Abstract
Aims
Resveratrol (RV), an antioxidant, inhibits angiotensin II (Ang II)-induced hypertrophy and Ang II- or epidermal growth factor (EGF)-induced Akt phosphorylation in rat vascular smooth muscle cells (VSMCs). Both signalling pathways are reported to utilize reactive oxygen species (ROS). The aim of this study was to show whether RV reduces the ROS level in Ang II- or EGF-activated VSMCs and whether reduction of ROS causes the impeded signalling towards Akt in the presence of RV.
Methods and results
We show here that RV reduces intracellular ROS and extracellular H2O2 release from VSMCs as measured using 2′,7′-dichlorodihydrofluorescein-diacetate and Amplex Red™. Since NADPH oxidases (Nox) 1 and 4 are major ROS sources in VSMCs, we examined their need for Akt phosphorylation in response to Ang II or EGF. Experiments using the blocking peptide gp91ds-tat verified a role for Nox1 in Ang II signalling towards Akt, but excluded a role for Nox1 in the respective EGF signalling. A small interfering RNA-mediated knock-down of Nox4 showed that Nox4 was not required for Ang II- or EGF-induced Akt phosphorylation. Use of the flavoprotein inhibitor diphenyleneiodonium, N-acetyl-cysteine, and non-antioxidant RV derivatives revealed that the antioxidant capacity of RV is not required for the inhibition of Akt phosphorylation, in both rat and human VSMCs.
Conclusion
Thus, although RV acts as an antioxidant, the antihypertrophic response of RV in VSMCs and the signalling downstream of the EGF receptor towards Akt seem to be largely redox independent.
Keywords: Vascular smooth muscle cells, Angiotensin II, Epidermal growth factor, Redox-regulation
1. Introduction
The small polyphenol resveratrol (RV), found in various plant-derived sources, including grapes, is considered to contribute to the beneficial effect of red wine in cardiovascular diseases.1–3 Pharmacological activities of RV relevant for a putative cardio- and vasoprotective effect include the reduction of platelet aggregation, low-density lipoprotein oxidation, prostaglandin synthesis, and tumour necrosis factor-α-induced activation of endothelial cells, as well as the promotion of endothelial nitric oxide (NO) synthase expression and activity.2,3 In addition, RV has been shown to inhibit growth-related signalling pathways in cardiac fibroblasts and to protect against ischaemia–reperfusion injury.2,3 Previous work of our group has demonstrated an antihypertrophic action of RV in angiotensin II (Ang II)-activated vascular smooth muscle cells (VSMCs), which is mediated by a selective inhibition of Akt phosphorylation.4,5 Angiotensin II is a potent vasoconstrictor, but is also involved in inflammatory processes, vascular oxidative stress, cell growth, and extracellular matrix deposition, promoting endothelial dysfunction and vascular remodelling.6,7 In the vessel wall, VSMCs are the principal target cells of Ang II in which it promotes contraction contraction, cell growth (hypertrophy), migration, and increased extracellular matrix deposition via activation of the Ang II type 1 AT1 receptor.7 In VSMCs, the cellular signalling pathways mediating hypertrophy are complex. Important steps are the activation of NAD(P)H oxidase, a major source of cellular reactive oxygen species (ROS), and the transactivation of the epidermal growth factor (EGF) receptor (EGF-R) in a ROS- and c-Src-dependent manner.6–9 Downstream of the EGF-R, Akt and extracellular signal-regulated kinase (ERK) signalling pathways are activated.9 We have previously shown that RV does not interfere with the transactivation of the EGF-R in Ang II-activated VSMCs when inhibiting Akt phosphorylation. In accordance with this, EGF-induced Akt phosphorylation was also inhibited by RV in VSMCs.5 Thus, RV interferes with the signalling towards Akt downstream of the EGF-R in Ang II- or EGF-activated VSMCs. The exact mode of action of RV in VSMCs has remained elusive, although some data have suggested activation of the redox-sensitive protein-tyrosine phosphatase (PTP) Shp2 (Src homology-2-containing protein-tyrosine phosphatase-2) as a mechanism.5
Many activities of RV that are thought to contribute to its cardio- and vasoprotective effect, including the activation of redox-sensitive PTPs, such as Shp2, may be explained by its antioxidant activity.2 ROS are involved in cell signaling, leading to cell growth and migration, activation of MMPs, altered function of many kinases and phosphatases, and activation of pro-inflammatory transcription factors.10 In addition, ROS oxidize low-density lipoprotein and may inactivate NO.11 In VSMCs, the most important source of ROS are the NAD(P)H oxidases Nox1 and Nox4.12–14 Nox1 is highly expressed in proliferating VSMCs and is activated by a variety of growth factors, such as Ang II and platelet-derived growth factor, contributing to their activation of p38 mitogen-activated protein kinase and Akt.13,15,16 The function of Nox4, the only constitutively active NAD(P)H oxidase, is less clear.15,16 In VSMCs, Nox4 was found within distinct subcellular locations, such as the nucleus or the endoplasmic reticulum, but also at focal adhesions where the transactivated EGF-R and phosphatidylinositol 3-kinase (PI3K) can be found after Ang II stimulation.15–18 While Nox1 is known to be required for Ang II-induced EGF-R transactivation, little is known how the EGF-activated EGF-R induces ROS production19,20 that may lead to PTP oxidation and signal transduction.21
The aim of this study, therefore, was to investigate the following: (i) whether RV reduces the ROS level in or H2O2 released from Ang II- or EGF-activated VSMCs; (ii) whether Nox1 or Nox4 proteins, or ROS in general, are required for the phosphorylation of Akt downstream of the EGF-R; and thus (iii) whether RV may act directly or indirectly as an ‘antioxidant’ when inhibiting the phosphorylation of Akt.
