaoui S., Giroux S., Ezan E., Sainteny F., Corvol P., Vainchenker W. (2004) Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 104, 978–985 [DOI] [PubMed] [Google Scholar]
- 21. Esther C. R., Jr., Howard T. E., Marino E. M., Goddard J. M., Capecchi M. R., Bernstein K. E. (1996) Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab. Invest. 74, 953–965 [PubMed] [Google Scholar]
- 22. Davies J. Q., Gordon S. (2005) Isolation and culture of murine macrophages. Methods Mol. Biol. 290, 91–103 [DOI] [PubMed] [Google Scholar]
- 23. Scholzen T. E., Luger T. A. (2004) Neutral endopeptidase and angiotensin-converting enzyme–key enzymes terminating the action of neuroendocrine mediators. Exp. Dermatol. 13(Suppl. 4), 22–26 [DOI] [PubMed] [Google Scholar]
- 24. De Giorgio R., Tazzari P. L., Barbara G., Stanghellini V., Corinaldesi R. (1998) Detection of substance P immunoreactivity in human peripheral leukocytes. J. Neuroimmunol. 82, 175–181 [DOI] [PubMed] [Google Scholar]
- 25. Friedman A. D. (2007) C/EBPalpha induces PU. 1 and interacts with AP-1 and NF-kappaB to regulate myeloid development. Blood Cells Mol. Dis. 39, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwasaki H., Akashi K. (2007) Myeloid lineage commitment from the hematopoietic stem cell. Immunity 26, 726–740 [DOI] [PubMed] [Google Scholar]
- 27. Zhang D. E., Zhang P., Wang N. D., Hetherington C. J., Darlington G. J., Tenen D. G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 94, 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang P., Iwama A., Datta M. W., Darlington G. J., Link D. C., Tenen D. G. (1998) Upregulation of interleukin 6 and granulocyte colony-stimulating factor receptors by transcription factor CCAAT enhancer binding protein alpha (C/EBP alpha) is critical for granulopoiesis. J. Exp. Med. 188, 1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen X. Z., Lukacher A. E., Billet S., Williams I. R., Bernstein K. E. (2008) Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J. Biol. Chem. 283, 9957–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haznedaroglu I. C., Ozturk M. A. (2003) Towards the understanding of the local hematopoietic bone marrow renin-angiotensin system. Int. J. Biochem. Cell Biol. 35, 867–880 [DOI] [PubMed] [Google Scholar]
- 31. Tsubakimoto Y., Yamada H., Yokoi H., Kishida S., Takata H., Kawahito H., Matsui A., Urao N., Nozawa Y., Hirai H., Imanishi J., Ashihara E., Maekawa T., Takahashi T., Okigaki M., Matsubara H. (2009) Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler. Thromb. Vasc. Biol. 29, 1529–1536 [DOI] [PubMed] [Google Scholar]
- 32. Pascual D. W., Bost K. L. (1990) Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology 71, 52–56 [PMC free article] [PubMed] [Google Scholar]
- 33. Greeno E. W., Mantyh P., Vercellotti G. M., Moldow C. F. (1993) Functional neurokinin 1 receptors for substance P are expressed by human vascular endothelium. J. Exp. Med. 177, 1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia G. E. ANG II receptor antagonists as modulators of macrophages polarization. (2010) Am. J. Physiol. Renal Physiol. 298, F868–F869 [DOI] [PubMed] [Google Scholar]
- 35. Alonso F., Krattinger N., Mazzolai L., Simon A., Waeber G., Meda P., Haefliger J. A. (2010) An angiotensin II- and NF-κB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc. Res. 87, 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagai Y., Garrett K. P., Ohta S., Bahrun U., Kouro T., Akira S., Takatsu K., Kincade P. W. (2006) Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ueda Y., Cain D. W., Kuraoka M., Kondo M., Kelsoe G. (2009) IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J. Immunol. 182, 6477–6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yao Z., Li P., Zhang Q., Schwarz E. M., Keng P., Arbini A., Boyce B. F., Xing L. (2006) Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J. Biol. Chem. 281, 11846–11855 [DOI] [PubMed] [Google Scholar]
- 39. Riviere G., Michaud A., Corradi H. R., Sturrock E. D., Ravi Acharya K., Cogez V., Bohin J. P., Vieau D., Corvol P. (2007) Characterization of the first angiotensin-converting like enzyme in bacteria: ancestor ACE is already active. Gene 399, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonnet D., Lemoine F. M., Khoury E., Pradelles P., Najman A., Guigon M. (1992) Reversible inhibitory effects and absence of toxicity of the tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (AcSDKP) in human long-term bone marrow culture. Exp. Hematol. 20, 1165–1169 [PubMed] [Google Scholar]
- 41. Rodgers K., Xiong S., DiZerega G. S. (2003) Effect of angiotensin II and angiotensin(1–7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother. Pharmacol. 51, 97–106 [DOI] [PubMed] [Google Scholar]
- 42. Bramucci M., Miano A., Quassinti L., Maccari E., Murri O., Amici D. (1999) Presence and comparison of angiotensin converting enzyme in commercial cell culture sera. Biochem. Mol. Biol. Int. 47, 107–115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.