Abstract
Background
Oral ethanol self-administration procedures in rats are useful pre-clinical tools for the evaluation of potential new pharmacotherapies as well as for the investigation of the etiology of alcohol abuse disorders and addiction. Determination of the effects of a potential treatment on a full ethanol dose-response curve should be essential to predict its clinical efficacy. Unfortunately, this approach has not been fully explored due to the aversive taste reaction to moderate to high doses of ethanol, which may interfere with consumption. In the present study, we set out to determine whether a meaningful dose-response curve for oral ethanol self-administration can be obtained in rats.
Methods
Long-Evans rats were trained to self-administer a 20% ethanol solution in an operant procedure following a history of excessive voluntary ethanol intake. After stabilization of ethanol self-administration the concentration of the solution was varied from 2.5 to 60% (v/v) and operant and drinking behaviors as well as blood ethanol concentration (BEC) were evaluated following the self-administration of a 20, 40 and 60% ethanol solution.
Results
Varying the concentration of ethanol from 2.5% to 60% after development of excessive ethanol consumption led to a typical inverted U-shape dose-response curve. Importantly, rats adapted their level and pattern of responding to changes in ethanol concentration to obtain a constant level of intake and BEC, suggesting that their operant behavior is mainly driven by the motivation to obtain a specific pharmacological effect of ethanol.
Conclusion
This procedure can be a useful and straightforward tool for the evaluation of the effects of new potential pharmacotherapies for the treatment of alcohol abuse disorders.
Keywords: Ethanol, Self-administration, Dose-response Curve, Consumption, Addiction
Introduction
The regulation of self-administration of drugs of abuse depends on several complex interrelated mechanisms including the motivational state, the conditioned and unconditioned reinforcing effects of the drug, as well as adverse effects induced by exposure to drugs of abuse such as sedation or stereotypies (Lynch and Carroll, 2001; Madden, 2001; Mello and Negus, 1996; Yokel, 1987). In this regard, varying the unitary dose of drugs of abuse in rodents and monkeys generally leads to a typical, non-monotonic, inverted U-shape dose-response curve (Carney et al., 1976; Glick et al., 1975; Mello and Negus, 1996; Yokel, 1987). Low doses of the drug fail to maintain a high level of responding (ascending limb of the curve) while, within a certain range of doses (descending limb of the curve), responding progressively decreases as the dose increases in order to achieve or maintain a relatively constant level of intake (Ahmed and Koob, 1999; Gerber and Wise, 1989; Lynch and Carroll, 2001). Determining the effects of a potential anti-addictive therapy on a full dose-response function for the self-administration of a drug of abuse provides crucial information about its clinical efficacy (Mello and Negus, 1996). Specifically, and as documented for opioids and cocaine (Barrett et al., 2004; Mello and Negus, 1996), horizontal, left or right, shift usually reflects a decrease in the apparent potency of the self-administered drug, while downward shifts are indicative of a specific attenuation of the motivation to self-administer drugs of abuse (Ahmed and Koob, 1998; Graham et al., 2007; Maisonneuve and Glick, 1999; Piazza et al., 2000). It is noteworthy to consider that a downward shift may be therefore viewed as ideal from a therapeutic perspective, while a mere alteration in the potency of the self-administered drug that shifts the curve horizontally and consequently leads to an increase in self-administration and intake at some doses is less desirable (Mello and Negus, 1996; Piazza et al., 2000). This approach has also been shown to be powerful in determining whether excessive drug intake is due to an increase in motivation, or to a change in the pharmacological sensitivity (i.e., tolerance or sensitization), to the drug (Ahmed and Koob, 1998; Piazza et al., 2000).