2. Methods
2.1. Reagents
Materials were obtained from the following suppliers: antibodies against phospho-Akt (Ser473) and phospho-p38 were from Cell Signaling Technology, anti-tubulin antibody was obtained from Santa Cruz Biotechnology. Resveratrol, Ang II, diphenyleneiodonium (DPI) and N-acetyl-cysteine (NAC) were purchased from Sigma-Aldrich. Epidermal growth factor was obtained from Upstate. 2′,7′-Dichlorodihydrofluorescein-diacetate (H2DCF-DA) and the Amplex Red™ reagent were from Molecular Probes/Invitrogen. The blocking peptide for Nox1 (Nox2 is not expressed in rat VSMCs;22 and own unpublished results), gp91ds-tat, was [H]-RKKRRQRRRCSTRIRRQL-NH2, and the control peptide used was [H]-RKKRRQRRRAGAGAGAGA-NH2, both from CASLO Laboratory ApS. All chemicals used for the synthesis of RV derivatives, unless otherwise stated, were analytical grade and obtained from Sigma-Aldrich Chemie GmbH (Schnelldorf, Germany), TCI Europe (Zwijndrecht, Belgium) and Apollo Scientific Limited (Bredbury, UK) and used without further purification.
2.2. Cultivation of cells
The VSMCs were provided by Drs Kathy K. Griendling and Dan Sorescu (Emory University School of Medicine) and were obtained by three independent isolations from male Sprague–Dawley rat (200–250 g, 5–8 weeks old) thoracic aortas by enzymatic digestion as described previously.8,23 Cells were grown in phenol red-free Dulbecco's modified Eagle's medium supplemented with 10% calf serum, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (growth medium). For experiments, cells between passage 7 and 15 were used at 70% confluence. Prior to all growth factor stimulation experiments, cells were starved for 24 h in Dulbecco's modified Eagle's medium containing 0.1% calf serum supplemented with 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (starve medium).
2.3. SDS–PAGE and western blotting
For experiments, cells were grown in 60 mm dishes and serum starved overnight in starve medium, then treated with Ang II (100 nM) or EGF (100 ng/mL) for the indicated time. Resveratrol (50 µM), vehicle control [dimethyl sulfoxide (DMSO) 0.1%], gp91ds-tat or the respective control peptide (100 µM) were added 30 min before stimulation. The VSMCs were pretreated with DPI (10 µM) for 1 h and NAC (10 mM) for 2 h before stimulation. Cells were then suspended in lysis buffer (50 mM HEPES, 50 mM NaCl, 5 mM EDTA, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride and 1× complete protease inhibitor cocktail). Western blot analysis was performed as described previously.4 Proteins of interest were visualized by the use of specific antibodies, an ECL system and a CCD camera (Fuji LAS 3000). Densitometric evaluation was performed by employing the AIDA image analyser 4.06 software (Raytest).
2.4. Measurement of intracellular ROS
For detection of the intracellular total ROS level, cells were grown in six-well plates and treated as indicated. Quiescent cells were washed once with pre-warmed HEPES buffered saline solution and incubated at 37°C for 30 min with 20 µM H2DCF-DA before stimulation with EGF (100 ng/mL) or Ang II (100 nM) for the indicated periods of time. Cells were then immediately analysed by flow cytometry (FACSCalibur, BD Bioscience). The mean fluorescence of 10 000 analysed cells (corrected for autofluorescence) was detected and used as a measurement for total ROS.
