Abstract
Purpose
Capecitabine has shown similar efficacy to 5-fluorouracil (5-FU); a regimen containing 2 weeks of capecitabine/oxaliplatin (CapOx) has demonstrated noninferiority to infusional 5-FU/oxaliplatin/leucovorin (FOLFOX) for the treatment of metastatic colorectal cancer (mCRC). This phase II study explores the efficacy and safety of a 2-day course of oxaliplatin/capecitabine (2DOC), with oxaliplatin given on day 1 and capecitabine given orally every 8 hours in high doses over 6 doses, mimicking FOLFOX6.
Patients and Methods
This phase II study was conducted by the University of Wisconsin Carbone Cancer Center. Eligible patients with mCRC received oxaliplatin 100 mg/m2 intravenously (I.V.) over 2 hours followed by leucovorin 20 mg/m2 I.V. bolus and 5-FU 400 mg/m2 I.V. bolus on day 1 and day 15. Capecitabine was administered at 1500 mg/m2 orally every 8 hours over 6 doses starting on day 1 and day 15.
Results
A total of 45 patients were enrolled; 44 were evaluated for response. Seventeen patients (39%) had objective responses. Median time to progression was 6.8 months, and median overall survival (OS) was 17.5 months. The most common side effects were grade 1/2 neuropathy, fatigue, and nausea. Severe hand-foot syndrome (HFS) was rare.
Conclusion
The overall response rate with the 2DOC regimen is similar to published CapOx regimens, and time to progression and OS are similar. The incidence of HFS, diarrhea, and mucositis were lower compared with published results of 2-week schedules of capecitabine. The 2DOC regimen merits further study as a more convenient regimen than infusional 5-FU with less HFS when compared with a 2-week administration of capecitabine.
Keywords: 2DOC, FOLFOX6, Irinotecan, Neutropenia, Thrombocytopenia
Background
Chemotherapy has been shown to improve quality of life and prolong overall survival (OS) in patients with metastatic colorectal cancer (mCRC).1 Further advances in survival have been demonstrated in third-generation regimens of bolus/infusional 5-fluorouracil (FU), leucovorin (LV), and oxaliplatin (FOLFOX) or irinotecan (FOLFIRI), with median survival reaching more than 20 months.2 Administration of infusional regimens can be cumbersome, requiring indwelling catheters, infusion supplies, and home nursing visits. Indwelling catheters are associated with pneumothorax or malposition during placement and late complications, which can range from inconvenient (malfunction, discomfort) to life-threatening (thromboembolism, pocket-site infection, catheter embolization).3 Simplified regimens are sought to maintain efficacy while minimizing prolonged infusions.
Capecitabine is an oral prodrug of 5-FU that is converted enzymatically to active 5-FU by thymidine phosphorylase, which is more active in colorectal cancer (CRC) cells than surrounding normal cells.4,5 Single-agent capecitabine has been shown to have equivalent efficacy when compared with bolus 5-FU/LV (Mayo regimen) in phase III studies with mCRC.6 Given this efficacy, capecitabine is an attractive option to avoid infusional 5-FU. Published protocols combining capecitabine and oxaliplatin (CapOx) involved 2 weeks of twice-daily capecitabine combined with oxaliplatin in varying schedules.7-13 In a study that targeted older adult patients, grade 3/4 toxicities occurred in 28% of patients receiving CapOx, which appeared better than the 42%-80% in other published studies of older adults who received a FOLFOX-type regimen.14,15
During 2-week administrations of capecitabine, palmar-plantar erythrodysesthesia, or hand-foot syndrome (HFS), was reported in about one third of patients, and diarrhea in 15%-20%.9 These side effects are best managed by treatment interruption, as they are thought to be secondary to prolonged exposure to capecitabine.16 If the duration of capecitabine treatment could be reduced, the side-effect profile of the regimen should be improved. A randomized phase II study explored the use of higher-dose capecitabine with 85 mg/m2 of oxaliplatin every other week of a 4-week cycle. No statistically significant change in toxicity was identified, and both the response rate (RR; 54.5% vs. 42.2%) and progression-free survival (PFS; 10.5 months vs. 6.0 months; P = .0013) were higher in the dose-intense arm.17 Based on these data, a more dose-intense strategy of capecitabine and oxaliplatin is safe and feasible, with potential benefits in terms of efficacy.
