Abstract
Background & aims
CXCL5 (also known as Epithelial neutrophil-activating peptide 78 or ENA-78) belongs to the CXC chemokine family and has been shown to have promitotic effects on hepatocytes. The aim of our study was to assess CXCL5 plasma levels in patients with chronic liver disease.
Materials & Methods
CXCL5 plasma levels were measured in 111 patients with chronic liver disease patients and 98 healthy controls. Gene expression of CXCL5 and its main receptor CXCR2 were also determined in liver biopsies from 46 patients.
Results
CXCL5 levels were correlated with clinical presentation, laboratory parameters and liver histology. Plasma CXCL5 levels in patients with liver cirrhosis were lower than in healthy controls, and correlated with the hepatic biosynthetic capacity, Child-Pugh and MELD scores. Patients with hepatic necroinflammation and fibrosis on liver histology showed lower plasma CXCL5. In patients with typical clinical complications of cirrhosis, CXCL5 levels were found to be decreased. Intrahepatically, CXCL5 expression was increased in patients with advanced fibrosis and cirrhosis. The isolation of different cellular compartments from mouse livers suggested that hepatic stellate cells and sinusoidal endothelial cells are the main sources of hepatic CXCL5.
Conclusions
Plasma CXCL5 levels are lower in patients with chronic liver disease, suggesting that CXCL5 might be involved in the pathogenesis of chronic liver disease. CXCL5 could serve as an additional biomarker for hepatic necroinflammation and fibrosis.
Keywords: ENA78, chemokine, hepatocytes, Kupffer cells, Child
Introduction
Liver cirrhosis is an endstage disease and can be caused by several etiologies including viral hepatitis, biliary disease, autoimmune disorders and chronic alcohol abuse. Although considerable progress has been made to understand the molecular mechanism for progression from acute liver injury to fibrosis and cirrhosis, there is no antifibrotic therapy currently available for patients with chronic liver disease. The mainstay is to treat the underlying liver disease. If this can not be achieved and the patient progresses to liver cirrhosis with decompensation, liver transplantation is the only treatment option 1.
Following an acute liver insult, hepatocytes are damaged and undergo necrotic or apoptotic cell death. This is accompanied by a considerable inflammatory reaction, which is partly mediated by resident Kupffer cells or infiltrating inflammatory cells such as monocytes and neutrophils 2. Hepatic stellate cells and other fibrogenic cell types are activated by chemokines and cytokines such as TGF-β1 to produce large amounts of extracellular matrix proteins. Several chemokines have been implicated in the pathogenesis of liver fibrosis. For example the CCR2 ligand MCP-1 is produced by Kupffer cells and HSCs, which promotes hepatic fibrosis by recruitment of macrophages that are associated with HSC activation 3.
The role of CXCL5 (also known as Epithelial neutrophil-activating peptide 78 or ENA-78) has not been elucidated in liver fibrosis and cirrhosis. CXCL5 is best known for its function as neutrophil chemoattractant and activator, and it shares structural and biological features with IL-8 4, 5. CXCL5 is produced by a variety of cell types including monocytes, neutrophils, epithelial cells, fibroblasts and smooth-muscle cells, and its gene expression is induced by proinflammatory stimuli such as TNF, IL-1β and LPS 4, 6, 7. Serum levels of CXCL5 were increased in animals with adjuvant-induced arthritis and correlated with progression of joint inflammation 4. Beside rheumatoid arthritis, CXCL5 has been implicated in the pathogenesis of inflammatory bowel disease 8, renal allograft rejection 9 and obesity-induced insulin resistance 10. In addition, CXCL5 has angiogenic properties that are essential for non-small cell lung cancer 11. Most interestingly, CXCL5 has proliferative effects on rat hepatocytes in vitro and in vivo. Antibody mediated neutralization of CXCL5 slowed the rate of liver regeneration after partial hepatectomy 12.