2.5. Determination of extracellular H2O2 level
The VSMCs were grown in 24-well plates and serum starved for 24 h. Cells were washed once with prewarmed phosphate-buffered saline, then krebs ringer phosphate glucose buffer containing the Amplex Red™ reagent was applied to the cells. Plates were incubated for 15 min at 37°C before VSMCs were stimulated as indicated. Resorufin, the fluorescent product, was measured in triplicate by a fluorescence multi-well plate reader with an excitation wavelength of 535 nm and an emission wavelength of 590 nm (Genios Pro, Tecan, Switzerland).
2.6. Transfection with small interfering RNA and quantitative real-time PCR
Five hours after seeding, VSMCs were transfected with small interfering RNA (siRNA; siNox4 from ThermoFisher Scientific, 50 nM; or scrambled siRNA control from Invitrogen) using Oligofectamine (Invitrogen) and Opti-MEM 1 (Gibco). For Nox4, the sequences used were sense 5′-ACUGAGGUACAGCUGGAUGUU-3′, and antisense 5′-CAUCCAGCUGUACCUCAGUUU-3′. Experiments were performed 72 h after siRNA transfection. The knock-down efficiency was verified by quantitative real-time PCR (qPCR). For this, mRNA was isolated using the pegGOLD total RNA Kit (Peqlab), and reverse transcription was performed using Superscript™ First-Strand Synthesis System (Invitrogen) according to the manufacturer's protocol. PCR was carried out with a LightCycler™ LC480 SYBR Green I Master reagent (Roche Diagnostics) and the LightCycler™ LC480 (Roche Diagnostics) system.
2.7. Synthesis of RV derivatives
Methylated resveratrol derivatives were synthesized according to the literature.24 Both 1H and 13C nuclear magnetic resonance spectra were recorded on a Bruker Advance DPx200 (200 and 50 MHz). Mass spectra (MS) were obtained by a Shimadzu (GC-17A; MS-QP5050A) spectrometer.
2.8. Statistical analysis
Results are expressed as means ± SEM. Statistical analysis was performed by ANOVA followed by Dunnett's multiple comparison test or by one-sample t-test using GraphPad PRISM, version 4.0 (GraphPad Software, San Diego, CA, USA). Differences with P < 0.05 were considered significant.
3. Results
3.1. Resveratrol reduces intracellular total ROS level and H2O2 release from VSMCs
First, we asked whether RV reduces intracellular total ROS level and/or H2O2 released from VSMCs and thereby leads to inhibition of Akt phosphorylation as described earlier.4,5 Since NAD(P)H oxidases play a pivotal role in VSMC-derived ROS production, DPI, an unspecific inhibitor of flavin-containing enzymes including NAD(P)H oxidases,25 was used as positive control. For detection of intracellular ROS, we loaded VSMCs with 20 µM H2DCF-DA. Angiotensin II (100 nM) was able to induce ROS production twofold compared with the vehicle control. Resveratrol (50 µM) and DPI (10 µM) pre-treatment reduced this ROS production almost to the basal level (Figure 1A). In contrast, treatment of cells with 100 ng/mL EGF, a concentration that readily induced Akt phosphorylation, did not lead to a significant activation of intracellular ROS production in comparison with the vehicle control. Resveratrol and DPI, however, were able to inhibit basal intracellular ROS level by about 50% (Figure 1B), verifying a strong ROS reducing activity for both compounds. Activation of the EGF-R has been reported to lead to increased extracellular H2O2 levels in A431 cells.19 Therefore, we also measured extracellular H2O2 level using Amplex Red™. Both Ang II and EGF were able to induce the extracellular release of H2O2 in a time-dependent manner (Figure 1C and D). Whereas EGF treatment led to a continuous accumulation of H2O2, which was highest after 15 min (20% above basal level), H2O2 production after Ang II stimulation peaked at 10 min (15% above basal level). Resveratrol reduced Ang II- and EGF-induced H2O2 but also basal levels far below control values (Figure 1C and D). These findings demonstrate that RV blocks both H2O2 detected in the extracellular environment and intracellular total ROS produced in VSMCs.
Figure 1.