The current phase II study was conducted to evaluate the efficacy and safety of a regimen containing oxaliplatin, bolus 5-FU/LV, and a 2-day course of oral capecitabine repeated every 2 weeks. We abbreviated the name of the regimen to “2DOC” for “two-day oxaliplatin/capecitabine.” The dosing schedule is meant to replicate that of mFOLFOX6, which involves 46 hours of continuous-infusion 5-FU.2 In the phase I study exploring this regimen, only 1 of 36 patients experienced HFS. The dose-limiting toxicities of the regimen were neutropenia, gastrointestinal side effects, and 2 episodes of confusion. Of 7 patients with advanced, pretreated CRC, 1 partial response was seen, and stable disease was manifest in 3 patients, which prompted further investigation.18
Patients and Methods
Study Design
This study was a single-arm open-label phase II study conducted as a multi-institutional study through the University of Wisconsin Carbone Cancer Center (UWCCC) and the Wisconsin Oncology Network (WON). The WON is a consortium of academic, university-affiliated, and community-practice sites that have agreed to participate in clinical trial enrollment through the University of Wisconsin. The institutions of each participating site were required to obtain local institutional review board approval. Scientific review committee approval was also required where applicable.
The primary endpoint was overall response rate. Secondary objectives included time to progression, OS, response of tumor markers, and toxicity. A Simon-Optimal 2-stage accrual design was used. After 24 patients, the threshold for proceeding (≥ 5 responses) was met, and 21 additional patients were enrolled.
Patients
Patients were eligible if they were over 18 years of age; had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, 1, or 2; and were able to provide informed consent for the study. Pathologically confirmed metastatic colorectal cancer, with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST), was required.19 Previous systemic therapy for advanced CRC was not allowed, but adjuvant therapy with oxaliplatin-containing regimens was allowed. The timing of adjuvant therapy was not specified as part of the protocol. Laboratory exclusion criteria included white blood cell count < 3000 cells/μL; absolute neutrophil count < 1500 cells/μL; platelets < 100,000 cells/μL; total bilirubin > 1.5 mg/dL; aspartate aminotransferase (AST) > 2.5 U/L × upper limit of normal (ULN); alanine aminotransferase (ALT) > 2.5 U/L × ULN; and estimated creatinine clearance < 50 mL/min as calculated by the Cockroft-Gault formula. Patients with known central nervous system metastases were ineligible, as were patients with grade 2 or greater peripheral neuropathy. Pregnant or lactating women were excluded from the study. Women of childbearing potential and sexually active males were required to agree to hormonal or barrier methods of contraception.
Patients who received a full treatment cycle were considered evaluable for efficacy. Patients who received a single dose of chemotherapy were evaluable for safety.
Study Assessments
Before registration, screening assessments included a full history and physical, complete blood count, coagulation parameters, carcinoembryonic antigen (CEA), and blood chemistries. Complete blood counts and blood chemistries were checked on day 1 and 15 of each cycle. CEA was rechecked every cycle. Disease evaluation and tumor measurement were conducted at registration with computed tomography (CT) scan and/or magnetic resonance imaging (MRI), and repeated every 8 weeks. Tumor response was assessed according to RECIST and confirmed with repeated imaging 4 weeks following initial documentation of an objective response.
Treatment
Based on the results from the phase I trial, the recommended phase II dose used in this trial was oxaliplatin 100 mg/m2 intravenously (I.V.) over 2 hours followed by LV 20 mg/m2 I.V. bolus and 5-FU 400 mg/m2 I.V. bolus on day 1 and day 15. Capecitabine was administered at 1500 mg/m2 orally every 8 hours for 6 doses starting on day 1 and day 15. The first dose of capecitabine was given concurrently with oxaliplatin. Cycle duration was defined as 28 days.
Toxicity
Toxicity grades were assigned using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0.20 Dose modifications for toxicity were based on the worst toxicity observed during the previous course. For grade 3 leukopenia/neutropenia, the dose of bolus 5-FU was reduced to 200 mg/m2 for subsequent treatments. If grade 3 hematologic toxicity persisted, bolus 5-FU was discontinued. For further hematologic toxicity after this modification, the oxaliplatin dose was reduced by 20%. For any grade 4 hematologic toxicity, the dose of oxaliplatin, 5-FU, and capecitabine were all reduced by 20%. For grade 3 or 4 gastrointestinal or hepatic toxicities (excluding nausea and vomiting), therapy was held until toxicity returned to baseline, and retreatment commenced with a 20% dose reduction of all 3 drugs. For grade 2 HFS, the next treatment was delayed until toxicity resolved to grade 1 or less. For grade 3 HFS, the same schema applied, and the capecitabine dose was reduced by 25%.