Therefore, the aim of the current study was to analyze plasma CXCL5 levels in 111 patients with chronic liver disease. We demonstrate that CXCL5 levels are lower in patients with liver cirrhosis, correlate with the Child's and MELD score, liver function capacity and histological necroinflammation as well as fibrosis.
Patients and Methods
Study population
Written informed consent was obtained from all participants, and the study protocol was approved by the local ethics committee. We studied 111 patients (66 male, 45 female) with chronic liver diseases, who were evaluated as inpatients for a potential liver transplantation. 93 patients (55 male, 38 female) had a liver cirrhosis staged Child A, B or C according to Child-Pugh criteria (Table 1) 13. 18 patients (11 male, 7 female) without liver cirrhosis also underwent the liver transplant evaluation program, mainly because of hereditary metabolic disorders or benign (e.g. liver and kidney cysts) or malignant tumors (e.g. hepatocellular carcinoma without cirrhosis, liver metastases). In addition, 98 healthy volunteers (42 male, 56 female, median age 48 years, range 20-75 years) with normal ALT, negative HBV, HCV and HIV markers and no history of liver disease served as controls.
Table 1. Patient demographics and CXCL5 measurements.
Median value and range in parenthesis is given, if not otherwise indicated.
| All | Stages of cirrhosis | |||||
|---|---|---|---|---|---|---|
| No cirrh. | Child A | Child B | Child C | |||
| (n) | 111 | 18 | 35 | 44 | 14 | |
|
| ||||||
| Sex (male/female) | (n) | 66 / 45 | 11 / 7 | 18 / 17 | 30 / 14 | 7 / 7 |
|
| ||||||
| Age | (years) | 46 (18-70) |
46 (18-65) |
41 (18-64) |
48 (20-70) |
40 (26-69) |
|
| ||||||
| Etiology of liver disease | (n) | |||||
| Virus hepatitis | 32 | 1 | 9 | 17 | 5 | |
| Biliary or autoimmune | 27 | 1 | 14 | 10 | 2 | |
| Alcohol or cryptogenic | 29 | 1 | 11 | 10 | 7 | |
| Other origin | 23 | 15 | 1 | 7 | 0 | |
|
| ||||||
| CXCL5 | (pg/ml) | 86.6 (12.4- 2004) |
178.2 (68.4- 2004) |
84.5 (12.4- 1330) |
71.7 (24.6- 390.3) |
44.9 (28.8- 149.3) |
Laboratory parameters
At our center, the evaluation for a potential liver transplantation is a highly standardized routine procedure including analysis of blood chemistry, complete differential blood count, serological markers for virus hepatitis, autoantibodies, tumor markers, serum and lipid electrophoresis, and others. For measurement of experimental parameters, plasma was obtained in EDTA separator tubes, centrifuged at 2000 g at 4 °C for 10 min. and stored at −80°C until analysis. Plasma CXCL5 concentrations were determined by ELISA (Human CXCL5/ENA-78 DuoSet, R&D Systems, Wiesbaden, Germany) according to manufacturer's instructions.
Liver histology
In 65/111 patients, a liver biopsy was performed at time of study entry and semi-quantitatively evaluated for hepatic necroinflammation, cholangitis, cholestasis, steatosis and fibrosis / cirrhosis by a blinded and experienced pathologist 14.
Clinical complications
Typical clinical complications due to portal hypertension, e.g. splenomegaly, ascites, hypertensive gastropathy or esophageal varices, were evaluated by complete abdominal ultrasound (in 109/111 patients) and upper gastrointestinal endoscopy (in 107/111 patients). The medical history of gastrointestinal bleedings (variceal bleeding, hematemesis, melena, hematochezia, hemorrhoidal bleeding, intraabdominal bleeding, and other serious bleedings) and the current systemic bleeding tendency unrelated to portal hypertension (epistaxis, prolonged posttraumatic bleeding, gingival hemorrhage, spontaneous hematoma, and hypermenorrhea / menorrhagia) were assessed by a standardized interview in 105/111 patients, as previously described 15.
