Abstract
Aims
Angiotensin II (Ang II) stimulates cardiac remodelling and fibrosis in the mechanically overloaded myocardium. Although Rho GTPases regulate several cellular processes, including myocardial remodelling, involvement in mediating mechanical stretch-induced regulation of angiotensinogen (Ao), the precursor to Ang II, remains to be determined. We, therefore, examined the role and associated signalling mechanisms of Rho GTPases (Rac1 and RhoA) in regulation of Ao gene expression in a stretch model of neonatal rat cardiac fibroblasts (CFs).
Methods and results
CFs were plated on deformable stretch membranes. Equiaxial mechanical stretch caused significant activation of both Rac1 and RhoA within 2–5 min. Rac1 activity returned to control levels after 4 h, whereas RhoA remained at a high level of activity until the end of the stretch period (24 h). Mechanical stretch initially caused a moderate decrease in Ao gene expression, but was significantly increased at 8–24 h. RhoA had a major role in mediating both the stretch-induced inhibition of Ao at 4 h and the subsequent upregulation of Ao expression at 24 h. β1 integrin receptor blockade by Tac β1 expression impaired acute (2 and 15 min) stretch-induced Rac1 activation, but increased RhoA activity. Molecular experiments revealed that Ao gene expression was inhibited by Rac1 through both JNK-dependent and independent mechanisms, and stimulated by RhoA through a p38-dependent mechanism.
Conclusion
These results indicate that stretch-induced activation of Rac1 and RhoA differentially regulates Ao gene expression by modulating p38 and JNK activation.
Keywords: Cardiac fibroblasts, Mechanotransduction, Rac1, RhoA, Angiotensinogen
1. Introduction
Mechanical stress is a major stimulus responsible for the functional and structural changes that occur in the haemodynamically overloaded myocardium. The renin–angiotensin system (RAS) is activated in the pressure-overloaded myocardium and has a major role in mediating both hypertrophy and remodelling of the heart.1 Several clinical and experimental studies have demonstrated that angiotensin-converting enzyme (ACE) inhibitors and Ang II type I receptor (AT1R) antagonists prevent and/or reverse the cardiac hypertrophy and myocardial remodelling caused by hypertension and mechanical load.2,3 All components of the RAS (renin, Ao, ACE, Ang II, Ang II receptors) are present in the ventricular myocardium and produced by cardiac fibroblasts (CFs).1,4,5 Targeted overexpression of Ao in the myocardium results in increased cardiac Ang II and ventricular hypertrophy,6 suggesting that locally produced Ao can induce heart failure.
The mechanisms by which mechanical stress regulates Ao gene expression in CFs remain to be determined. Like their myocyte counterparts, cultured CFs display immediate signalling responses to mechanical stretch.7,8 β1 integrin is a major mechanosensor in cardiac cells and couples to effector systems involved in the regulation of cellular growth and gene expression.9,10 Primary effectors activated by β1 integrin include Rho GTPases11 and stress-activated protein kinases (SAPKs).12 SAPKs (such as JNK and p38) have been shown to be downstream targets of Rho GTPases in non-cardiac cells,13 whereas JNK and p38 are important for stretch-induced regulation of Ao expression in cardiac myocytes and fibroblasts.14
Rho GTPases, Rac1 and RhoA, have been implicated in pathophysiology of many major cardiovascular diseases, such as hypertension, heart failure, myocardial infarction, and atherosclerosis.15 Inhibition of Rho-kinase, a potent RhoA effector, blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure.16,17 However, the role of Rho GTPases in stretch-induced Ao gene expression is not known. Thus, in the present study, we examined the role of Rac1 and RhoA in mediating β1 integrin-induced JNK and p38 activation, as well as regulation of Ao gene expression in stretched CFs.
2. Methods
2.1. Preparation of neonatal rat CF cultures
CFs were prepared from hearts of newborn (0–2 day old) Sprague–Dawley rat pups, as described.14 Cells were passaged, attached to deformable membranes coated with collagen-IV and serum-starved for experiments as described in the Supplementary material online, Section 1. For all the stretch experiments, cells were exposed to 20% equiaxial stretch, which mimics in situ pathological conditions. This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and approved (protocols 2002–011; 2008–040) by the Texas A&M Health Science Center Institutional Animal Care and Use Committee.