Statistical Analysis
The primary endpoint was overall response rate, defined as complete response (CR) or partial response (PR). On the basis of efficacy data for 5-FU and oxaliplatin combinations, we estimated that 45 patients would need to be enrolled to demonstrate an overall response rate (ORR) of at least 40% with 90% power. Progression-free survival was calculated based on the date of treatment initiation and estimated by Kaplan-Meier analysis.
Results
Patient Demographics
A total of 45 patients (25 women, 20 men) were enrolled between June 2004 and October 2007. The median age of patients was 64 years (range, 41-76 years); the mean age was 61.56 years (SD = 9.87). ECOG PS was 0 or 1 in 34 patients (Table 1). Mean baseline CEA was 328 ng/mL (range, 0.7-7041 ng/mL). Of the 32 patients with documentation of the primary site, 27 were colon primaries, and 5 were rectal primaries.
Table 1. Patient Demographicsa.
| Demographic | Number of Patients (%) |
|---|---|
| Sex | |
| Female | 25 (56) |
| Male | 20 (44) |
| Race | |
| White | 43 (96) |
| Other | 2 (4) |
| Age, Yearsb | |
| 40-49 | 7 (16) |
| 50-59 | 11 (24) |
| 60-69 | 15 (33) |
| 70-79 | 12 (27) |
| ECOG Baseline PS | |
| 0 | 14 (40) |
| 1 | 20 (57) |
| 2 | 1 (3) |
Total number of patients = 45.
Median age = 64 years.
Abbreviations: ECOG = Eastern Cooperative Oncology Group; PS = performance status
Efficacy
A total of 44 patients were included in the efficacy analysis based on intention to treat. One patient, who withdrew consent before receiving a cycle of chemotherapy, was excluded from the efficacy and safety analyses. Three patients received 1 dose of therapy but had to be stopped because of toxicity. Response data are shown in Table 2. Seventeen patients (39%) achieved a PR to the 2DOC regimen; PR was confirmed by a central radiographic review. Seventeen patients (39%) maintained stable disease, with median duration of stable disease of 4.96 months (95% CI, 4.17-6.60 months). Median time to disease progression was 6.8 months (95% CI, 4.14-9.92 months), and median OS was 17.5 months (95% CI, 14.32-27.86 months; Table 2 and Figures 1 and 2).
Table 2. Patient Response to the 2DOC Regimena.
| Best Response | Number of Patients (%) |
|---|---|
| Partial Response | 17 (39) |
| Progressive Disease | 7 (16) |
| Stable Disease | 17 (39) |
Total number of patients = 44.
Abbreviation: 2DOC = 2-day oxaliplatin/capecitabine
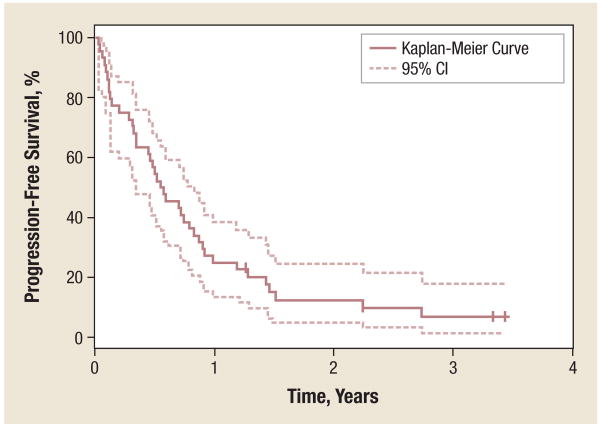
Figure 1. Progression-Free Survival.
Of 41 patients, 57% achieved 6-month PFS (95% CI, 41%-70%); 25% achieved 1-year PFS (95% CI, 14%-38%); and 13% achieved 2-year PFS (95% CI, 5%-25%). Median PFS was 6.8 months (95% CI, 4.14-9.92 months).