Intravenous (i.v) self-administration of ethanol in rats and nonhuman primates conforms to an inverted U-shape dose-response curve function (Carney et al., 1976; Kuzmin et al., 1999; Lyness and Smith, 1992), and it has been shown that animals adjust their rate and pattern of lever-responding to obtain and maintain a stable BEC during the self-administration session (Karoly et al., 1978). Oral self-administration is the most common model of ethanol self-administration used in rodents (Sanchis-Segura and Spanagel, 2006). While a range of ethanol concentrations from 3–5 to 20–30% (v/v) is used in self-administration studies (e.g., Blednov et al., 2005; Martinetti et al., 2006; Nowak et al., 1999; Rhodes et al., 2005; Sharpe and Samson, 2003), a full dose-response curve of oral ethanol self administration, which requires higher concentrations, has not been fully explored due to the innate taste aversion for moderate to high concentrations of ethanol (Meisch and Stewart, 1994; Samson et al., 1988) which can induce a bias in the dose-response curve (Le and Shaham, 2002). However, studies in which the taste reactivity of a wide range of concentrations (5 to 40%, v/v) of ethanol in rats were evaluated, surprisingly found that the number of ingestive, but not aversive, orofacial responses tend to increase with the increase in ethanol concentration (Bice and Kiefer, 1990; Kiefer et al., 1994), and taste reactivity to ethanol was found to be enhanced after a history of ethanol drinking (Kiefer et al., 1994; Kiefer et al., 2005). In line with these data, outbred rats tested with a concurrent choice between two ethanol concentrations during operant self-administration tend to respond more on the lever associated with the higher (20 or 40%, v/v) than the lower (10%, v/v) ethanol concentration (Samson et al., 1988), and access to these higher concentrations led to even greater intakes (Meisch and Thompson, 1974; Samson et al., 1988). Similarly, rats develop within a few days a preference for the highest ethanol concentration under a home-cage free choice drinking procedure between ethanol solutions ranging from 5 to 20% (v/v) (Wolffgramm, 1990). In addition, rats with long-term exposure to ethanol have been shown to adapt their consumption to ethanol concentrations ranging from 5 to 25% in order to obtain a constant daily ethanol intake (Wahltrom, 1987). Moreover, intermittent-access to a 20% ethanol (v/v) solution in a two-bottle choice procedure rapidly leads to the voluntary intake of large amounts of ethanol without the need for initiation procedures like sucrose fading or food and water deprivation (Carnicella et al., 2009b; Simms et al., 2008; Wise, 1973). Taken together, these data suggest that the orosensory properties of ethanol are not an obstacle to perform a meaningful dose-response curve for oral ethanol self-administration, especially in rats habituated to consume large amounts of ethanol. Therefore, we set out to test whether a full dose-response curve of operant ethanol self-administration can be obtained in rats with a history of excessive voluntary ethanol intake.
Materials and Methods
Ethanol preparation and Reagents
Ethanol was purchased from Gold Shield (Hayward, CA) and was diluted in filtered tap water to obtain the appropriate concentration (in v/v). β-nicotinamide adenine dinucleotide (NAD) and alcohol dehydrogenase (ADH) were purchased from Sigma (St. Louis, MO).
Animals
Male Long-Evans rats (280–300 g at the beginning of the experiment) were purchased from Harlan (Indianapolis, IN). Animals were single-housed under a 12 hrs light:dark cycle with lights on at 7:00 a.m., food and water available ad libitum, and kept in conditions of constant temperature (23°C) and humidity (50%). All animal procedures in this report were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996.
Intermittent-access 20% ethanol 2-bottle choice drinking paradigm
The procedure is similar to the one described in (Carnicella et al., 2009b). Animals were given 24-hr concurrent access to 1 bottle of 20% ethanol in water and 1 bottle of water, starting at 11:00 a.m. on Monday, Wednesday and Friday, with 24 or 48 hrs ethanol-deprivation periods in-between the ethanol-drinking sessions. The placement (left or right) of each solution was alternated between each session to control for side preference. The water and ethanol bottles were weighed after 24 hrs of access. A bottle containing water in a cage without rats was used to evaluate the spillage that was always ≤ 0.5 ml (< 2.5% of the total fluid intake). This protocol leads to a robust escalation in ethanol intake over 21 ethanol-access sessions (7 weeks) and a stable baseline of 5–6 g/kg/24hrs in parallel to a significant increase in ethanol preference over water (Fig. 1) (Carnicella et al., 2009b). This intermittent procedure also leads to the voluntary consumption of large amounts of ethanol in a short period of time at the beginning of the ethanol-drinking session that generates BECs over 80 mg% in rats (Carnicella et al., 2009b). These BECs correspond to human binge-drinking as defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA, 2004) and as such, the ethanol intake under this paradigm is referred to as excessive. Animals consuming less than 4 g/kg/24hrs at the baseline were excluded from the study.
Figure 1. Escalation of ethanol intake and preference in an intermittent access two-bottle choice paradigm.
Mean ± SEM of ethanol intake (A) and preference (B), and water intake (C) during acquisition of voluntary consumption of a 20% ethanol solution.
Operant ethanol self-administration
Rats were trained to self-administer a 20% ethanol solution in operant self-administration chambers as described in (Carnicella et al., 2008). Briefly, the chambers contain two levers: an active lever, for which presses result in delivery of 0.1 ml of the ethanol solution, and an inactive lever, for which responses are counted but no programmed events occur. After 2 to 3 nights in the chambers to allow acquisition of a lever-press response for ethanol under a fixed ratio 1 (FR1), operant sessions were conducted 5 days per week, with the schedule requirement increased to FR3 and the length of session shortened from 60 to 30 min over the first 2 weeks. Animals pressing for less than 0.4 g/kg/30 min at the baseline were excluded from the study.
