Abstract
Drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway are a rapidly growing chemotherapy class for treatment of solid tumors. This “targeted therapy” is more specific than traditional chemotherapy, causing fewer side effects. However, VEGF-targeted therapies cause hypertension in 30 – 80% of patients. Unlike traditional “off-target” side effects, hypertension is a mechanism-dependent, “on-target” toxicity – reflecting effective inhibition of the VEGF signaling pathway rather than non-specific effects on unrelated signaling pathways. In this article, we review current understanding of the mechanisms of VEGF-targeted therapy-induced hypertension, discuss similarities with preeclampsia, review implications for therapy of this increasingly common clinical problem and discuss the potential use of blood pressure rise as a biomarker for proper drug dosing and effective VEGF pathway inhibition.
Keywords: VEGF, targeted therapy, hypertension, nitric oxide, thrombotic microangiopathy
Therapeutic Targeting of the VEGF Signaling Pathway
The introduction of chemotherapies that target the VEGF signaling pathway, also called anti-angiogenic chemotherapies, is an exciting advance in oncology based on the original hypothesis proposed by Dr. Judah Folkman in 1971 that tumor growth and metastases are angiogenesis-dependent processes1. This hypothesis proposed that tumor cells communicate with vascular endothelial cells (EC) within developing neoplasms via “diffusible” growth factors, leading to increased vascularization, which further facilitates tumor growth. Interrupting pro-angiogenic signaling pathways is a principle objective of novel anti-neoplastic strategies, and Vascular Endothelial Growth Factor (VEGF) is a main target.
Vascular endothelial growth factor was discovered as a tumor-derived soluble factor capable of inducing endothelial cell permeability2 and angiogenesis3. Since that initial discovery, seven members of the VEGF family have been identified: VEGF-A, -B, -C, -D, -E and Placental Growth Factors (PlGF) 1 and 2. VEGF mediates its effects through VEGF receptors (VEGFR-1, VEGFR-2 and VEGFR-3), with VEGFR-2 as its primary cognate receptor expressed on endothelial cells. VEGF binding to VEFR-2 activates intrinsic tyrosine kinase activity of VEGFR-2, leading to activation of numerous downstream signaling cascades. Most notably, VEGFR-2 activation recruits PI3-kinase, which in turn activates AKT which directly phosphorylates eNOS causing increased NO production. NO diffuses in a paracrine fashion to adjacent vascular smooth muscle cells, activating guanylate cyclase with increased cGMP production and vasodilation.
VEGF exerts pleiotropic effects on ECs including migration and invasion into the basement membrane, proliferation, and formation of fenestrations.4 VEGF is expressed in virtually every tissue during development, and is required for development-related angiogenesis, causing embryonic lethality while under-5, 6 or over-7 expressed. VEGF plays important roles in adult life as well, being required for physiologic angiogenesis during the female reproductive cycle8, wound healing9, bone repair10 and muscular adaptation to exercise11. It is increasingly appreciated that VEGF signaling is required for homeostasis of adult vasculature as well12.
The first anti-VEGF chemotherapy to be approved for use in cancer treatment was bevacizumab, in 2004 (REFERENCE). A humanized monoclonal antibody that binds circulating VEGF-A, bevacizumab was first approved for the treatment of colon cancer. Subsequently the multi-targeted kinase inhibitors (MTKIs) sorafenib, sunitinib, and pazopanib were approved for the treatment of metastatic renal cell carcinoma (mRCC). MTKIs are small molecules that target the VEGF receptor VEGFR-2, the platelet-derived growth factor (PDGF) receptor, RAS, and c-KIT.13 Use of these medications has since expanded to many different solid tumors, with ongoing clinical trials of newer formulations of the medications as well as use with an expanding number of solid tumors. Their use is growing rapidly. They are now first line therapy for cancers such as mRCC, which accounts for 2.5% of all new cancer diagnoses. With increased use of these medications, and many highly potent formulations in development, there is an increasing interest in and clinical need to understand VEGF inhibitor toxicities.