Abbreviation: PFS = progression-free survival
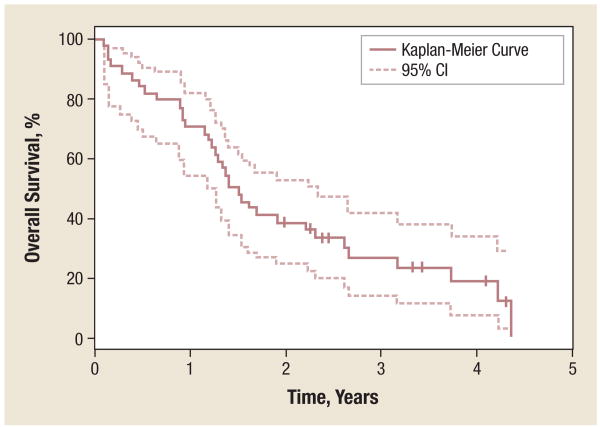
Figure 2. Overall Survival.
Of 41 patients, 84% achieved 6-month OS (95% CI, 70%-92%); 71% achieved 1-year OS (95% CI, 55%-82%); 39% achieved 2-year OS (95% CI, 25%-53%); and 27% achieved 3-year OS (95% CI, 14%-41%). Median OS was 17.50 months (95% CI, 14.32-27.86 months).
Abbreviation: OS = overall survival
Four patients (9%) underwent liver metastasectomy following completion of 2DOC therapy. All four of these patients are alive at the time of this article's submission. One of the four is receiving single-agent cetuximab for liver and lung recurrence, 2 remain on observation only (> 2 years from surgery), and 1 patient moved out of state but is still alive.
An exploratory analysis regarding the effect of the 2DOC regimen on CEA was also performed. Mean CEA decreased by 144 ng/mL at the end of cycle 2 (range, 0.8-4437 ng/mL). A mean decline in CEA was manifest in patients receiving the regimen, but this decline did not correlate with response.
Safety Analysis
A safety analysis of 44 patients who received therapy is presented in Tables 3 and 4. The median number of cycles administered was 4 (range, 1-11 cycles). Nine patients discontinued treatment because of adverse events. The most common treatment-related adverse event was sensory neuropathy in 24 patients (55%), which were mostly grade 1/2. Other common toxicities included nausea (45%), fatigue (42%), thrombocytopenia (36%), anemia (33%), neutropenia (27%), leukopenia (29%), and diarrhea (23%). Of particular relevance, there were only 4 patients (9%) with HFS; only 1 instance of grade 2 HFS was reported in this study. The confusion present in the phase I study of 2DOC was not seen in this trial. Mucositis was very rare as well, with only 1 patient (2%) reporting grade 1 mucositis.
Table 3. Adverse Events in Patients Receiving the 2DOC Regimena.
| Adverse Event | Number of Patients (%) | Number of Adverse Events |
|---|---|---|
| Gastrointestinal | ||
| Nausea | 20 (45) | 22 |
| Anorexia | 11 (25) | 13 |
| Diarrhea | 10 (23) | 11 |
| Vomiting | 8 (18) | 8 |
| Constipation | 7 (16) | 7 |
| Neurology | ||
| Neuropathy-sensory | 24 (55) | 44 |
| Blood/Bone Marrow | ||
| Thrombocytopenia (platelets) | 16 (36) | 24 |
| Anemia (hemoglobin) | 15 (33) | 21 |
| Leukopenia (leukocytes; total WBC) | 13 (29) | 18 |
| Neutropenia/granulocytopenia (ANC/AGC) | 12 (27) | 16 |
| Constitutional Symptoms | ||
| Fatigue | 19 (42) | 25 |
| Dermatology/Skin | ||
| Hand-foot skin reaction | 4 (9) | 6 |
| Pain | ||
| Pain-other | 8 (18) | 9 |
| Abdominal pain or cramping | 4 (9) | 4 |
| Hepatic | ||
| AST | 8 (18) | 10 |
| Alkaline phosphatase | 7 (16) | 8 |
| Metabolic/Laboratory | ||
| Hyperglycemia | 6 (14) | 10 |
| Pulmonary | ||
| Dyspnea | 5 (11) | 6 |
Total number of patients = 44. All toxicity grades are included.