Dose-response curve of ethanol self-administration
After 1 month of training under these parameters (FR3, 30 min), which resulted in a stable baseline of operant ethanol self-administration, ethanol concentration was decreased from 20% to 2.5%. A new baseline was considered to be reached when operant responding varied less than 20% within 3 consecutive days. Operant self-administration was therefore performed with this ethanol concentration during 2 weeks in order to obtain a stable baseline (Fig. 2). Then, different concentrations of ethanol were presented in the following ascending order: 5, 10, 20, 40 and 60%. These concentrations were tested during 5 consecutive days only (i.e., one week of self-administration) since a new baseline was obtained in 2 or 3 sessions (Fig. 2). To determine whether this ascending order influences the operant responding for ethanol, 10, 20 and 40% were further tested in an unsystematic order at the end of the experiment (from 60 to 20%, from 20 to 40%, from 40 to 10%). Data from the ninth (2.5%) or fourth (5–60%) session of self-administration, when level of responding was stable (Fig. 2), were used for graphic representations and analysis.
Figure 2. Dose-response curve time-course.
Mean ± SEM number of ethanol deliveries during the 30-min operant sessions for each ethanol concentration.
BEC measurements
At the end of a 30-min operant ethanol self-administration session, rats were briefly anesthetized with isoflurane and blood was collected from the lateral tail vein with heparinized capillary tubes. Serum was extracted with 3.4% trichloroacetic acid and a 5-min centrifugation at 2000 rpm, and then assayed for ethanol content using the NAD-NADH enzyme spectrophotometric method (Weiss et al., 1993; Zapata et al., 2006). BECs were determined by using a standard calibration curve.
Statistical analysis
The experiments were conducted in a within-subjects design and data were analyzed using one-way or two-way ANOVA with repeated measures followed by the Student-Newman-Keuls method or paired t-test when indicated.
Results
History of intermittent-access 20% ethanol 2-bottle choice drinking paradigm produces a reliable acquisition of operant ethanol self-administration
As previously reported (Carnicella et al., 2009b; Simms et al., 2008), rats showed a significant escalation of ethanol intake (F(8, 160) = 77.96 p < 0.001) and preference (F(8, 160) = 88.95, p < 0.001) across sessions of access to 20% ethanol (Fig. 1A, B), with a concomitant decrease in water consumption (Fig. 1C; F(8, 160) = 92.33, p < 0.001). Rats reached a stable baseline of ethanol consumption and preference after 12 to 15 sessions (ethanol intake and preference averaged from the last 6 sessions: 5.82 ± 0.17 g/kg/24hrs and 52.2 ± 0.2 %, respectively). Two rats out of 11 failed to escalate their ethanol intake and were therefore removed from the experiment and the analysis. Eight out of 9 rats successfully acquired operant self-administration for a 20% ethanol solution. Therefore, out of 11 initial subjects, 8 reached the criteria of inclusion, leading to a success rate of 73% (Table 1).
Table 1. Characteristics of the acquisition of operant self-administration for a 20% ethanol solution in rats with or without a history of ethanol consumption.
Acquisition of operant ethanol self-administration in rats with a previous exposure to ethanol in an intermittent-access 20% ethanol 2-bottle-choice drinking paradigm (n = 11), or no previous exposure to ethanol (n = 12).
| Previous Exposure to ethanol | Success rate of acquisition | Ethanol/inactive lever responding | Ethanol deliveries | Ethanol intake (g/kg) |
|---|---|---|---|---|
| + | 73% (8/11 rats) | 76.25 ± 12.13/2.50 ± 0.89 | 25.10 ± 3.08 | 0.87 ± 0.15 |
| − | 42% (5/12 rats) | 67.8 ± 4.59/9.8 ± 3.6 | 22.4 ± 1.57 | 0.82 ± 0.05 |
To determine whether the history of excessive ethanol consumption positively influences the acquisition of operant ethanol self-administration, we tested the success rates to acquire operant ethanol self-administration in a group of ethanol naive rats which did not have prior history of excessive ethanol consumption. In contrast to the ethanol intermittent-access experienced group, only 5 subjects out of 12 acquired operant responding for a 20% ethanol solution, corresponding to a success rate of 42% (Table 1). Interestingly, the level of ethanol intake during operant self-administration among these 5 subjects (0.82 ± 0.05 g/kg) corresponded to the level obtained after the intermittent-access procedure (0.87± 0.15 g/kg) (Table 1). Therefore, these data suggest that the history of excessive ethanol consumption induced by the intermittent-access procedure did not directly increase subsequent ethanol intake during operant self-administration but rather increased the number of ethanol responders. These animals also began to be subjected to the ascending order of ethanol concentrations, but since the number of animals that acquired lever press responding was low and the response for each rat was highly variable, the experiment was not carried forward.