Hypertension with VEGF Inhibition
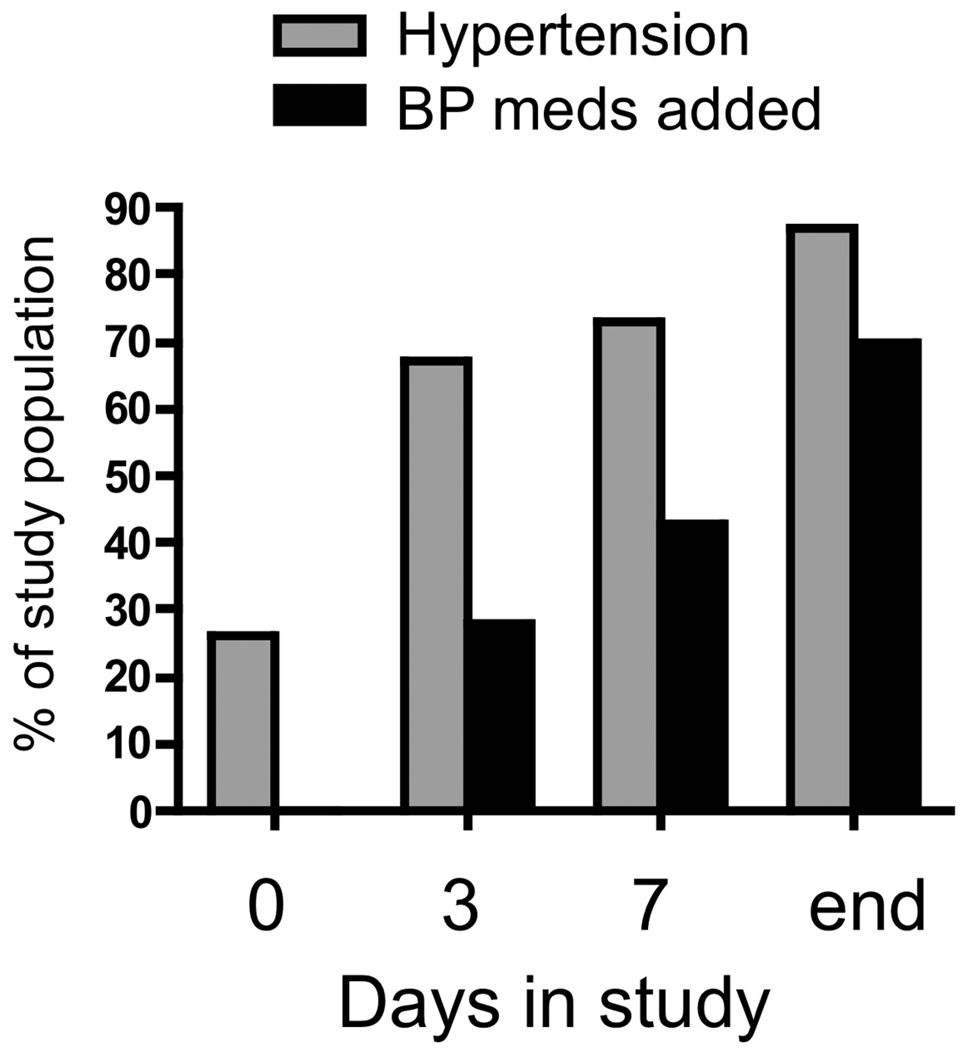
As VEGF-targeted therapies entered the clinic, it became clear that hypertension and proteinuria were major toxicities of this drug class – both the biologics and the small molecules. Proteinuria as a consequence of VEGF inhibition is discussed by Eremina and Quaggin in an accompanying article in this issue, and this article will focus on hypertension in patients treated with VEGF-targeted therapy. Hypertension occurs in up to 80% of patients on some forms of these medications 14 and nearly all patients taking these drugs experience a rise in blood pressure, even if not to hypertensive levels. Preclinical and clinical studies evaluating bevacizumab revealed the dose-dependent development of hypertension in up to two-thirds of patients 15. Sorafenib and sunitinib are also associated with hypertension and a preeclampsia-like syndrome 16. The kinetics of blood pressure rise vary, but with some high-potency VEGF inhibitors, it can be rapid. In one recent study examining sorafenib, among 54 patients initiating therapy, 93% had a rise in blood pressure by day 6, and most experienced a rise in blood pressure over the first 24 hours of therapy, as assessed by ambulatory blood pressure monitoring (ABPM) 16, 17. Similarly, we found a very rapid rise in blood pressure among women initiating Cediranib therapy, a high-potency small molecule VEGF-targeted therapy, with 67% of patients developing hypertension over the first three days of therapy and 87% by the end of the study (Figure 1.) 14. 43% of these patients developed Grade 3 (> 150/100 mmHg not controlled for 48 hours despite treatment) or Grade 4 hypertension (hypertensive crisis). Posterior leukoencephalopathy related to therapy-induced hypertension is described, and is not rare in clinical practice, often requiring discontinuation of therapy 18.
Figure 1. Rapid development of hypertension after starting Cediranib.
Blood pressure and antihypertensive medication addition in 46 women with ovarian cancer were tracked before (Day 0) and after starting Cediranib, a high potency VEGF-targeted therapy. The mean length of time in the study was 115 days and the median was 84 days. Reproduced with permission 14.
Since hypertension is a common, and often dose-limiting toxicity, it would be clinically useful to predict which patients are at the highest risk of developing this complication after starting anti-angiogenic therapy. The risk factors for development of hypertension on these agents are, however, largely undefined. We found that age ≥57 years predicted development of hypertension in the first 3 days of treatment with cediranib, and also predicted a larger average increase in SBP at day 3 than in those < 57 years (15.9mmHg vs. 7.0mmHg; p=0.02). Being aged ≥65 years showed a trend toward rapid development of hypertension over the first 3 days (RR 1.41; 95% CI: 0.98–2.02). BMI, eGFR, family history of hypertension or cardiovascular disease, and prior hypertension did not predict development of hypertension, nor did starting dose of cediranib 14. Maitland and colleagues analyzed blood pressure 10 days after starting sorafenib, and observed a mean systolic increase of +10.8 mm Hg; (95% confidence interval (95% CI), 8.6–13.0; range, −5.2 to +28.7 mm Hg). The considerable variability in degree of blood pressure rise did not correlate with age, body size, sex, self-reported race, baseline blood pressure, or steady-state sorafenib plasma concentration. Smoking and hypercholesterolemia have been proposed as risk factors in a small study 19 and our clinical experience indicates that pre-existing hypertension is an important risk factor, but clearly large studies are needed in this area.