Resveratrol (RV) diminishes intracellular reactive oxygen species (ROS) level and attenuates both basal and angiotensin II (Ang II)- or epidermal growth factor (EGF)-induced extracellular H2O2. Quiescent vascular smooth muscle cells (VSMCs) were pre-incubated with dichlorodihydrofluorescein-diacetate (H2DCF-DA; 20 µM, 15 min) and then incubated with 50 µM RV or vehicle control for 30 min or 10 µM diphenyleneiodonium (DPI) for 1 h and finally stimulated with 100 nM Ang II for 15 min (A) or 100 ng/mL EGF for 10 min (B). ROS production (equivalent to the amount of formed DCF) was analysed by flow cytometry. The average fluorescence signal obtained in the presence of Ang II or EGF was set to 100% (**P < 0.01; one-way ANOVA vs. Ang II or EGF treatment, n = 3). Serum-starved VSMCs were stimulated in Amplex Red™ buffer with Ang II (100 nM; C) or EGF (100 ng/mL; D) for 5–15 min after pre-incubation with RV (50 µM) or vehicle control for 30 min. H2O2 production was quantified by fluorescence measurement. Absolute values were correlated to catalase-treated cells used as a negative control. Graphs show the relative extracellular H2O2 production expressed as a percentage of vehicle-treated control cells (open square, vehicle-treated cells; filled squares, RV-treated cells; ***P < 0.001; t-test, n = 4).
3.2. A Nox4 knock-down does not influence the phosphorylation of Akt in Ang II- or EGF-activated VSMCs
To investigate whether Nox4 is important for the EGF- or Ang II-induced signalling towards Akt in our cell system, Nox4 was knocked down by siRNA. After 72 h, Nox4 mRNA level was analysed by qPCR. The siRNA reduced the Nox4 mRNA level to 15% compared with scrambled control, whereas the mRNA level of Nox1 was unchanged (Figure 2A and B). As expected, knock-down of Nox4 had no impact on the phosphorylation of Akt after Ang II (100 nM, 10 min) stimulation. Moreover, the inhibitory effect of RV on Akt phosphorylation remained unaffected (Figure 2C). Interestingly, the EGF-induced Akt phosphorylation and the inhibitory effect of RV also remained unaltered in Nox4 knock-down cells (Figure 2D). Thus, Nox4 seems not to be involved in Ang II- or EGF-mediated phosphorylation of Akt, and therefore Nox4-derived ROS are not a potential target of RV.
Figure 2.
Ang II- and EGF-induced Akt phosphorylation and its inhibition by RV occur independent of Nox4. Knock-down of Nox4 was achieved by treating cells with 50 nM small interfering RNA (siRNA) against Nox4 or 50 nM scrambled control siRNA for 72 h. The mRNA was isolated, and expression of Nox4 and Nox1 detected by qPCR. Graphs (A and B) show mean + SEM of Nox4 mRNA (A) or Nox1 mRNA (B) level relating to 18S mRNA. Data were normalized by setting scrambled control to 100% (***P < 0.001; n = 3). Serum-deprived Nox4 siRNA-treated cells (50 nM, 72 h) were pre-treated with RV (50 µM, 30 min) or vehicle control followed by stimulation with 100 nM Ang II for 10 min (C) or 100 ng/mL EGF for 5 min (D). Akt phosphorylation and tubulin (tub, serving as a loading control) were detected by western blot analysis. One representative blot out of three, which were densitometrically analysed, is shown. The graphs represent the mean densitometric values + SEM of three independent experiments. Akt phosphorylation in response to Ang II or EGF of scrambled control was set to 100% (*P < 0.05, **P < 0.01; one-way ANOVA, n = 3).
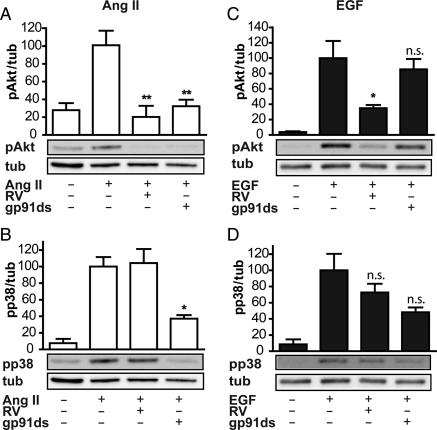
3.3. Down-regulation of Nox1 inhibits Akt phosphorylation in response to Ang II but not EGF stimulation
In Ang II-activated VSMCs, Nox1 has been shown to be the major ROS-producing component necessary for the transactivation of the EGF-R, leading finally to Akt activation.16 To the best of our knowledge, however, it is unknown whether EGF utilizes Nox1 to transduce its growth signal, although in transactivated settings prolonged Nox1 activation seems to need Rac1 activation via PI3K downstream of the EGF-R.16 To inhibit Nox1 we employed 100 µM of the blocking peptide gp91ds-tat that prevents assembly of the Nox protein with cofactors12 or a control peptide. As expected, gp91ds-tat pre-treatment led to an inhibition of Ang II-mediated Akt phosphorylation (Figure 3A). In contrast to RV, gp91ds-tat also inhibited the phosphorylation of p38, a known redox-sensitive kinase, by more than 50% (Figure 3B). In EGF-activated VSMCs, gp91ds-tat, in contrast to RV, had no effect on Akt phosphorylation (Figure 3C). A putative effect of gp91ds-tat on p38 phosphorylation did not reach significance (Figure 3D). The unspecific control peptide used had no influence on Akt or p38 phosphorylation in response to either stimulus (data not shown). These data show that Nox1 is important for Ang II- but not for EGF-mediated phosphorylation of Akt and p38. Inhibition of Nox1 is not able to mimic the effect of RV in our setting, and thus renders Nox1-derived ROS an unlikely target of RV.