Dose-response curve for operant ethanol self-administration in rats with a history of high levels of ethanol consumption
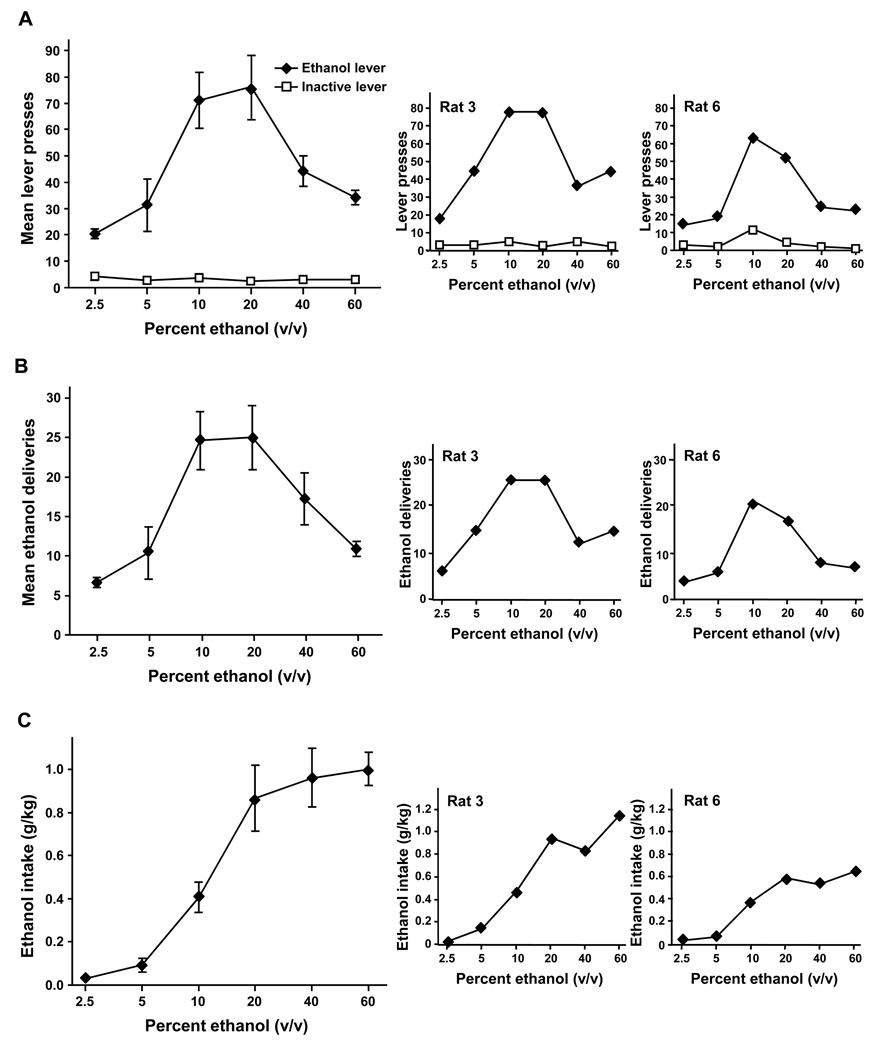
To determine whether a meaningful dose-response curve for oral ethanol self-administration can be obtained in rats, the concentration of the ethanol solution was varied from 2.5% to 60% after acquisition of operant ethanol self-administration following a history of excessive voluntary ethanol intake. As shown in Fig. 3A and B, we obtained the characteristics of a typical inverted U-shape dose-response curve in which the number of presses on the ethanol lever and the number of ethanol deliveries increased from concentrations of 2.5% to 10% ethanol and then gradually decreased from 20% to 60% ethanol (main effect of ethanol concentration: F(5, 35) = 11.98, p < 0.001 and F(5, 35) = 14.56, p < 0.001 for the number of lever presses and of ethanol deliveries respectively). Post hoc analysis using the Student-Newman-Keuls method on the number of lever presses and of ethanol deliveries isolated 10 and 20% from the other concentrations (no significant difference between 10 and 20%: p = 0.39, but all others concentrations are significantly different from 10 and 20%: ps < 0.05). In contrast, the number of inactive lever presses was unaffected by the change in ethanol concentration (Fig. 3A; main effect of the lever: F(1, 35) = 53.48, p < 0.001 and significant interaction between ethanol concentration and lever: F(5, 35) = 15.62, p < 0.001) and the inactive lever condition can be isolated from the ethanol lever condition (no difference within the inactive lever condition: ps > 0.91, but all numbers of lever presses of the inactive lever condition significantly differ from the lever presses on the ethanol lever condition: ps < 0.05). Importantly, ethanol intake conformed to a sigmoid curve (Fig. 3C; main effect of ethanol concentration: F(5,35) = 36.54, p < 0.001) with a similar amount of ethanol consumed when concentrations of 20%, 40% and 60% of ethanol were presented (ps > 0.36, and all different from 2.5, 5 and 10%: ps < 0.001). This asymptotic level of ethanol intake was confirmed by the BEC measurements at the end of the session, showing remarkably constant BECs in rats consuming a solution of 20%, 40% or 60% of ethanol (58.07±12.16, 62.04±9.28, 59.77±7.35 mg% respectively; no effect of the ethanol concentration: F(2, 14) = 0.06, p = 0.95). These data strongly suggest that the progressive decrease in the level of operant responses within this range of concentrations was not due to an increased aversion to the taste of ethanol but directly resulted from an adjustment of the operant behavior to obtain a constant amount of ethanol.