Isolation of hepatocytes, Kupffer cells, sinusoidal endothelial cells and hepatic stellate cells
Isolation of liver cell fractions from normal mouse liver using magnetic cell sorting (MACS) has been described 16. These cell fractions were homogenized immediately for RNA extraction. Purity of the cells was routinely checked for contaminating liver cell populations by RT-QPCR using primers specific for albumin, desmin, F4/80 and CD31.
Realtime-PCR analysis
RNA was extracted from using Trizol (Invitrogen). RNA was digested with DNase using the DNA-free kit (Ambion) and reverse transcribed using the High Capacity cDNA Reverse Transcription kit (ABI). Realtime QPCR was performed with Sybr Green as described 17 using primer sets obtained from the NIH qPrimerDepot. mRNA expression analyses were performed from fresh frozen liver biopsies and explanted cirrhotic livers after liver transplantation 18.
Statistical analysis
Due to the skewed distribution of the parameters, data are given as median, minimum, maximum, and shown graphically by box-and-whiskers plots. The box-and-whiskers plots display a statistical summary of the median, quartiles, range and extreme values. The whiskers extend from the minimum to the maximum value excluding outside (>1.5 times upper/lower quartile, open circle) and “far out” (>3 time upper/lower quartile, asterixes) values which are displayed separately. The degree of association between two variables was assessed by the Spearman rank correlation test. Comparisons of parameters between two different groups were conducted with the Mann-Whitney-U-test and multiple comparisons with the Kruskal-Wallis analysis of variances (ANOVA) and Mann-Whitney-U-tests for post hoc analysis. All statistical analyses were performed using SPSS version 12 (SPSS, Chicago, IL, USA).
Results
CXCL5 plasma concentrations are lower in patients with advanced liver disease
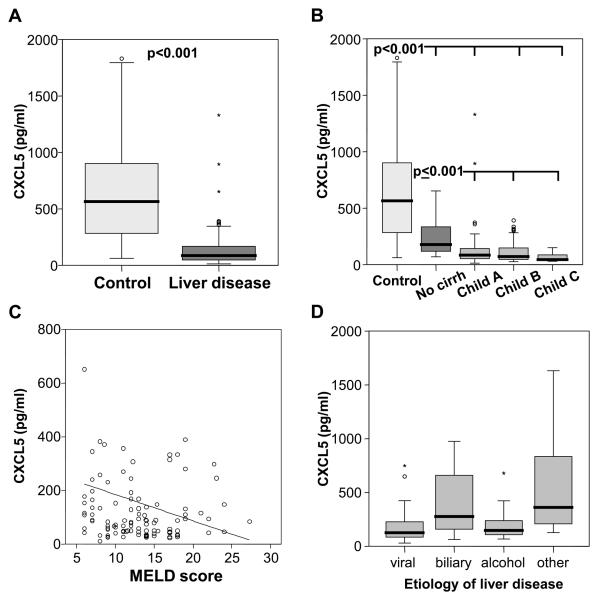
We evaluated 111 patients (66 male, 45 female) with chronic liver diseases who were considered for potential liver transplantation (Table 1). Plasma CXCL5 levels in patients with chronic liver disease were significant lower than in healthy controls (median 86.6 vs. 564.8 pg/ml, p<0.001; Fig. 1A). CXCL5 plasma levels decreased with the stage of cirrhosis according to the Child-Pugh classification (Fig. 1B). Patients without liver cirrhosis (n=18, median 178.2 pg/ml) had significantly lower levels than controls (p<0.001), but higher levels than patients with Child A-C cirrhosis (n=93, median 70.1, p<0.001). The level of decompensation was also assessed by the MELD scoring system, and CXCL5 showed a negative correlation with the MELD score (r=−0.208, p=0.03; Fig.1C). CXCL5 was lower in patients with viral hepatitis or alcoholic liver disease as compared to patients with biliary/autoimmune disease or other causes of liver disease (Fig. 1D), but this association was fully explained by the higher prevalence of advanced disease stages in these two etiology subgroups (Table 1). There was a significant correlation between CXCL5 plasma levels and the hepatic biosynthetic capacity as assessed by various laboratory parameters such as albumin, cholinesterase activity, prothrombin time or single hepatic coagulation factors (Table 2). However, we did not observe correlations between plasma CXCL5 and inflammatory cytokines such as TNF or IL-6 (data not shown).