Figure 3.
Inhibition of Nox1 blunts phosphorylation of Akt and p38 induced by Ang II but does not alter EGF-induced Akt and p38 phosphorylation. Quiescent VSMCs were pre-treated with 100 µM of the gp91ds-tat blocking peptide (gp91ds), 50 µM RV or vehicle control for 30 min, and were then stimulated with 100 nM Ang II for 10 min (A and B) or 100 ng/mL EGF (C and D) for 5 min. Akt (A and C) and p38 phosphorylation (B and D) as well as tubulin (tub, as loading control) were detected by western blot analysis. One representative blot out of three, which were densitometrically analysed, is shown. The graphs depict the mean densitometric values + SEM of three independent experiments. Signals obtained in response to Ang II or EGF were set to 100% (*P < 0.05, **P < 0.01; n.s., not significant; one-way ANOVA vs. Ang II or EGF treatment, n = 3).
3.4. Both DPI and NAC inhibit Akt phosphorylation upon Ang II but not EGF stimulation
To further clarify the general dependency of EGF- and Ang II-induced signalling towards Akt and p38 on ROS we applied the Nox/flavoprotein inhibitor DPI (10 µM) and the precursor of glutathione, NAC (10 mM). We found that Ang II-induced phosphorylation of Akt was blocked by NAC as effectively as by RV, while DPI was less effective (Figure 4A). In contrast to RV, which had no effect on the phosphorylation of p38 after Ang II stimulation, NAC and DPI were able to block the phosphorylation to basal levels (Figure 4B). Phosphorylation of Akt upon EGF stimulation was inhibited effectively only by RV; NAC and DPI had no consistent inhibitory effect (Figure 4C). Resveratrol, NAC or DPI did not inhibit p38 phosphorylation in response to EGF stimulation (Figure 4D). These data indicate that Ang II, as described, utilizes ROS for its signal transduction towards Akt and p38, but EGF does not. Moreover, RV seems not to require its antioxidant activity to inhibit Akt phosphorylation.
Figure 4.
DPI and NAC inhibit Ang II-induced phosphorylation of Akt and p38. Serum-deprived cells were pre-treated with 50 µM RV or vehicle control for 30 min, 10 µM DPI for 1 h or 10 mM NAC for 2 h, and then stimulated with 100 nM Ang II for 10 min (A and B), or 100 ng/mL EGF for 5 min (C and D). Lysates were subjected to western blot analysis using antibodies against pAkt (A and C), pp38 (B and D) and tubulin (tub). Representative blots out of three are shown. The graphs depict the mean + SEM of the densitometric analyses. Signals obtained in response to Ang II or EGF were set to 100% (*P < 0.05, **P < 0.01; n.s., not significant; one-way ANOVA vs. Ang II or EGF treatment, n = 3).
3.5. A non-antioxidant RV derivative inhibits Ang II and EGF-mediated Akt phosphorylation
To confirm that RV, although redox-active, does not act as an antioxidant when inhibiting Akt phosphorylation we employed the following two non-antioxidant derivatives of RV: trans-3,5,4′-trimethoxystilbene (RV-3M) with all three hydroxyl groups methylated (Figure 5A) and thus blunted redox activity,26 and trans-3,5-dihydroxy-4′-methoxystilbene (RV-1M), methylated at the 4′-OH group only (Figure 5B). Mono-methylation at the 4′-OH group renders RV largely redox inactive, since Queiroz et al. have recently shown that the prime antioxidant pharmacophore of RV is 4′-hydroxystilbene,27 a finding that is also supported by others26 and confirmed by our synthesized derivatives in vitro (see Supplementary material online, Figure S1). To test their redox activity in the presence of cells we used Amplex Red™ to detect extracellular H2O2 in the presence of RV or its derivatives, RV-3M and RV-1M. As expected, neither of the two derivatives was found to reduce extracellularly detected H2O2 significantly after stimulation of VSMCs with Ang II (Figure 6A). Using Akt phosphorylation as read-out, RV-3M inhibited neither Ang II- nor EGF-induced Akt phosphorylation. Most interestingly, however, the derivative RV-1M, despite its lack of influence on H2O2, reduced phosphorylation of Akt in response to Ang II treatment by over 50%, which was comparable to RV-mediated inhibition. The EGF-induced phosphorylation of Akt was inhibited equally well by RV-1M compared with RV (Figure 6B and C). These data clearly show that RV inhibits Akt phosphorylation by a mechanism that is not related to its antioxidant activity in both Ang II- and EGF-stimulated VSMCs. In contrast, the 3- and 5-OH groups of RV seem to be critically involved in mediating Akt inhibition. A consistent picture of redox-independent inhibition of Akt phosphorylation by RV and RV-1M was observed in human primary aortic smooth muscle cells (see Supplementary material online, Figure S2).