Figure 3. U-shape dose-response curve for ethanol in rats with a history of high levels of ethanol consumption.
Left, Mean ± SEM number of lever presses (A), ethanol deliveries (B) and ethanol intake (C), during a 30-min operant session in function of the ethanol concentration of the solution presented. Right, Individual values of two representative rats. n = 8.
Effects of ethanol concentration on the pattern and rate of operant self-administration
In order to characterize the influence of ascending ethanol concentrations on operant behavior, we analyzed the pattern and rate of operant responding for each dose of ethanol. As indicated by the cumulative number of ethanol deliveries (Fig 4A and 6B), the rate of responding is low for an ethanol concentration of 2.5% or 5% and substantially increased for higher concentrations. However, the rate of responding for ethanol concentrations of 40% and 60% progressively declined over the session (Fig. 4B). A two-way ANOVA found a significant effect of the ethanol concentration (F(5, 98) = 16.08, p < 0.001), a significant effect of time (F(14, 98) = 32.11, p < 0.001), and a significant interaction (F(14, 98) = 8.43, p < 0.001). Consistently, ethanol concentrations of 2.5% and 5% led to a long latency to initiate the operant response and an early termination of the self-administration, as shown in Fig. 4C which represents the period of operant activity during the 30 min session. The duration of the self-administration activity significantly increased with the presentation of a 10% ethanol solution (F(5, 35) = 8.48, p < 0.001; 10% different from 2.5 and 5%: ps < 0.001), reflecting both a decrease in the latency to initiate the operant response (F(5, 35) = 5.49, p < 0.001; 10% different from 5%: p < 0.001, but not from 2.5%: p = 0.36) and a later termination of responding (F(5, 35) = 5.10, p < 0.001; 10% significantly different from 2.5%: p < 0.01, but not from 5% = 0.13) (Fig. 4C). The latency to initiate the operant response remained short under the presentation of higher ethanol concentration (20–60%; ps > 098), but the self-administration period was terminated earlier as the dose increased (60% significantly different from 10%: p < 0.001, but not 20%: p = 0.16, or 40%: p = 0.08) (Fig. 4C). These data, together with the abovementioned results, suggest that low concentrations of ethanol failed to initiate and maintain efficient operant responding, while for higher concentrations animals stop responding when a specific level of ethanol is reached.
Figure 4. Modification of the pattern of responding to changes in ethanol concentrations.
A&B, Mean ± SEM cumulative ethanol deliveries during a 30-min operant session in function of the ethanol concentration of the presented solution. Data in panels A and B were divided into two separate parts for clarity. C, Mean ± SEM period of operant activity during the 30-min self-administration session in function of the ethanol concentration of the solution presented. Period of operant activity is determined by the latencies to the first (bottom of bars) and the last (top of bars) delivery. n = 8.
Effect of the order of presentation of the ethanol concentration on the dose-response function
To determine whether the order of presentation influences the operant responding for ethanol, 10, 20 and 40% were further tested in an unsystematic order at the end of the experiment. This order of presentation led to a dose-response function that was similar to the one observed for the ascending order of presentation. Specifically, compared to the presentation of a 20% ethanol solution, the 40% ethanol solution induced a decrease in the number of presses on the ethanol lever (Fig. 5A; main effect of the lever: F(1, 14) = 79.31, p < 0.001), main effect of ethanol concentration: F(2, 14) = 9.50, p < 0.01, and significant interaction: F(2, 14) = 7.71, p < 0.01). Consequently, a decrease in the number of ethanol deliveries was obtained (Fig. 5B; F(2, 14) = 9.20, p < 0.01), but no change in ethanol intake (Fig. 5C; F(2, 14) = 103.71, p < 0.001, and no difference between 20 and 40%: p = 0.28). Importantly, the ethanol intakes were equivalent to those obtained with the ascending order presentation (Ts(7) < 1.41, ps > 0.33). Moreover, the patterns of operant responding in function of the ethanol concentration (Fig. 5D; main effect of ethanol concentration: (F(2, 98) = 18.45, p < 0.001), main effect of time: (F(14, 98) = 24.88, p < 0.001), and significant interaction (F(14, 98) = 6.53, p < 0.001) are also similar between the two procedures of presentation. These data therefore suggest that the order of presentation of the ethanol concentrations did not influence the shape of the dose-response curve.