Fig. 1. CXCL5 plasma levels in patients with chronic liver disease.
(A) In patients with chronic liver disease (n=111), CXCL5 plasma concentrations are lower as compared to healthy controls (n=98) (median 86.6 pg/ml, range 12.4-2004, vs. median 564.8, range 61.2-2367; p<0.001, U-Test). (B) CXCL5 plasma concentrations decrease with Child-Pugh's stage of liver cirrhosis. Significant p-values are given. (C) CXCL5 plasma levels correlate with the disease severity as assessed by the MELD (model of end stage liver disease) score. r=−0.208, p=0.03, Spearman rank correlation test. (D) CXCL5 plasma levels according to the underlying etiology of liver disease (viral hepatitis [n=32]: median 50.9 pg/ml, range 12.4-299.4; biliary/autoimmune [n=27]: median 111.2, range 25.5-1330; alcohol [n=29]: median 59.5, range 27.2-895; other [n=23]: median 145.2, range 50.6-2004; p<0.001, U-Test).
Table 2. Correlation analysis for CXCL5.
Spearman rank correlation test. aPTT, activated thromboplastin time; MELD, model of end stage liver disease.
| CXCL5 | ||
|---|---|---|
| r | p | |
| Cholinesterase | 0.484 | < 0.001 |
| Albumin | 0.234 | 0.014 |
| Platelet count | 0.611 | < 0.001 |
| Prothrombin time | − 0.432 | < 0.001 |
| aPTT | − 0.514 | < 0.001 |
| Fibrinogen | 0.480 | < 0.001 |
| Factor II | 0.540 | < 0.001 |
| Factor V | 0.527 | < 0.001 |
| Factor VII | 0.452 | < 0.001 |
| Factor XIII | 0.555 | < 0.001 |
| Child stage | − 0.390 | < 0.001 |
| MELD score | − 0.208 | 0.030 |
Histological inflammation and fibrosis are associated with low plasma levels of CXCL5
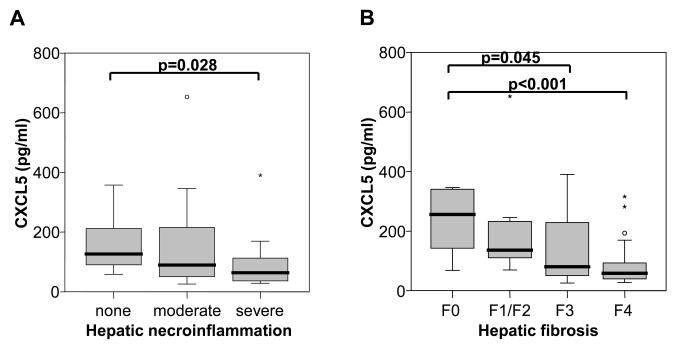
Liver histology was available in 65 out of 111 patients at the time of study entry and was semi-quantitatively evaluated by a blinded and experienced pathologist. Although CXCL5 is a chemoattractant and activator of neutrophils and has been implicated in the pathogenesis of a variety of inflammatory and autoimmune diseases 19, CXCL5 plasma concentrations were significantly lower in patients with severe hepatic necroinflammatory activity as compared to patients with no necroinflammation on liver biopsy (Fig. 2A). CXCL5 plasma levels decreased with the degree of liver fibrosis and cirrhosis (Fig. 2B). No significant correlation between CXCL5 concentration and cholestasis or steatosis was found (data not shown).