Abbreviations: 2DOC = 2-day oxaliplatin/capecitabine; AGC = absolute granulocyte count; ANC = absolute neutrophil count; AST = aspartate aminotransferase; WBC = white blood cell count
Table 4. Grade 3/4 Adverse Events in Patients Receiving 2DOCa.
| Adverse Event | Grade 3 Number of Patients (%) | Grade 4 Number of Patients (%) |
|---|---|---|
| Leukocytopenia (Total WBC) | 1 (2) | 0 (0) |
| Neutropenia/Granulocytopenia | 5 (11) | 2 (4) |
| Platelets | 1 (2) | 0 (0) |
| Coagulation, Other | 1 (2) | 0 (0) |
| Fatigue | 2 (4) | 0 (0) |
| Fever | 2 (4) | 0 (0) |
| Anorexia | 1 (2) | 0 (0) |
| Dehydration | 1 (2) | 1 (2) |
| Diarrhea Patients With a Colostomy | 1 (2) | 0 (0) |
| Diarrhea Patients Without Colostomy | 0 (0) | 1 (2) |
| Nausea | 0 (0) | 1 (2) |
| Vomiting | 0 (0) | 1 (2) |
| AST | 1 (2) | 0 (0) |
| Infection Without Neutropenia | 3 (7) | 0 (0) |
| Neuropathy, Motor | 1 (2) | 0 (0) |
| Neuropathy, Sensory | 5 (11) | 0 (0) |
| Pain, Other | 1 (2) | 0 (0) |
| Fistula or GU Fistula (eg, Vaginal, Vesicovaginal) | 0 (0) | 1 (2) |
Total number of patients = 44.
Abbreviations: 2DOC = 2-day oxaliplatin/capecitabine; AST = aspartate aminotransferase; GU = genitourinary; WBC = white cell blood count
The most severe adverse events were neutropenia (grade 4, 2 events) and dehydration, diarrhea, nausea, and vomiting (grade 4, 1 event each). One patient developed a grade 4 genitourinary fistula that required cessation of therapy before first disease reevaluation. Common side effects are listed in Table 3.
Discussion
The 2DOC regimen demonstrated antitumor activity in metastatic/advanced CRC. The response rate of 39% is consistent with other published capecitabine/oxaliplatin combinations. Phase II studies of oxaliplatin and twice-daily capecitabine for 14 days demonstrated objective response rates of 27%-55% as first-line therapy, and 15% ORR as second-line therapy.9,11,12,21 Phase III studies comparing capecitabine-containing regimens to FOLFOX-type regimens showed a consistent trend of slightly decreased RR in CapOx, but OS and PFS were not statistically significantly different.10,13 A meta-analysis of all of the above studies reaffirmed the findings from the individual trials: similar PFS and OS, but decreased RR.7 The phase III NO16966 trial was the largest of all included studies, with a total of 2034 patients, and had a 2 × 2 design to allow for the inclusion of bevacizumab in each regimen. The trial showed a RR of 47% in the CapOx arm compared with 48% with FOLFOX4. This response rate was unchanged with the addition of bevacizumab to both regimens. CapOx was shown to be noninferior to the FOLFOX4 regimen in terms of PFS and OS in this trial.8
Importantly, all 4 of the patients whose disease was controlled to the point that they could proceed with metastasectomy are still alive. Only 1 patient had confirmed recurrence and required active antineoplastic therapy postoperatively. A Surveillance, Epidemiology, and End Results database review from 2007 demonstrated that only 6.1% of patients with liver metastases were able to undergo metastasectomy; of those patients, 5-year survival was 32.8%.22 The 2DOC regimen appears to produce positive results consistent with this cohort, with the caveat that the 2DOC sample size is small and 5-year follow-up has not been completed.
2DOC has toxicities similar to other capecitabine/oxaliplatin combinations, with some important exceptions. Neuropathy was the most common side effect, likely secondary to oxaliplatin, and not worse on this regimen than previously reported with other capecitabine schedules. The incidence of severe HFS was lower for 2DOC than in published reports of 2-week capecitabine dosing schedules. Given that HFS is often a treatment-limiting toxicity with longer courses of capecitabine, the near absence of severe HFS is encouraging. This factor makes the 2DOC schedule an attractive alternative when designing treatment schedules combining fluoropyrimidines with other drugs that cause HFS, such as the multikinase inhibitors sorafenib and sunitinib.23,24 To that end, we have designed a trial that is currently enrolling patients that will use 2DOC in combination with sorafenib for the treatment of pancreas and biliary-tract cancers. Other systemic limiting toxicities of capecitabine (diarrhea, mucositis) were noticeably decreased compared with other published historical controls.