Figure 5.
Non-antioxidant derivatives of RV, trans-3,5,4′-trimethoxystilbene (RV-3M; A), and trans-3,5-dihydroxy-4′-methoxystilbene (RV-1M; B).
Figure 6.
Non-antioxidant trans-3,5-dihydroxy-4′-methoxystilbene (RV-1M) inhibits phosphorylation of Akt after Ang II and EGF stimulation. (A) Starved VSMCs were stimulated in Amplex Red™ buffer with Ang II (100 nM) for 10 min after pre-incubation with RV or RV derivatives (RV, RV-3M, RV-1M; all 50 µM), or vehicle control for 30 min. H2O2 production was quantified by fluorescence measurement. The graph shows the relative extracellular H2O2 production (**P < 0.01; one-way ANOVA vs. Ang II or EGF treatment, n = 3). (B and C). Serum-deprived cells were pre-treated with RV or RV derivatives, RV-3M or RV-1M, for 30 min (50 µM), followed by stimulation with 100 nM Ang II for 10 min (B), or 100 ng/mL EGF for 5 min (C). Protein levels were detected by western blot using antibodies against pAkt and tubulin. One representative blot out of three per stimulation is shown. Graphs show the mean + SEM of the densitometric signals; Ang II or EGF stimulation is set to 100% (**P < 0.01; one-way ANOVA vs. Ang II or EGF treatment, n = 3).
4. Discussion
We show here for the first time that although RV reduces total intracellular ROS level and extracellular H2O2 release, its antioxidant activity is not required for the inhibition of Akt phosphorylation in Ang II- or EGF-activated VSMCs. Moreover, we show that in VSMCs, EGF leads to an increase of extracellular H2O2 level but not of intracellular ROS production. This H2O2 seems not to play a major role for the signal transduction towards Akt and p38. Furthermore, we confirm that the Ang II-induced signalling pathways towards Akt and p38 require Nox1, and clearly show that Nox4 is not required for Ang II- or EGF-induced Akt activation.
Many pharmacological effects of RV are believed to be related to its antioxidant activity. In the field of cardiovascular diseases, Opie & Lecour recently summarized several of the RV effects that could be linked to an antioxidant action, including inhibition of low-density lipoprotein oxidation, inhibition of prostaglandin synthesis, down-regulation of intercellular adhesion molecule-1 and nuclear factor-κB, reduction of platelet activation and inhibition of Ang II-induced cardiomyocyte hypertrophy, and they speculated that even more RV effects might be redox related, although not proven yet.2 Especially the report showing that RV inhibits Ang II-induced cardiomyocyte hypertrophy by its antioxidant activity may be of interest in our context. The authors show that RV (1–100 µM) inhibits Ang II-induced [3H]leucine incorporation as a measure for hypertrophy, beta-myosin heavy chain promoter activity, intracellular ROS level and ERK phosphorylation (although not examining other kinases) in cardiomyocytes. To link the antioxidant activity to the antihypertrophic effect of RV, they show that RV and the antioxidant NAC inhibit Ang II- or H2O2-induced [3H]leucine incorporation in cardiomyocytes comparably.28 In some parts, the above report is in agreement with this and previous studies of our group, although we used VSMCs instead of cardiomyocytes; we found an inhibition of Ang II-induced hypertrophy measured by [3H]leucine incorporation4 and now also ROS production by RV. Next to a minor effect on ERK phosphorylation, we found a strong inhibition of the PI3K–Akt pathway leading more downstream to an effective inhibition of p70 S6 kinase important for cellular hypertrophy.4 Here we show, however, that inhibition of ROS and Akt phosphorylation are two parallel events mediated by RV that could not be causally linked in our system.