Fig. 2. CXCL5 concentrations according to liver histology.
(A) CXCL5 is decreased in patients with severe (median 64 pg/ml, range 28.1-390.3) hepatic necroinflammation compared to patients without necroinflammation on liver biopsy (median 126.9, range 58-357.6; p=0.028). (B) CXCL5 plasma concentrations decrease with the degree of hepatic fibrosis/cirrhosis when patients with no (F0, median 256 pg/ml, range 68.4-1330), mild (F1/F2, median 136.2 pg/ml, range 69.3-652.7) or severe fibrosis (F3, median 80.6 pg/ml, range 25.5-390.3) and complete cirrhosis (F4, median 58.7 pg/ml, range 27.2-316.1) were compared. Significant p-values are given.
CXCL5 is lower in chronic liver disease patients with clinical signs of decompensation
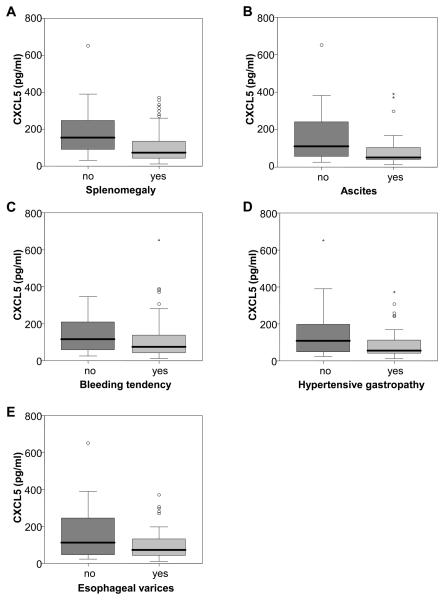
Patients with liver cirrhosis are susceptible to a variety of clinical complications such as splenomegaly, ascites, bleeding tendency, hypertensive gastropathy and esophageal varices. Patients with splenomegaly (Fig. 3A), ascites (Fig. 3B), bleeding tendency unrelated to portal hypertension (Fig. 3C), portalhypertensive gastropathy (Fig. 3D) and esophageal varices (Fig. 3E) showed lower plasma levels of CXCL5. Thus, CXCL5 concentrations correlate well with clinical signs of decompensation.
Fig. 3. CXCL5 and clinical complications.
CXCL5 is lower in patients with compared to patients without (A) splenomegaly (median 73.3 vs. 154.1 pg/ml, p=0.003), (B) ascites (median 62 vs. 110.3 pg/ml, p=0.003), (C) a current bleeding tendency unrelated to portal hypertension (median 74.8 vs. 125.5 pg/ml, p=0.017), (D) portalhypertensive gastropathy (median 53.6 vs. 95.3 pg/ml, p=0.006), and (E) esophageal varices (median 70.1 vs. 110.3 pg/ml, p=0.046).