This trial is relatively small and does not have sufficient power to reliably assess OS. Consequently, it was not designed as a direct comparison between the existing CapOx regimens and 2DOC. Nevertheless, the toxicity data are different from the 2-week CapOx regimen in terms of HFS and mucositis, but the response rates and survival times are similar. Further, this 2DOC design does not include bevacizumab, the VEGF-inhibiting agent that has now become part of the standard of care for mCRC. However, the NO 16966 trial demonstrated similar effects on OS between the CapOx arm and FOLFOX4 arms when bevacizumab was added.25
Conclusion
In summary, the 2DOC regimen uses a shorter duration but higher dose of capecitabine, with similar efficacy to previously published capecitabine-containing regimens. Based on the results of this trial, a phase III trial comparing the 2DOC strategy versus either FOLFOX or CapOx could be performed to look for noninferiority; however, powering it for any statistically significant difference would be difficult. The data presented here are not sufficient to argue that 2DOC should supplant FOLFOX or CapOx based on the amount of phase III data that have been published using those dosing strategies. That said, this high-dose, short-course capecitabine schedule represents a potential therapeutic alternative for patients with mCRC who cannot tolerate infusional chemotherapy or suffer excessive toxicity from prolonged administration of capecitabine. Furthermore, combining 2DOC with multikinase inhibitors in future studies could help avoid dose-limiting or confounding HFS and other toxicities.
Acknowledgments
This work was supported by sanofi-aventis U.S.
Footnotes
Disclosures: Noelle K. LoConte, Daniel L. Mulkerin (institutional), William Schelman, and Kyle D. Holen have received research funding from sanofi-aventis U.S. All other authors have no relevant relationships to disclose.
References
- 1.Glimelius B, Hoffman K, Graf W, et al. Quality of life during chemotherapy in patients with symptomatic advanced colorectal cancer. Cancer. 1994;73:556–62. doi: 10.1002/1097-0142(19940201)73:3<556::aid-cncr2820730310>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Biffi R, Debraud F, Orsi F, et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol. 1998;9:767–73. doi: 10.1023/a:1008392423469. [DOI] [PubMed] [Google Scholar]
- 4.Schuller J, Cassidy J, Dumont E, et al. Preferential activation of capecitabine in tumor following oral administration in colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45:291–7. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Findlay M, Osterwalder B, et al. Capecitabine, an oral fluoropyrimidine carbamate with substatial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol. 2000;18:1337–45. doi: 10.1200/JCO.2000.18.6.1337. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 7.Arkenau HT, Arnold D, Cassidy J, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol. 2008;26:5910–7. doi: 10.1200/JCO.2008.16.7759. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–12. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084–91. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Rubio E, Tabernero J, Gomez-Espana A, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: Final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224–30. doi: 10.1200/JCO.2006.09.8467. [DOI] [PubMed] [Google Scholar]
- 11.Hochster HS, Hart LL, Ramantathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol. 2008;26:3523–9. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 12.Martoni AA, Pinto C, Di Fabio F, et al. Capecitabine plus oxaliplatin (XELOX) versus protracted 5-fluorouracil venous infusion plus oxaliplatin (pviFOX) as first-line treatment in advanced colorectal cancer: a GOAM phase II randomised study (FOCA trial) Eur J Cancer. 2006;42:3161–8. doi: 10.1016/j.ejca.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Porschen R, Arkenau HT, Kubicka S, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO colorectal study group. J Clin Oncol. 2007;25:4217–23. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 14.Feliu J, Salud A, Escudero P, et al. XELOX as first-line treatment for elderly patients over 70 years of age with advanced colorectal cancer. Br J Cancer. 2006;94:969–75. doi: 10.1038/sj.bjc.6603047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aparicio T, Desrame J, Lecomte T, et al. Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly. Br J Cancer. 2003;89:1439–44. doi: 10.1038/sj.bjc.6601310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract. 2006;12:131–41. doi: 10.1177/1078155206069242. [DOI] [PubMed] [Google Scholar]
- 17.Scheithauer W, Kornek GV, Raderer M, et al. Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2003;21:1307–12. doi: 10.1200/JCO.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Mulkerin D, LoConte N, Holen K, et al. A phase I study of an oral simulated FOLFOX with high dose capecitabine. Invest New Drugs. 2009;27:461–8. doi: 10.1007/s10637-008-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck S, Elizabeth A, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006 Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 21.Lopez-Vivanco G, Munoz A, Mane JM, et al. Combination of oxaliplatin and capecitabine (CAPOX) in first and second-line treatment for metastatic colorectal carcinoma (MCRC) J Clin Oncol. 2004;22(suppl) abstract 3701. [Google Scholar]
- 22.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–26. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 23.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–61. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 24.Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–92. doi: 10.1001/archderm.144.7.886. [DOI] [PubMed] [Google Scholar]
- 25.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin based chemotherapy as first line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]