Figure 5. Dose-response function following random presentation of different ethanol concentrations.
A–C, Mean ± SEM number of lever presses (A), ethanol deliveries (B), ethanol intake (C) and cumulative ethanol deliveries (D) during a 30-min operant session in function of the ethanol concentration of the solution presented. **p < 0.01, ***p < 0.001 compared with the other ethanol concentrations.
Discussion
In the present study we show that varying the concentration of the ethanol solution over a wide range (2.5 to 60%) in an operant self-administration procedure led to a typical inverted U-shape dose-response curve in rats with a history of excessive ethanol intake. Moreover, we found that rats adapted their level, rate and pattern of responding to changes in ethanol concentration to obtain a constant level of ethanol intake and BEC.
Intermittent-access to ethanol in rats produces robust and stable voluntary ethanol drinking without using a prior sucrose fading procedure (Carnicella et al., 2009b; Simms et al., 2008; Wahlstrom and Nordberg, 1987; Wise, 1973). Here we show that an intermittent-access procedure to a 20% ethanol solution promotes the subsequent acquisition of operant ethanol self-administration. It is noteworthy however, that while this procedure increases the number of ethanol responders, it does not directly increase ethanol intake compared to animals with no history of excessive ethanol consumption and a shorter ethanol experience, suggesting a possible differential effect of the intermittent-access procedure on drinking- and seeking-behaviors. While the underlying mechanisms remain to be elucidated, habituation or changes in the aversive effects of ethanol, such as taste (Kiefer et al., 1994; Kiefer et al., 2005), are likely to play a role in the development of elevated ethanol intake and the subsequent acquisition of operant ethanol self-administration, as well as to facilitate consumption of higher ethanol concentrations (Wolffgramm, 1990). Specifically, it may account for the increasing number of operant ethanol responders following the intermittent-access procedure. It should also be noted that the intermittent-access 20% ethanol 2-bottle choice drinking paradigm leads to neuroadaptations, such as an increase in AMPA receptors function in the ventral tegmental area (Stuber et al., 2008), and such changes may also contribute to the acquisition of operant ethanol self-administration by increasing the motivation to seek and consume ethanol.
As the innate taste aversion of rats for moderate to high doses of ethanol may interfere with oral self-administration of ethanol, only a few studies have examined adaptations in operant behaviors to changes from low to high ethanol concentrations (Meisch and Thompson, 1974; Samson et al., 1988). Here, we demonstrate that a meaningful dose-response curve can be obtained in rats habituated to voluntarily drink large amounts of ethanol, with an ascending limb for low to moderate ethanol concentrations (2.5 to 10%) and a descending limb for higher concentrations (20 to 60%). Within the descending limb, the volume of ethanol solution self-administered progressively decreases as the concentration increases. However, ethanol intake remains relatively constant, with remarkably similar BECs for the 3 concentrations tested. Therefore, this inverted relationship between operant responding and ethanol concentrations is likely to be due to a tight regulation of the level of ethanol consumed rather than to an increased taste aversion to the solution delivered. Consistently, operant responding terminates when a specific amount of ethanol is consumed, leading to shorter self-administration periods for higher ethanol concentrations as ethanol is cumulated more rapidly. Moreover, the decrease in the latency to initiate operant behavior with high ethanol concentrations is not consistent with a negative influence of the taste of ethanol that would be expected to delay the initiation of responding as the ethanol concentration increases. Indeed, the adaptation in the level and pattern of responding to changes in ethanol concentration converges to the point of obtaining a desired level of ethanol as observed for i.v. self-administration of ethanol, amphetamine and cocaine (reviewed in Lynch and Carroll, 2001). Our results therefore suggest that the taste of ethanol, even for very high doses such as 60%, is not an obstacle to perform a meaningful dose-response curve for oral self-administration in rats with a history of excessive ethanol intake.