Hepatic CXCR2 expression does not change in fibrosis or cirrhosis
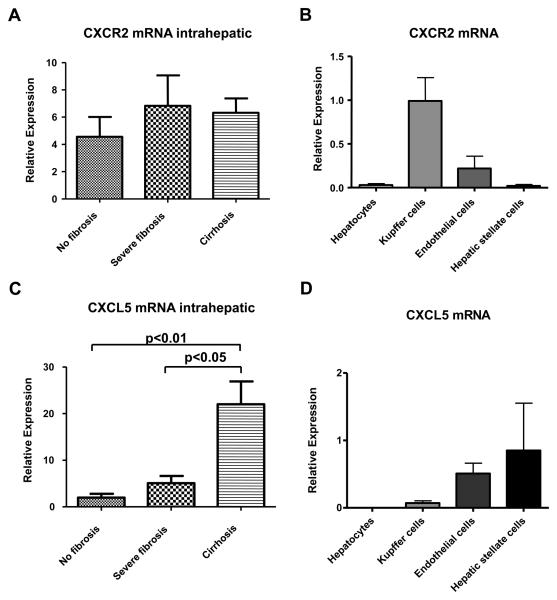
CXCR2 is the cellular receptor for CXCL5 10. As CXCR2-deficient mice show a significantly reduced liver weight after partial hepatectomy as compared to wildtype mice 20, we investigated hepatic levels of CXCR2 using realtime QPCR. Gene expression of CXCR2 was similarly expressed in human liver without fibrosis, severe fibrosis or cirrhosis (Fig. 4A). To determine the cellular source of hepatic expression of CXCR2 in vivo, we isolated parenchymal and non-parenchymal liver cells from normal mouse liver. CXCR2 mRNA was expressed predominantly in Kupffer cells and sinusoidal endothelial cells, and minimally in hepatocytes and hepatic stellate cells (Fig. 4B). In addition, hepatic gene expression of CXCL5 was assessed. Surprisingly and in contrast to circulating plasma CXCL5, hepatic CXCL5 mRNA expression was significantly higher in liver biopsies from patients with severe fibrosis and cirrhosis as compared to human liver without fibrosis (Fig. 4C). We next addressed which cellular compartments could be responsible for the hepatic CXCL5 expression by using isolated murine liver cells. Murine hepatocytes did not express CXCL5 mRNA, whereas hepatic stellate cells and sinusoidal endothelial cells expressed highest gene levels of CXCL5 in normal mouse liver (Fig. 4D).
Fig. 4. Hepatic gene expression of CXCR2 and CXCL5.
(A and C) Expression of CXCR2 and CXCL5 were analyzed by RT-qPCR in human liver without fibrosis (n=5), with severe fibrosis (n=8) or with evidence of cirrhosis (n=33) and normalized to β-actin. (B and D) Liver cell fractions were isolated from a normal mouse liver. CXCR2 and CXCL5 mRNA were analyzed by RT-qPCR and normalized to 18S. The mean of three independent isolations is shown.
Discussion
Liver fibrosis is a scarring process that arises in response to chronic liver injury of any etiology. After an acute liver injury parenchymal cells regenerate and replace necrotic and apoptotic hepatocytes, which is accompanied by a mild inflammatory response and a limited deposition of extracellular matrix proteins. If the injury persists and becomes chronic, liver regeneration fails and hepatocytes are replaced by scarring tissue. There is substantial evidence that stimulation of hepatocyte regeneration limits the fibrotic process 21, 22.
The exact pathogenic role of CXCL5 in patients with chronic liver disease is yet unknown. Acute liver injury precipitated by hepatectomy or ischemia reperfusion in mice is associated with a hepatic and systemic increase in CXCL5 12, 23. CXCL5 is not expressed in a normal murine liver, however, synthesis is strongly induced in hepatocytes following liver injury 23. Antibody-mediated neutralization of CXCL5 significantly impairs normal liver regeneration after an acute liver insult 12, 23. Antibodies directed against the receptor of CXCL5, CXCR2, enhance hepatic damage in an acetaminophen-induced liver injury model, while exogenous addition of CXCL5 enhanced liver regeneration 24. CXCL5 also exerts direct CXCR2-dependent mitogenic effects on cultured hepatocytes 12, 24. Together, these studies demonstrate that CXCL5 is a hepatic regenerative factor and is important for the induction of hepatocyte proliferation in the setting of acute liver injury.