2.2. Adenovirus infection of cells
Twelve hours after plating, CFs were infected for 24 h with an optimal MOI of adenovirus [45 MOI for green fluorescent protein (GFP), dominant-negative Rac1-N17 (Rac1-DN) and dominant-negative RhoA-N19 (Rho-DN); constitutively active Rac1-V17 (Rac1-CA) and constitutively active RhoA-V19 (RhoA-CA); 300 MOI for LacZ and Tac β1] in serum-free DMEM/Medium 199. Determination of expressed proteins in CFs was done using flow cytometry and western blot analysis. Amplification of adenoviruses is described in the Supplementary material online, Section 2.
2.3. Preparation of cell lysates and western blot analysis
Cell lysates for western blot analysis were obtained by scrapping CFs into assay lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) supplemented with 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM 4- (2-aminoethyl)-benzenesulfonyl fluoride hydrochloride, and 1 mM sodium orthovanadate (see Supplementary material online, Section 3). Insoluble cell debris was removed by centrifugation (25 000 g) for 10 min at 4°C and cell lysates were boiled with Laemmli sample buffer [0.5 mol/L Tris–HCl (pH 6.8), 10% SDS, 10% glycerol, 4% β-merceptoethanol, and 0.05% Bromphenol Blue]. Western blot analysis was performed as described in the Supplementary material online, Section 3.
2.4. Rac1 and RhoA activity assays
Procedures for performing Rac1 and RhoA activity assays are detailed in the Supplementary material online, Section 4. CFs were stretched for various times after adenovirus-assisted transfection. Immediately after stretch, CFs were lysed in the commercially available (Boston BioProducts, Worcester, MA, USA) RIPA lysis buffer supplemented with protease inhibitor cocktail, followed by centrifugation (25 000 g) for 10 min at 4°C. Equal volumes of cell lysate were incubated with GST–PBD and GST–RBD beads (prepared as described in the Supplementary material online, Section 5) and incubated for 60 min at 4°C as previously described.18 Briefly, beads containing fusion-protein bound Rho GTPase were washed with bead wash buffer and eluted in Laemmli sample buffer. GTP-bound Rac1 and RhoA were detected by 12% SDS–PAGE and western blotting, using respective antibodies. Parallel sets of lysates were separated by SDS–PAGE and western blots were probed using Rac1 (BD Transduction laboratory, Franklin Lakes, NJ, USA) or RhoA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibodies. Bands of interest were quantified using scanning densitometry. Signals for active Rac1/RhoA were normalized by total (lysate) of corresponding samples and represented as fold change.
2.5. Quantitative measurement of Ao mRNA using real-time PCR
Absolute levels of Ao mRNA in total RNA from CFs were determined using real-time RT-PCR.14 Procedures for isolation of RNA and multiplex real-time RT-PCR are given in the Supplementary material online, Section 6.