Mechanisms of VEGF-associated hypertension: Clues from Preeclampsia
Recent advances in our understanding of preeclampsia give reason to believe that hypertension in both patients with preeclampsia and patients taking anti-angiogenic therapy share a common pathogenesis. Preeclampsia is a disease of pregnancy affecting 5–7% of women in their 2nd and 3rd trimester, and is marked by hypertension, proteinuria and edema. This condition is characterized by systemic endothelial dysfunction, and if left untreated, ascites, pulmonary edema, thrombocytopenia, headaches, disseminated intravascular coagulation, and even blindness may develop20. In severe cases, the condition may progress to eclampsia, characterized by seizures and other neurologic manifestations, including strokes. Soluble VEGFR-1 (also called sFlt1) is found at high levels in the blood of women with preeclampsia 21, 22. sFlt1 is produced via alternative splicing of the Flt1 transcript, resulting in a deletion of the intracellular and transmembranous domains of Flt1, rendering the truncated protein soluble. sFLT1 binds and sequesters VEGF in mechanism analogous to bevacizumab, resulting in inhibition of VEGF signaling. Systemic administration of sFlt1 to pregnant rats reproduces several symptoms of preeclampsia, including hypertension, proteinuria and renal thrombotic microangiopathy 21, 23.
While the pathophysiology of hypertension in preeclampsia remains incompletely understood, one characteristic of preeclampsia is deficient production of nitric oxide (NO) 24. VEGF stimulates NO production by ECs 25, 26, suggesting that decreased free VEGF levels in preeclampsia might lead to suppressed NO signaling, causing systemic vasoconstriction and hypertension. In support of this hypothesis, NO metabolites are inversely related to sFlt1 in preeclampsia 27. These results suggest that elevated levels of sFlt1 in preeclampsia bind and sequester VEGF, inhibiting signaling through EC VEGFR-2, reducing NO production and causing systemic vasoconstriction and elevated blood pressure.
Inhibition of Endothelium-Derived Relaxing Factors
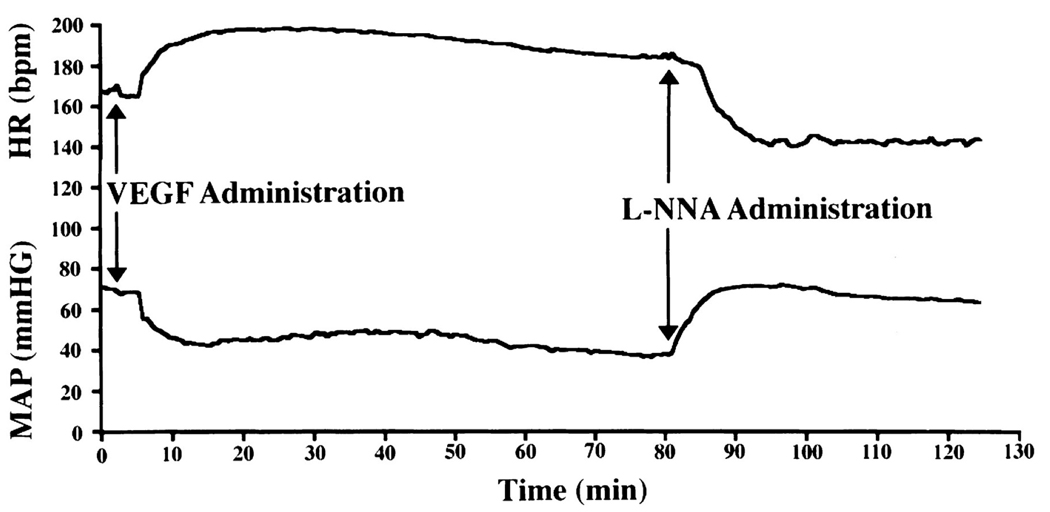
High dose VEGF infusion into rabbits induces systemic vasodilation and causing an immediate drop in blood pressure 28 that can be inhibited by the NOS inhibitor, L-NAME (Figure 2) 26. VEGF, acting through VEGFR-2, activates endothelial NO synthase (eNOS) activity through AKT26, leading to increased vasodilatory NO production (Figure 3) Bevacizumab has been shown to decrease NO levels in vitro 29, and given that preeclampsia is characterized by deficient NO production, these observations together suggest the hypothesis that anti-angiogenic therapies might also cause systemic deficiency of NO with consequent hypertension by inhibiting VEGF signaling.
Figure 2. VEGF Infusion induces NO-dependent hypotension.
VEGF infusion activates VEGFR-2 expressed on vascular endothelial cells, resulting in activation of eNOS and nitric oxide-dependent vasodilation. In this experiment, VEGF infusion causes immediate fall in blood pressure with a concomitant rise in heart rate. The fall in blood pressure is mediated by production of nitric oxide because addition of the NOS inhibitor L-NNA reverses the fall in blood pressure. Reproduced with permission 26.