In this study we showed in a large number of patients with chronic liver disease that CXCL5 plasma levels are reduced in liver cirrhosis, correlate with hepatic synthetic capacity, fibrosis and disease progression. Moreover, reduced CXCL5 levels are associated with the occurrence of clinical complications of liver cirrhosis. Based on the above studies in mice, CXCL5 is mainly induced in hepatocytes following acute liver injury to enhance the regenerative capacity of the liver. This is supported by a recent study of patients with acute hepatitis demonstrating a higher hepatic expression of CXCL5 as compared to controls 25, but levels return quickly to baseline after cessation of the acute insult 23. Chronic liver disease is characterized by a progressive decrease in the number of hepatocytes as evidenced by a decreased capacity to synthesize albumin or clotting factors. There are no studies assessing hepatic CXCL5 expression in chronic liver disease and cirrhosis. We found that hepatic gene expression of CXCL5 increases in chronic liver disease as compared to normal liver. Hepatic stellate cells and endothelial cells synthesize highest levels of CXCL5 mRNA in normal murine liver. As liver fibrosis goes along with angiogenesis and proliferation of hepatic stellate cells 16, it is therefore conceivable that hepatic CXCL5 gene expression increases with liver fibrosis in whole liver samples. Obviously other cell types than hepatic stellate cells and endothelial cells must express and secrete higher amounts of CXCL5, because systemic plasma levels are lower in patients with chronic liver disease. We can only speculate about the cellular source for systemic CXCL5 levels, but neutrophils and monocytes secrete CXCL5 and could be the extrahepatic source for systemic CXCL5 19. As the infiltration of blood monocytes and neutrophils into the liver critically participates in experimental hepatic fibrosis 2, the reduced circulating CXCL5 levels might be related to an altered distribution or activation of these innate immune cell subsets in patients with advanced liver disease. Of note, plasma CXCL5 correlated with total WBC (r=0.312, p=0.001), with neutrophil (r=0.242, p=0.022) and lymphocyte counts (r=0.353, p<0.001), corroborating that WBC subtypes might be a source of CXCL5 in the plasma of patients (detailed data not shown). Future studies have to address this important issue and provide a more detailed answer.
CXCL5 is a chemokine that exerts its effects through the chemokine receptor CXCR2 10. We have assessed CXCR2 expression quantitatively in human liver and found no significant difference in the total amount of CXCR2 gene expression in normal human liver as compared to fibrotic or cirrhotic liver. To determine the expression pattern of CXCR2 we purified liver cell fractions from normal mouse liver using MACS sorting, which allows us to achieve highly pure parenchymal and non-parenchymal cell populations. We confirmed the expression of CXCR2 on hepatocytes. Although the highest expression of CXCR2 was found on Kupffer cells as resident macrophages, it is unlikely that Kupffer cells are the main target of CXCL5 in the liver, as we found the lowest plasma concentration of CXCL5 in patients with the most severe necroinflammatory activity on liver biopsy. Other chemokines, such as ligands targeting the chemokine receptors CCR2 or CCR1 on macrophages, may be functionally dominant, as suggested by murine studies 2, 26.
Our study also provides data on the potential usefulness of plasma CXCL5 as a clinical and non-invasive biomarker in liver fibrosis and cirrhosis. Notably, CXCL5 concentrations directly correlated with the degree of fibrosis in liver histology. It may therefore help to include CXCL5 into current models to predict histological changes by non-invasive procedures. In addition, we also demonstrate a correlation between CXCL5 plasma levels and clinical signs of decompensation in patients with liver cirrhosis, such as portal hypertension, esophageal varices and bleeding disorders. It may therefore be useful to include CXCL5 in future diagnostic algorithms for gauging the clinical course of patients with chronic liver disease. However, larger prospective studies must validate the relevance of plasma CXCL5 measurements in chronic liver disease.
In conclusion, plasma CXCL5 levels are lower in patients with chronic liver disease. CXCL5 is an emerging chemokine that exerts mitogenic properties on hepatocytes. Though decreasing CXCL5 concentrations appears to be a good marker for fibrosis and clinical signs of decompensation, further studies are required to determine if CXCL5 supplementation could be used as therapeutic intervention in chronic liver disease and fibrosis.
Acknowledgements
We thank Marina Fink for excellent technical assistance. This study was supported in part by NIH grants K08 DK081830 (to BS), by the German Research Foundation Ta434/2-1 and SFB-TRR57 (to FT), by the UCSD Digestive Diseases Research Development Center (DK080506) (to BS), and by the AGA Fellowship to Faculty Transition Award (FFTA) (to BS).