Different mechanisms can underlie the regulation of drug intake. Among them, maintenance of the reinforcing effect the drug at an optimal level is thought to play a critical role (Killeen and Reilly, 2001; Lynch and Carroll, 2001; Yokel, 1987; Zittel-Lazarini et al., 2007) and the regulation of ethanol intake and operant behavior observed in the present study is consistent with this hypothesis. A second possible mechanism is the “direct effect”, which refers to a general decrease in operant performance due to disruptive effects of high levels of a drug of abuse, such as alteration of motor skills, confusion or sedation (Lynch and Carroll, 2001; Madden, 2001; Mello and Negus, 1996; Yokel, 1987). Accordingly, drug levels would be maintained at a threshold above which the ability of the animal to respond is impaired. In our study, the maximal ethanol intake leads to a BEC of 60 mg%, while ethanol-induced sedative and ataxic effects in rats appear for BECs of 100 mg% or higher (Little, 1999). Therefore, such depressant effects of ethanol are unlikely to contribute significantly to the tight regulation of ethanol intake we observed.
As suggested by behavioral economic models, the ascending limb may be controlled by the complex relation between the dose delivered and the rate of self-administration required to obtain a desired drug level (Oleson and Roberts, 2009; Sizemore and Martin, 2000; Zittel-Lazarini et al., 2007). For i.v. cocaine self-administration in rats (Sizemore and Martin, 2000; Zittel-Lazarini et al., 2007), subjects press for an ideal level of intake or do not press when the rate of responding is perceived as too high to obtain this level, in an all or nothing manner (Sizemore and Martin, 2000; Zittel-Lazarini et al., 2007). In our study however, individual responding gradually increases as the concentration of ethanol increases within the ascending limb and subjects maintain a certain level of responding even for intake below the desired level (see Fig. 3). This divergence with cocaine self-administration is likely to be due to the different route of self-administration. Intravenous self-administration of cocaine can be considered as purely pharmacologic while during oral self-administration of ethanol, animals also experience the exteroceptive properties of the drug that function as discriminative stimuli and conditioned reinforcers (Carnicella et al., 2008; Meisch, 2001; Meisch and Stewart, 1994). Therefore, the taste and odor of ethanol is likely to play an important role in maintaining operant responding for low doses. Testing operant responding for each dose of ethanol in extinction as well as using a progressive ratio procedure would be of great interest for further studies that will provide an in depth insight into the influence of the ethanol concentration, i.e. the unit, on motivational processes.
Testing the effects of a potential new pharmacotherapy on a full dose-response function for the self-administration of a drug of abuse improves its preclinical evaluation; this approach has been fully validated for opioid and psychostimulant self-administration in rats and monkeys (reviewed in Mello and Negus, 1996). For example, mu-selective opioid and dopaminergic antagonists produce a rightward shift of the dose-response curve for self-administration of opioids and cocaine, respectively (Barrett et al., 2004; Mello and Negus, 1996). Conversely, D2-like agonists that substitute for the reinforcing effect of cocaine (Wise et al., 1990) shift the dose-response curve to the left (Barrett et al., 2004). In contrast, a downward shift, as induced by systemic injection of the Ibogaine-derivative 18-methoxycoronaridine (Maisonneuve and Glick, 1999) or intra-nucleus accumbens infusion of BDNF inhibitory antibodies (Graham et al., 2007) for morphine and cocaine self-administration respectively, indicates a direct inhibitory effect on the motivation to consume the drug. Therefore, the procedure we developed in the present study for oral ethanol self-administration may be an important tool for preclinical evaluation of existing and new pharmacotherapies for alcohol abuse disorders that can be used in combination with other procedures such as models of relapse (Carnicella et al., 2009a; Le and Shaham, 2002). This procedure may also be useful to evaluate the motivation to consume ethanol in strains of rats selected for high ethanol intake or following behavioral manipulation such as ethanol deprivation. Specifically, if the increase in consumption results from a change in the sensitivity to the pharmacological effect of ethanol (i.e., tolerance or sensitization), a horizontal shift would be observed. In contrast, if it results from an actual increase in motivation, an upward shift would be observed (see Ahmed and Koob, 1999 and Zernig et al., 2007 for a similar discussion about cocaine self-administration).
In summary, we demonstrate that a bitonic dose-response curve can be obtained for oral operant ethanol self-administration in rats, with an ascending limb mainly governed by the conditioned reinforcing properties of ethanol, and a descending limb tightly regulated by pharmacological effects. In combination with other approaches, we suggest that this procedure can be a useful and straightforward tool for the prediction of the clinical efficacy of potential new medications for the treatment of alcohol use and abuse disorders.
Acknowledgements
We thank Drs. Jerome Jeanblanc and Ronald Keiflin for helpful discussions and Dr. Segev Barak for critically reviewing the manuscript.
This work was supported by the NIH-NIAAA, R01 AA014366 (D.R.), R01 AA016848 (D.R.), R01 AA013438 (D.R.), P50 AA017072 (D.R.), and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (D.R.).