Our data also imply that the transduction of the signal induced by EGF in VSMCs is less redox dependent than that of Ang II, at least towards Akt and p38. Indeed, data regarding the ROS production of the EGF-activated (not transactivated) EGF-R and their importance for subsequent signal transduction are limited. The first report showing an intracellular increase in H2O2 production in response to EGF used epithelial A431 cells and concentrations of EGF as high as 500 ng/mL for stimulation.29 Halvey et al.,20 also detecting an increase of intracellular ROS after EGF stimulation in HaCaT cells, still used 200 ng/mL EGF. In agreement with our data, DeYulia et al.19 showed in 293T cells transiently transfected with EGF-R that the addition of EGF (50 ng/mL) results in elevated extracellular H2O2. They even found that solely the receptor–ligand interaction using isolated proteins was sufficient to produce H2O2. The exact mechanism by which ROS is produced, however, remained elusive. Regarding the ROS source that might be used by the EGF-R to transduce the EGF signal, Chen et al. proposed Nox4 that is localized at the endoplasmic reticulum and is critical for the regulation of PTP1B. Using human aortic endothelial cells (HAECs), and for transfection studies COS-7 cells, they suggest that Nox4-mediated oxidation and inactivation of PTP1B in the endoplasmic reticulum serves as a regulatory switch for EGF-R trafficking that terminates the EGF signal.30 Moreover, both the activated EGF-R as well as Nox4 were shown to be localized at focal adhesions in VSMCs.31 Knocking down Nox4 in our system, however, had no influence on EGF- as well as Ang II-induced Akt phosphorylation, which excludes Nox4 as essential mediator of signal transduction towards Akt downstream of the AT1- and EGF-R as well as a potential target of RV.
While the impact of Nox4-derived ROS for a variety of physiological processes, including cell growth, death, and differentiation is not completely understood, the role of Nox1 in VSMC is much better defined.16 Nox1-derived ROS was shown to regulate smooth muscle cell growth (hypertrophy and hyperplasia) and migration. The best-studied mechanism by which Nox1 is activated is that via the AT1 receptor by Ang II, including activation of phospholipase C and subsequently protein kinase C that phosphorylates p47phox, a regulatory subunit of Nox1. Transactivation of the EGF-R leads to a PI3K-dependent activation of Rac1 that is required for continued activation of Nox1 by Ang II.16 Our data presented here again verify this model; the blocking peptide gp91ds-tat effectively inhibited the Ang II-induced signalling towards Akt and p38. In contrast, EGF-mediated phosphorylation of Akt and p38 was not significantly altered. We, therefore, excluded a Nox1-dependent mechanism activating Akt or p38 downstream of the EGF-R in our cells.
Several reports suggest an influence of RV on NAD(P)H oxidases in non-phagocytic cells.32–34 In our experimental set-up, however, neither knock-down of Nox4 nor inhibition of Nox1 assembly could mimic the RV effect. In addition, previous studies showed that RV was unable to inhibit the transactivation of the EGF-R in Ang II-activated VSMCs,5 a process highly regulated by Nox1. Nevertheless, it remains conceivable that RV contributes to the inhibition of Nox1-derived ROS production by the inhibition of PI3K-dependent Rac1 at later time points.
The strongest argument against a redox-dependent mechanism of the inhibition by RV of Ang II- or EGF-induced Akt activation came from RV derivatives that were not acting as antioxidants but were highly effective in inhibiting Akt phosphorylation (Figure 6). Most interestingly, we found that the hydroxyl groups in the 3- and 5-positions are sufficient to block Ang II- or EGF-induced Akt phosphorylation. Although not detected, we cannot completely exclude the possibility that small or local rises in intracellular H2O2 are quenched by these remaining two hydroxyl groups.
In summary, we could verify that Nox1 but not Nox4 is important for the Ang II-induced activation of Akt and p38 in VSMCs. The signalling triggered by EGF downstream of the EGF-R towards Akt and p38 phosphorylation seems to occur independently of Nox1, Nox4 or in general of ROS. Most interestingly, we showed that RV, although reducing intracellular ROS and extracellular H2O2 levels in VSMCs, does not use an antioxidant mechanism to inhibit Akt phosphorylation in Ang II- or EGF-activated VSMCs.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
The work was supported by the Austrian Science Fund (FWF) [grant number P18982-B17 to V.M.D.]. Funding to pay the Open Access publication charge was provided by the Austrian Science Fund (FWF).