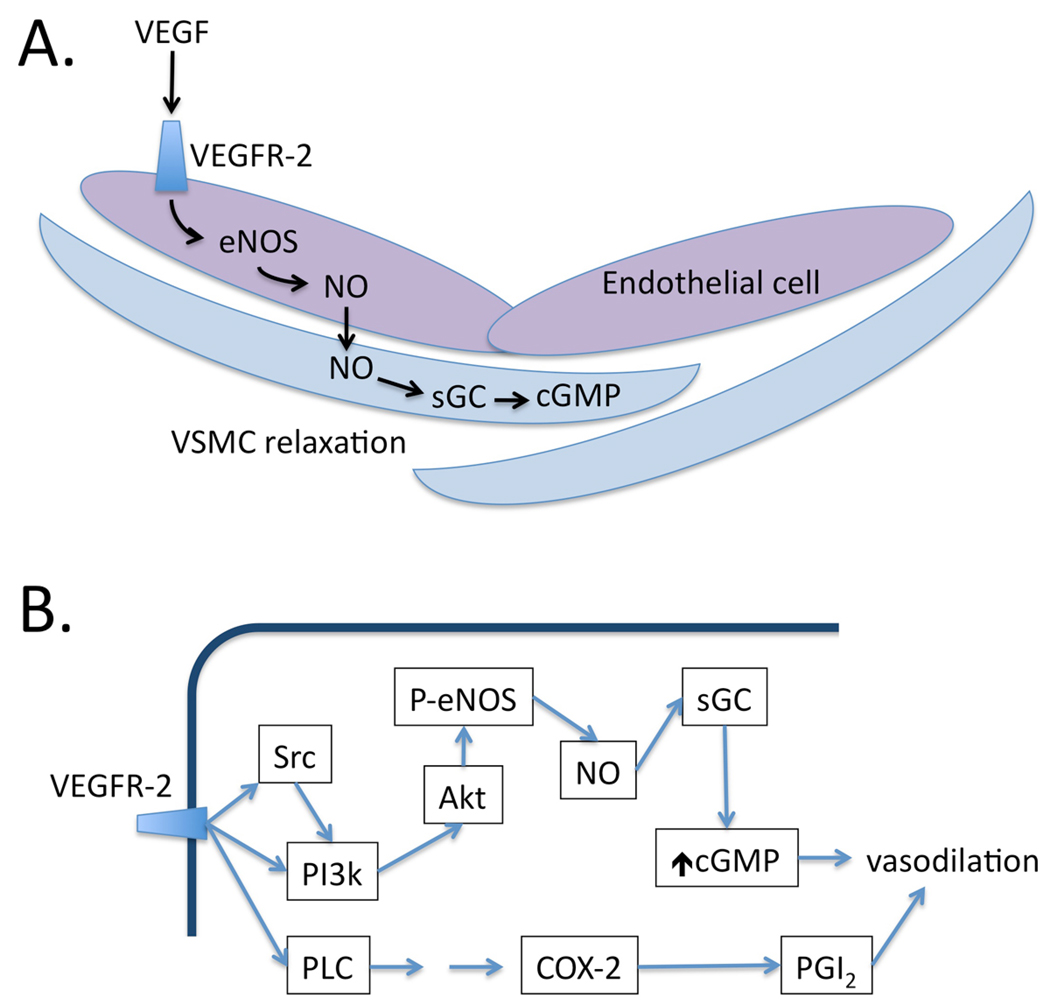
Figure 3. Vasodilatory signaling pathways induced by VEGF in the vasculature.
A. VEGF in the circulation binds to VEGFR-2 expressed on endothelial cells, triggering the production of NO by activation of eNOS. NO acts in a paracrine fashion on adjacent vascular smooth muscle cells (VSMC), where it activates soluble guanylate cyclase (sGC) to produce cGMP, which causes VSMC relaxation. B. Activation of VEGFR-2 on endothelial cells activates multiple downstream signaling pathways, including both SRC and PI3k. This results in activation of the kinase Akt, which directly phosphorylates and activates eNOS. Resulting NO binds to sGC in adjacent VSMC, increasing cGMP and causing vasodilation mediated by cGMP-dependent kinases (not shown). VEGFR-2 activation also activates phospholipase C (PLC), which triggers a signaling cascade resulting in the transcriptional activation of cyclooxygenase-2 (COX-2), which produces vasodilatory prostacyclin (PGI2), contributing to the vasodilatory reponse.
Providing the first direct support for an important role for suppressed NO in hypertension caused by VEGF-targeted therapies, Facemire and colleagues administered an antibody directed against VEGFR-2 to mice, and the mice subsequently developed hypertension. Animals receiving anti-VEGFR-2 antibody experienced reduced endothelial and neuronal NOS expression in kidney. Further, they showed that administration of the NOS inhibitor L-NAME abolished the difference in blood pressure between control and VEGFR-2 inhibition groups – suggesting that hypertension after VEGF blockade is indeed mediated by suppression of NO levels leading to vasoconstriction 30, 31. No results from human studies have been reported to date, but our preliminary evidence in a cross sectional study of mRCC patients receiving either VEGF-targeted therapy or other therapy indicates that those patients receiving anti-angiogenic therapy have reduced urinary nitrite and nitrate as well as cGMP compared to patients not receiving VEGF-targeted therapy (Robinson and Humphreys, in preparation).
While VEGF appears to regulate vascular tone primarily via NO, prostacyclin (PGI2) may also play role. VEGF activates PGI2 production in endothelial cells through heterodimerization between VEGFR-1 and VEGFR-2 32, and PGI2 is a potent vasodilator 33 (Figure 3). Some studies have measured reduced levels of PGI2 metabolites in preeclampsia, providing additional support 34. While there were no differences in stable metabolite levels of the prostanoids PGI2, PGE2 and thromboxane A2 in a preclinical study 30, determining the relative contribution of suppressed NO vs. PGI2 vasodilatory pathways in the pathogenesis of anti-angiogenic therapy-induced hypertension will likely require large, prospective studies measuring these biomarkers in patients before and after starting VEGF-targeted therapy.