References
- 1.Schnabl B, Scholten D, Brenner DA. What is the potential role of antifibrotic agents for the treatment of liver disease? Nat Clin Pract Gastroenterol Hepatol. 2008;5:496–7. doi: 10.1038/ncpgasthep1200. [DOI] [PubMed] [Google Scholar]
- 2.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 3.Seki E, de Minicis S, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–97. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halloran MM, Woods JM, Strieter RM, et al. The role of an epithelial neutrophil-activating peptide-78-like protein in rat adjuvant-induced arthritis. J Immunol. 1999;162:7492–500. [PubMed] [Google Scholar]
- 5.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–62. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strieter RM, Kunkel SL, Burdick MD, Lincoln PM, Walz A. The detection of a novel neutrophil-activating peptide (ENA-78) using a sensitive ELISA. Immunol Invest. 1992;21:589–96. doi: 10.3109/08820139209069393. [DOI] [PubMed] [Google Scholar]
- 7.Nasu K, Arima K, Kai K, Fujisawa K, Nishida M, Miyakawa I. Expression of epithelial neutrophil-activating peptide 78 in cultured human endometrial stromal cells. Mol Hum Reprod. 2001;7:453–8. doi: 10.1093/molehr/7.5.453. [DOI] [PubMed] [Google Scholar]
- 8.Z'Graggen K, Walz A, Mazzucchelli L, Strieter RM, Mueller C. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997;113:808–16. doi: 10.1016/s0016-5085(97)70175-6. [DOI] [PubMed] [Google Scholar]
- 9.Schmouder RL, Streiter RM, Walz A, Kunkel SL. Epithelial-derived neutrophil-activating factor-78 production in human renal tubule epithelial cells and in renal allograft rejection. Transplantation. 1995;59:118–24. doi: 10.1097/00007890-199501150-00021. [DOI] [PubMed] [Google Scholar]
- 10.Chavey C, Lazennec G, Lagarrigue S, et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9:339–49. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arenberg DA, Keane MP, DiGiovine B, et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102:465–72. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock. 1998;10:248–57. doi: 10.1097/00024382-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Tacke F, Brabant G, Kruck E, et al. Ghrelin in chronic liver disease. J Hepatol. 2003;38:447–54. doi: 10.1016/s0168-8278(02)00438-5. [DOI] [PubMed] [Google Scholar]
- 14.Tacke F, Wustefeld T, Horn R, et al. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol. 2005;42:666–73. doi: 10.1016/j.jhep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Tacke F, Schoffski P, Trautwein C, et al. Plasma P-selectin levels are elevated in patients with chronic liver disease. Blood Coagul Fibrinolysis. 2003;14:319–25. doi: 10.1097/00001721-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Taura K, De Minicis S, Seki E, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–38. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 17.Tacke F, Gabele E, Bataille F, et al. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig Dis Sci. 2007;52:3404–15. doi: 10.1007/s10620-007-9758-8. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walz A, Schmutz P, Mueller C, Schnyder-Candrian S. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol. 1997;62:604–11. doi: 10.1002/jlb.62.5.604. [DOI] [PubMed] [Google Scholar]
- 20.Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol. 2003;163:563–70. doi: 10.1016/S0002-9440(10)63684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueki T, Kaneda Y, Tsutsui H, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–30. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253–8. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 23.Colletti LM, Green ME, Burdick MD, Strieter RM. The ratio of ELR+ to ELR- CXC chemokines affects the lung and liver injury following hepatic ischemia/ reperfusion in the rat. Hepatology. 2000;31:435–45. doi: 10.1002/hep.510310225. [DOI] [PubMed] [Google Scholar]
- 24.Hogaboam CM, Bone-Larson CL, Steinhauser ML, et al. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J. 1999;13:1565–74. doi: 10.1096/fasebj.13.12.1565. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 26.Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–70. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]