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47 Suppl 1:256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Kiefer SW. Taste reactivity in alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 1990;14:721–727. doi: 10.1111/j.1530-0277.1990.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JM, Llewellyn ME, Woods JH. Variable interval responding maintained by intravenous codeine and ethanol injections in the rhesus monkey. Pharmacol Biochem Behav. 1976;5 doi: 10.1016/0091-3057(76)90272-0. 577-52. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, Ron D. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biol Psychiatry. 2009a;66:146–153. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009b;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol Biochem Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Glick SD, Cox RS, Crane AM. Changes in morphine self-administration and morphine dependence after lesions of the caudate nucleus in rats. Psychopharmacologia. 1975;41:219–224. doi: 10.1007/BF00428927. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Karoly AJ, Winger G, Ikomi F, Woods JH. The reinforcing property of ethanol in the rhesus monkey II. Some variables related to the maintenance of intravenous ethanol-reinforced responding. Psychopharmacology (Berl) 1978;58:19–25. doi: 10.1007/BF00426785. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Badia-Elder N. Alterations in taste reactivity to alcohol in rats given continuous alcohol access followed by abstinence. Alcohol Clin Exp Res. 1994;18:555–559. doi: 10.1111/j.1530-0277.1994.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37:167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Reilly MP. No thanks, I'm good. Any more and I'll be sick: comment on Lynch and Carroll (2001) Exp Clin Psychopharmacol. 2001;9:144–147. doi: 10.1037//1064-1297.9.2.144. discussion 160–162. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Semenova S, Zvartau E, De Vry J. Effects of calcium channel blockade on intravenous self-administration of ethanol in rats. Eur Neuropsychopharmacol. 1999;9:197–203. doi: 10.1016/s0924-977x(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Little HJ. The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol Ther. 1999;84:333–353. doi: 10.1016/s0163-7258(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL. Influence of dopaminergic and serotonergic neurons on intravenous ethanol self-administration in the rat. Pharmacol Biochem Behav. 1992;42:187–192. doi: 10.1016/0091-3057(92)90465-r. [DOI] [PubMed] [Google Scholar]
- Madden GJ. Drug-intake regulation and the interplay between economic costs and benefits: comment on Lynch and Carroll (2001) Exp Clin Psychopharmacol. 2001;9:148–150. doi: 10.1037//1064-1297.9.2.148. discussion 160–162. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. Eur J Pharmacol. 1999;383:15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Martinetti MP, Lowery EG, Vona SR, Wichnick AM, Adler RA, Finch DG. Limited-access consumption of ascending ethanol concentrations in alcohol-preferring, non-preferring, and Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:836–843. doi: 10.1111/j.1530-0277.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- Meisch RA. Oral drug self-administration: an overview of laboratory animal studies. Alcohol. 2001;24:117–128. doi: 10.1016/s0741-8329(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Stewart RB. Ethanol as a reinforcer: a review of laboratory studies of non-human primates. Behav Pharmacol. 1994;5:425–440. [PubMed] [Google Scholar]
- Meisch RA, Thompson T. Ethanol intake as a function of concentration during food deprivation and satiation. Pharmacol Biochem Behav. 1974;2:589–596. doi: 10.1016/0091-3057(74)90025-2. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- NIAAA. NIAAA Council approves definition of binge drinking. NIAAA Newsletter. 2004;3:2004. [Google Scholar]
- Nowak KL, McKinzie DL, McBride WJ, Murphy JM. Patterns of ethanol and saccharin intake in P rats under limited-access conditions. Alcohol. 1999;19:85–96. doi: 10.1016/s0741-8329(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12:591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Ethanol and sucrose self-administration components: effects of drinking history. Alcohol. 2003;29:31–38. doi: 10.1016/s0741-8329(02)00318-x. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore GM, Martin TJ. Toward a mathematical description of dose-effect functions for self-administered drugs in laboratory animal models. Psychopharmacology (Berl) 2000;153:57–66. doi: 10.1007/s002130000611. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom G. Ethanol exposure as inducer of stable voluntary ethanol drinking in the male rat. Drug Alcohol Depend. 1987;20:105–114. doi: 10.1016/0376-8716(87)90059-7. [DOI] [PubMed] [Google Scholar]
- Wahlstrom G, Nordberg A. Studies of stable voluntary intake of ethanol induced by intermittent ethanol exposure. Alcohol Alcohol Suppl. 1987;1:367–371. [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology. 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology (Berl) 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Intravenous self-administration: Response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 1–33. [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- Zittel-Lazarini A, Cador M, Ahmed SH. A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacology (Berl) 2007;192:337–346. doi: 10.1007/s00213-007-0724-0. [DOI] [PubMed] [Google Scholar]