Capillary Rarefaction
Substantial evidence exists to show that chronic VEGF inhibition causes capillary rarefaction, both in preclinical models and in humans. This capillary rarefaction may increase afterload and thereby contribute to the pathophysiology of hypertension induced by VSP inhibition. Chronic VEGF depletion causes reduced microvascular EC survival, ultimately leading to a net reduction in tissue microvessel density 35. The endothelial insult may additionally cause local thrombosis, leading to a further decrease in vascular perfusion, accelerating the process of EC apoptosis and microvessel obliteration. The cumulative effects of microvascular rarefaction may lead to increased systemic vascular resistance, resulting in a further increase in blood pressure. Early stages of capillary regression can be measured as early as 24 hours after drug initiation in mouse models. However, capillary density is not decreased by >50% until after at least 7–14 days 35, 36. In humans, evidence of decreased capillary density after 5 weeks of telatinib treatment has been observed, with regrowth of the microcapillary network after discontinuation of therapy 37. It is likely given this evidence in both mice and humans that capillary rarefaction does play a role in VEGF inhibitor-induced hypertension, but it is probably not the sole factor in the hypertension given that the rise in blood pressure develops more rapidly than this process would occur.
Alterations in the Pressure Natriuresis Relationship
Many decades of hypertension research have established the kidney’s ability to excrete sodium in response to chronic increases in arterial pressure (the pressure-natriuresis relationship) as a critical regulatory response to elevated blood pressure. For chronic hypertension to occur, a defect in renal sodium excretion has been seen in all forms of hypertension examined 38. At least two lines of evidence suggesting that VEGF inhibition might shift the pressure-natriuresis curve as an explanation for hypertension seen on VSP inhibitors. First, NO has a direct effect on regulation of pressure natriuresis and tubule-glomerular feedback. Disruption of proper eNOS functioning, through VEGF inhibition, may therefore cause sodium retention and extracellular fluid volume increase, perpetuating hypertension by changing the set point for sodium excretion 39. Support for this model is derived from rodent studies where VEGFR-2 inhibition caused a rightward shift in the chronic pressure-natriuresis relationship 30.
In other recent experiments, an alternate, novel mechanism for VEGF-mediated regulation of extracellular fluid volume has been proposed. In response to a high salt diet, production of VEGF-C by macrophages rises, binding to VEGFR-3 and stimulating lymphatic vessel growth. This lymphatic capillary network is hypothesized to form a compartment that buffers extracellular fluid volume in response to the increased sodium intake, blunting the expected rise in blood pressure. Inhibition of VEGF-C or macrophage depletion caused a decrease in lymphatic vessel density, and increased blood pressure in response to a high salt diet 40. While VEGF-targeted chemotherapy was designed to inhibit VEGF-A signaling through VEGFR-2, many of the MTKIs, including sunitinib and sorafenib also inhibit VEGF-C signaling through VEGFR-3. Thus, these agents might similarly limit lymphatic growth in response to salt intake, causing exacerbated hypertension in response to high salt diet.
The Renin-Angiotensin Axis
Although endothelial dysfunction might be expected to cause glomerular ischemia with upregulation of the renin-angiotensin-aldosterone axis, in fact no experimental evidence supports a role for this pathway. Facemire and colleagues, using DC101, a VEGF receptor inhibitor in mice, demonstrated that renin mRNA and aldosterone urinary excretion were actually decreased when compared with control mice 30. In the only published study examining this in humans, renin and aldosterone levels were not increased in 20 patients taking sorafenib 41. In our own study of epithelial ovarian cell cancer patients on cediranib, we found that body weight decreased while blood pressure increased 14. While the loss of weight may have been multifactorial, if the renin-angiotensin-aldosterone axis was upregulated, we would have expected an increase in extracellular fluid volume and hence body weight. These are all small studies and further investigation is needed, but so far these findings do not support a significant for the renin-angiotensin-aldosterone axis in mediating blood pressure elevation with VEGF inhibition. Indeed, circulating renin and aldosterone levels are suppressed in preeclampsia,42 consistent with the hypothesis that VEGF inhibition does not lead to up-regulation of the RAAS axis.
Other Vasoconstrictive Pathways
Emerging data supports a possible role for increased vasoconstrictive endothelin-1 (ET-1) in the pathogenesis of hypertension induced by anti-VEGF therapy. First, there is evidence that ET-1 plays a role in hypertension induced by a rat model of preeclampsia. Alexander and colleagues from the Granger group showed increased levels of preproendothelin mRNA in renal medulla during preeclampsia, and also showed marked attenuation of blood pressure by an endothelin type A receptor antagonist 43. In a follow up study from the same group, Murphy et al. measured elevated ET-1 in hypertension induced by infusion of sFlt-1 44. Further supporting a role for this polypeptide is the observation that endothelin receptor blockade abrogated hypertension induced by sFlt-1 in this model.