Acknowledgements
The authors would like to express their gratitude to H. Beres, B. Gindl and R. Leitner for expert technical assistance. We also wish to thank Drs Kathy K. Griendling and Dan Sorescu (Emory University School of Medicine) for providing rat VSMCs as well as for useful advice and fruitful discussions.
Conflict of interest: none declared.
References
- 1.Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. doi:10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Lecour S. The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J. 2007;28:1683–1693. doi: 10.1093/eurheartj/ehm149. doi:10.1093/eurheartj/ehm149. [DOI] [PubMed] [Google Scholar]
- 3.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. doi:10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Haider UG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol Pharmacol. 2002;62:772–777. doi: 10.1124/mol.62.4.772. doi:10.1124/mol.62.4.772. [DOI] [PubMed] [Google Scholar]
- 5.Haider UG, Roos TU, Kontaridis MI, Neel BG, Sorescu D, Griendling KK, et al. Resveratrol inhibits angiotensin II- and epidermal growth factor-mediated Akt activation: role of Gab1 and Shp2. Mol Pharmacol. 2005;68:41–48. doi: 10.1124/mol.104.005421. [DOI] [PubMed] [Google Scholar]
- 6.Heeneman S, Sluimer JC, Daemen MJAP. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. doi:10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 7.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. doi:10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 8.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–428. doi: 10.1042/CS20060342. doi:10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 10.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. doi:10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Thomas SR, Keaney JF., Jr Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic Biol Med. 2003;35:117–132. doi: 10.1016/s0891-5849(03)00239-9. doi:10.1016/S0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 12.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. doi:10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 13.Cave A. Selective targeting of NADPH oxidase for cardiovascular protection. Curr Opin Pharmacol. 2009;9:208–213. doi: 10.1016/j.coph.2008.10.001. doi:10.1016/j.coph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. doi:10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 15.Brandes RP, Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. doi:10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. doi:10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res. 2005;97:829–836. doi: 10.1161/01.RES.0000185322.46009.F5. doi:10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]
- 18.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. doi:10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 19.DeYulia GJ, Jr, Carcamo JM, Borquez-Ojeda O, Shelton CC, Golde DW. Hydrogen peroxide generated extracellularly by receptor–ligand interaction facilitates cell signaling. Proc Natl Acad Sci USA. 2005;102:5044–5049. doi: 10.1073/pnas.0501154102. doi:10.1073/pnas.0501154102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halvey PJ, Watson WH, Hansen JM, Go Y-M, Samali A, Jones DP. Compartmental oxidation of thiol–disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. doi:10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeYulia GJ, Jr, Cárcamo JM. EGF receptor–ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases. Biochem Biophys Res Commun. 2005;334:38–42. doi: 10.1016/j.bbrc.2005.06.056. doi:10.1016/j.bbrc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 22.Lassègue B, Sorescu D, Szöcs K, Yin Q, Akers M, Zhang Y, et al. Novel gp91phox homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. doi:10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 23.Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem. 1991;266:15498–15504. [PubMed] [Google Scholar]
- 24.Huang X-F, Ruan B-F, Wang X-T, Xu C, Ge H-M, Zhu H-L, et al. Synthesis and cytotoxic evaluation of a series of resveratrol derivatives modified in C2 position. Eur J Med Chem. 2007;42:263–267. doi: 10.1016/j.ejmech.2006.08.006. doi:10.1016/j.ejmech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Majander A, Finel M, Wikström M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) J Biol Chem. 1994;269:21037–21042. [PubMed] [Google Scholar]
- 26.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. doi:10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 27.Oueiroz AN, Gomes BAQ, Moraes WM, Jr, Borges RS. A theoretical antioxidant pharmacophore for resveratrol. Eur J Med Chem. 2009;44:1644–1649. doi: 10.1016/j.ejmech.2008.09.023. doi:10.1016/j.ejmech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Cheng TH, Liu JC, Lin H, Shih NL, Chen YL, Huang MT, et al. Inhibitory effect of resveratrol on angiotensin II-induced cardiomyocyte hypertrophy. Naunyn-Schmiedebergs Arch Pharmacol. 2004;369:239–244. doi: 10.1007/s00210-003-0849-6. doi:10.1007/s00210-003-0849-6. [DOI] [PubMed] [Google Scholar]
- 29.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. doi:10.1074/jbc.272.1.217. [PubMed] [Google Scholar]
- 30.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. doi:10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. doi:10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Zhang J, Ungvai Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. doi:10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. doi:10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 34.Orallo F, Alvarez E, Camina M, Leiro JM, Gomez E, Fernandez P. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol. 2002;61:294–302. doi: 10.1124/mol.61.2.294. doi:10.1124/mol.61.2.294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.