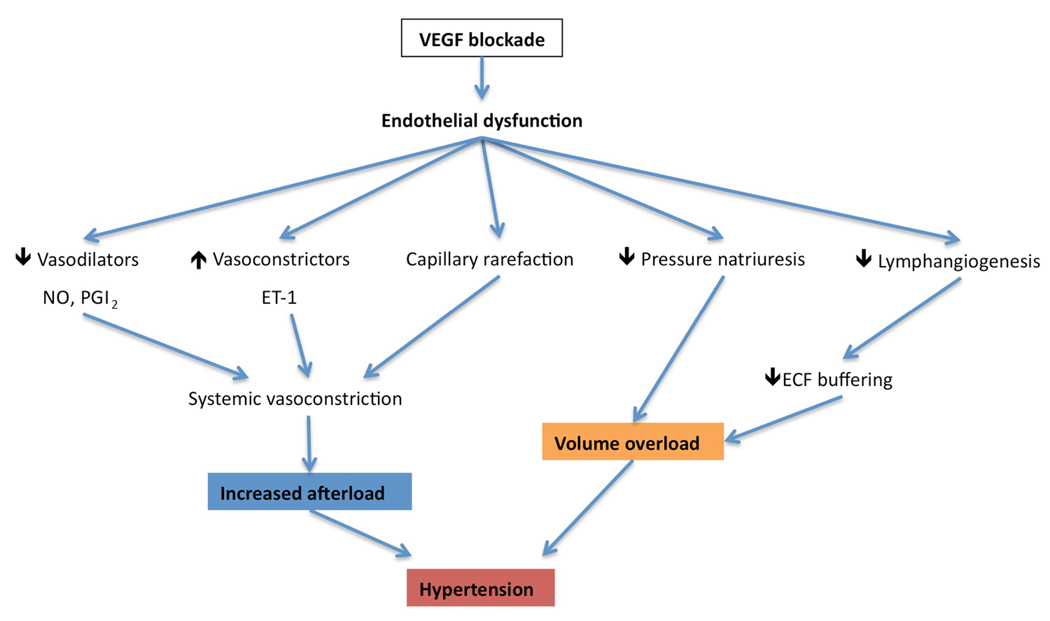
Oxidative stress could also be involved in endothelial changes observed when VEGF signaling is blocked. It has been demonstrated that exposure of endothelial cells to peroxide (H2O2) leads to increased mRNA levels for both VEGF and VEGFR-2 45. Since oxidative stress had been implicated in various forms of human hypertension, it is conceivable that loss of VEGF-related protection further contributes to blood pressure elevation. Finally, vasoconstrictive thromboxanes might play a role as well, although preclinical studies so far do not support this concept 30, there are no studies in humans to date. Figure 4 summarizes how the various mechanisms discussed above might contribute to the development of endothelial dysfunction and hypertension in patients treated with anti-angiogenic therapies (Figure 4).
Figure 4. Possible mechanisms of VSP inhibitor-induced hypertension.
VEGF blockade induces endothelial dysfunction because autocrine VEGF is required for endothelial cell health. This results in the inhibition of VEGF-dependent vasodilatory pathways such as NO and PGI2, as well as the possible upregulation of vasoconstrictive pathways such as endothelin-1 (ET-1). Together with the loss of microvascular capillary density through capillary rarefaction, these mechanisms cause systemic vasoconstriction, resulting in increased afterload and hypertension. Endothelial dysfunction and VEGF blockade also resets the pressure-natriuresis relationship resulting in inadequate renal sodium excretion in the face of elevated blood pressure. Finally, VEGF blockade may also block VEGFR-3 expressed on endothelial cells, decreasing lymphangiogenesis and reducing the capacity of the lymphatic network to buffer sodium and extracellular fluid (ECF) volume. Both of these latter two mechanisms will contribute to volume overload and exacerbate blood pressure rise.
Hypertension as a biomarker of tumor response
In addition to the importance of understanding these side effects for purposes of clinical management, there is increasing evidence that a rise in blood pressure in patients on these medications may predict better tumor response 46. The rationale is that hypertension is a mechanism-dependent effect of VEGF signaling pathway (VSP) inhibition – ie, it reflects effective in vivo inhibition of the VEGF signaling pathway 47. This raises a natural question: Are patients who do not develop hypertension on these drugs being underdosed? If they do not experience a rise in blood pressure, perhaps pro-angiogenic VEGF signaling in their tumor vasculature is not being effectively inhibited. In fact, a variety of observations suggest that this hypothesis may be at least partly true. Scartozzi, et al demonstrated that in a cohort of 39 patients with metastatic colorectal cancer on bevacizumab, partial remission was achieved in 75% of patients who developed grades 2–3 hypertension, but in only 32% of those who did not develop hypertension (p=0.04) 48. Several letters to the editor have reported similar results with bevacizumab, sorafenib and sunitinib 51–53. In an important study by Schneider and colleagues, VEGF genotypes, hypertension and overall survival was examined in patients on bevacizumab and paclitaxel compared with paclitaxel alone for metastatic breast cancer. They found that the VEGF-2578AA genotype was associated with superior median overall survival in the combination arm compared with the other genotypes. Intriguingly, this genotype was also associated with a higher incidence of bevacizumab-associated hypertension, and those who developed grade 3 or 4 hypertension had a higher median overall survival than those who did not develop hypertension (38.7 vs. 25.3 months, p=0.002) 49. By contrast, a different VEGF genotype, 634CC, was associated with significantly less grade 3 or 4 hypertension independent of VEGF expression levels in the tumor itself.
More recently, further evidence for a role for the VEGF-634 polymorphism on sunitinib-induced hypertension was presented by Kim et al 50 in 63 mRCC patients treated. These investigators also found that the VEGF-634CC genotype was associated with substantially decreased frequency and duration of hypertension compared to the VEGF-634GG genotype (8.9% vs 27.2%, p = 0.007). Collectively these reports support the notion that blood pressure rise on these drugs may be a biomarker for effective VEGF inhibition, and suggest an intriguing role for VEGF polymorphisms in modulating risk of hypertension. Based on these encouraging findings, a new trial (NCT00835978) is testing the hypothesis that patients who do not develop hypertension on standard dose axitinib may experience antitumor benefit with dose escalation.
Interestingly, albuminuria, which is also considered to be a mechanism-dependent toxicity of anti-angiogenic chemotherapy, has not been explored as a biomarker of VEGF responsiveness. Hypertension can be difficult to measure accurately without the use of ambulatory blood pressure monitoring (ABPM), which is not very practical in many clinical situations. However, measurement of the urinary albumin:creatinine ratio (ACR), a valid estimate of 24-hour urine albumin excretion, can be performed routinely and measured easily with a spot urine sample 54. The association between albuminuria and tumor response deserves further investigation, as albuminuria may be a potential biomarker of efficacy.
Management of Hypertension Induced by Anti-Angiogenic Therapy
While an abundance of data exists for managing essential hypertension, very little data exists to guide treatment of hypertension induced by VSP inhibitor blockade. Nevertheless, the condition is clinically important and several general recommendations can be made, based in part on a recent interdisciplinary working group convened to study this topic 55. First, patients should have controlled blood pressure before starting therapy, since they may be expected to have exacerbation of pre-existing hypertension after starting the drug. The clinical utility of medications that are known to raise blood pressure, such as NSAIDS or erythropoietin, should be re-evaluated (and the medications discontinued, if possible) before and during treatment with VSP inhibitors. Lifestyle recommendations can be made at this point as well, such moderating alcohol intake and reducing dietary salt, since these interventions will reduce blood pressure.
There is no simple algorithm to follow in managing hypertension induced by VSP inhibition and treatment will need to be individualized to each patient. However, active monitoring throughout treatment is required since this toxicity is so common. Antihypertensive therapy should be initiated when blood pressure rises above 140/90mmHg, as a general rule, but in high risk groups such as patients with diabetes or chronic kidney disease, the threshold is lower (130/80mmHg). Choice of antihypertensive agent should be individualized, and angiotensin converting enzyme inhibition or calcium channel blockers are reasonable first line options. Centrally acting antihypertensives or diuretics may be added to adequately control blood pressure. Close follow up is critical for appropriate titration to ensure efficacy and control any side effects. If blood pressure cannot be maintained below 140/90mmHg (or 130/89mmHg in high risk groups) then prompt referral to a specialist is indicated. When patients are taken off of VSP inhibitors, their blood pressure will return to their prior baseline with a variable time course, and close monitoring is required with tapering and discontinuation of added anti-hypertensive medications in order to avoid hypotension.
Conclusion
The use of anti-angioenic therapies is growing rapidly and nephrologists will increasingly be called upon to manage the renal and vascular side effects of these drugs. Hypertension on VEGF-targeted therapies is common and causes significant morbidity but it can be effectively managed and may also be a biomarker for tumor responsiveness. Understanding the mechanisms of VEGF inhibitor-induced hypertension will lead to rational treatment of this complication, and may illuminate our understanding of the role of VEGF in maintenance of vascular tone and endothelial health during homeostasis. Further studies are required to determine the risk factors for development of hypertension on these drugs and the possible use of blood pressure as an efficacy biomarker for prediction of treatment response.
Acknowledgements
This work was supported by DK073628, DK084316 and a pilot grant from Harvard Catalyst: NationalInstitutes of Health grant UL1 RR 025758-02 with financial contributions from participating institutions (all to B.D.H.) and a grant from the National Kidney Foundation (to E.S.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
S.A.K is listed as a coinventor on multiple patents held by the Beth Israel Deaconess Medical Center for the diagnosis and therapy of preeclampsia. These patents have been non-exclusively licensed to multiple companies. S.A.K is a consultant to Beckman Coulter, Johnson & Johnson, Roche and Abbott Diagnostics that are developing biomarkers for preeclampsia diagnosis/prediction.
Bibliography
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 4.Risau W. Development and differentiation of endothelium. Kidney Int Suppl. 1998 Sep;67:S3–S6. doi: 10.1046/j.1523-1755.1998.06701.x. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996 Apr 4;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 7.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000 Sep;127(18):3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 8.Otani N, Minami S, Yamoto M, Shikone T, Otani H, Nishiyama R, et al. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1999 Oct;84(10):3845–3851. doi: 10.1210/jcem.84.10.6025. [DOI] [PubMed] [Google Scholar]
- 9.Eming SA, Krieg T. Molecular mechanisms of VEGF-A action during tissue repair. J Investig Dermatol Symp Proc. 2006 Sep;11(1):79–86. doi: 10.1038/sj.jidsymp.5650016. [DOI] [PubMed] [Google Scholar]
- 10.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002 Jul 23;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mac Gabhann F, Ji JW, Popel AS. VEGF gradients, receptor activation, and sprout guidance in resting and exercising skeletal muscle. J Appl Physiol. 2007 Feb;102(2):722–734. doi: 10.1152/japplphysiol.00800.2006. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007 Aug 24;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broxterman HJ, Georgopapadakou NH. Anticancer therapeutics: "Addictive" targets, multi-targeted drugs, new drug combinations. Drug Resist Updat. 2005 Aug;8(4):183–197. doi: 10.1016/j.drup.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Robinson E, Matulonis UA, Ivy P, Berlin ST, Tyburski K, Penson RT, Humphreys BD. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor inhibitor. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.08111109. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003 Jul 31;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, et al. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008 Feb 20;100(4):282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 17.Maitland ML, Kasza KE, Karrison T, Moshier K, Sit L, Black HR, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009 Oct 1;15(19):6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007 Aug;50(2):203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Mir O, Mouthon L, Alexandre J, Mallion JM, Deray G, Guillevin L, et al. Bevacizumab-induced cardiovascular events: a consequence of cholesterol emboli syndrome? J Natl Cancer Inst. 2007 Jan 3;99(1):85–86. doi: 10.1093/jnci/djk011. [DOI] [PubMed] [Google Scholar]
- 20.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005 Feb 26–Mar 4;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 21.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003 Mar;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004 Feb 12;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 23.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003 Mar;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide. 2000 Aug;4(4):441–458. doi: 10.1006/niox.2000.0296. [DOI] [PubMed] [Google Scholar]
- 25.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999 Jun 10;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz JR, Rivard A, van der Zee R, Hariawala M, Sheriff DD, Esakof DD, et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997 Nov;17(11):2793–2800. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 27.Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008 Aug;52(2):402–407. doi: 10.1161/HYPERTENSIONAHA.108.115006. [DOI] [PubMed] [Google Scholar]
- 28.Yang R, Thomas GR, Bunting S, Ko A, Ferrara N, Keyt B, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996 Jun;27(6):838–844. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7(4):335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 30.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular Endothelial Growth Factor Receptor 2 Controls Blood Pressure by Regulating Nitric Oxide Synthase Expression. Hypertension. 2009 Aug 3; doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granger JP. Vascular endothelial growth factor inhibitors and hypertension: a central role for the kidney and endothelial factors? Hypertension. 2009 Sep;54(3):465–467. doi: 10.1161/HYPERTENSIONAHA.109.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neagoe PE, Lemieux C, Sirois MG. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J Biol Chem. 2005 Mar 18;280(11):9904–9912. doi: 10.1074/jbc.M412017200. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997 Dec 22;420(1):28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 34.Lewis DF, Canzoneri BJ, Gu Y, Zhao S, Wang Y. Maternal Levels of Prostacyclin, Thromboxane, ICAM, and VCAM in Normal and Preeclamptic Pregnancies. Am J Reprod Immunol. 2010 May 7; doi: 10.1111/j.1600-0897.2010.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004 Jul;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 37.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, et al. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008 Jun 1;14(11):3470–3476. doi: 10.1158/1078-0432.CCR-07-5050. [DOI] [PubMed] [Google Scholar]
- 38.Granger JP, Hall JE. Role of the kidney sodium and fluid secretion in hypertension. In: Lip G, Hall JE, editors. Comprehensive Hypertension. New York: Elsevier; 2007. pp. 241–263. [Google Scholar]
- 39.Zou AP, Cowley AW., Jr Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep. 1999 Apr–May;1(2):178–186. doi: 10.1007/s11906-999-0016-7. [DOI] [PubMed] [Google Scholar]
- 40.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009 May;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 41.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol. 2006 Mar 20;24(9):1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 42.August P, Lenz T, Ales KL, Druzin ML, Edersheim TG, Hutson JM, et al. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: deviations related to the development of superimposed preeclampsia. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1612–1621. doi: 10.1016/0002-9378(90)90639-o. [DOI] [PubMed] [Google Scholar]
- 43.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001 Feb;37(2 Part 2):485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 44.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010 Feb;55(2):394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Pacheco FR, Deudero JJ, Castellanos MC, Castilla MA, Alvarez-Arroyo MV, Yague S, et al. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1395–H1401. doi: 10.1152/ajpheart.01277.2005. [DOI] [PubMed] [Google Scholar]
- 46.Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res. 2009 Oct 1;15(19):5947–5949. doi: 10.1158/1078-0432.CCR-09-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snider KL, Maitland ML. Cardiovascular toxicities: clues to optimal administration of vascular endothelial growth factor signaling pathway inhibitors. Target Oncol. 2009 Apr;4(2):67–76. doi: 10.1007/s11523-009-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009 Feb;20(2):227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 49.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008 Oct 1;26(28):4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JJ, Vaziri SA, Elson P, Rini BI, Ganapathi MK, Ganapathi R. VEGF single necleotide polymorphisms and correlation to sunitinib-induced hypertension in metastatic renal cell carcinoma patients. J Clin Oncol. 2009;27 suppl abstr 5005. [Google Scholar]
- 51.Bono P, Elfving H, Utriainen T, Osterlund P, Saarto T, Alanko T, et al. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol. 2009 Feb;20(2):393–394. doi: 10.1093/annonc/mdn729. [DOI] [PubMed] [Google Scholar]
- 52.Levy BI. Blood pressure as a potential biomarker of the efficacy angiogenesis inhibitor. Ann Oncol. 2009 Feb;20(2):200–203. doi: 10.1093/annonc/mdp018. [DOI] [PubMed] [Google Scholar]
- 53.Ravaud A, Sire M. Arterial hypertension and clinical benefit of sunitinib, sorafenib and bevacizumab in first and second-line treatment of metastatic renal cell cancer. Ann Oncol. 2009 May;20(5):966–967. doi: 10.1093/annonc/mdp201. author reply 7. [DOI] [PubMed] [Google Scholar]
- 54.Eshoj O, Feldt-Rasmussen B, Larsen ML, Mogensen EF. Comparison of overnight, morning and 24-hour urine collections in the assessment of diabetic microalbuminuria. Diabet Med. 1987 Nov–Dec;4(6):531–533. doi: 10.1111/j.1464-5491.1987.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 55.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010 May 5;102(9):596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]