2.6. Flow cytometry and immunofluorescence microscopy
Adenoviruses used to express Rac1-DN and RhoA-DN in CFs also expressed GFP, allowing GFP to be a reporter for monitoring adenoviral vector expression by flow cytometry and immunofluorescent microscopy. For flow cytometry analysis, CFs infected for 24 h with GFP (control), Rac1-DN, or RhoA-DN adenovirus were harvested by incubation in 1 mM EDTA, washed with phosphate buffered saline (PBS, pH 7.4), resuspended at 1 × 106 cells/mL in FACS buffer (PBS containing 1% BSA and 1 mmol/L EDTA) analysed by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA, USA), using Cell Quest 5.2 software. Each analysis was based on a sample of 10 000 cells. CFs not expressing GFP were used as negative controls. Immunofluorescent staining for Rac-1, RhoA, and β1 integrin was performed on CFs plated onto 2-well chamber slides (Lab-Tek, Nunc, Nalge Nunc International, Rochester, NY, USA) (100 viable cells/mm2) coated with 1 µg/cm2 collagen IV. The CFs were grown to ∼60% confluence (2 days) in DMEM containing 10% newborn calf serum. The medium was changed to serum-free MEM and immunostaining was performed 24 h later. Prior to immunostaining, CFs were washed with PBS and fixed with Streck Tissue Fixative (Omaha, NE, USA) and permeabilized for 5 min using 0.05% Triton® 100. CFs were incubated for 1 h (22°C) with 5% bovine serum albumin to block non-specific binding. Cells were then incubated at 37°C for 1.5 h with primary antibodies for Rac-1, RhoA, or β1 integrin (Ha2/5, BD Transduction laboratory) and 45 min with the appropriate secondary antibody conjugated to Alexa-488 or Alexa 594 (Invitrogen). Samples were covered with mounting media [Prolong® Gold antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI), Invitrogen], overlaid with coverslips and examined on an Olympus Fluoview 1000 microscope.
2.7. Statistics analysis
Results are expressed as the means ± standard error of the means (SEM), computed from separate experiments. Comparisons between control and experimental groups were performed using the Student's group t-test. Multiple comparisons among treatment groups were performed using one-way analysis of variance (ANOVA) and by two-way ANOVA and levels of significance determined using the Tukey–Kramer multiple comparison post-hoc test (GraphPad prism Software, Inc., San Diego, CA, USA). P-values <0.05 were considered to be statistically significant.
3. Results
3.1. Time course of stretch-induced activation of Rac1 and RhoA in CFs
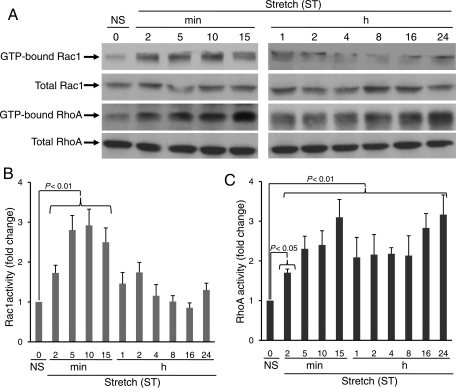
In order to characterize the temporal effects of mechanical stretch on Rac1 and RhoA activation in CFs, we measured Rac1 and RhoA activity from 2 min to 24 h. Rac1 was significantly activated within 2 min after stretch (fold change compared with no stretch; 2 min 1.73 ± 0.190, P < 0.01; 5 min 2.89 ± 0.365, P < 0.01; 10 min 2.92 ± 0.408, P < 0.01; 15 min 2.50 ± 0.359, P < 0.01) and declined after 15 min, which continued until 24 h (Figure 1A and B). Interestingly, RhoA was activated within 2 min after stretch (fold change compared with no stretch; 2 min 1.70 ± 0.092, P < 0.05; 5 min 2.30 ± 0.319, P < 0.01; 10 min 2.40 ± 0.358, P < 0.01; 15 min 3.10 ± 0.449, P < 0.01). In contrast to Rac1, RhoA demonstrated marked activation at later time points (fold change compared with no stretch; 16 h 2.84 ± 0.36, P < 0.01; 24 h 3.16 ± 0.495, P < 0.01) (Figure 1A and C).
Figure 1.
Time course of stretch-induced activation of Rac1 and RhoA in cardiac fibroblasts. CFs were exposed to uniaxial stretch (20%) for different times (2 min–24 h), as indicated. (A) After stretch, cells were harvested and lysates were incubated with respective PBD (Rac1 interactive binding domain of the PAK) and RBD (RhoA interactive binding domain of Rhotekin) beads for 60 min at 4°C, and immunoprecipitates were separated on 12% SDS–PAGE, transferred to PVDF membranes and immunoblotted using Rac1 and RhoA antibodies, as described in the methods. (B and C) Bar graphs show fold changes in Rac1 and RhoA activation after stretch (ST), compared with no stretch (NS). Acute stretch-induced activities (2–15 min) of both Rac1 and RhoA, whereas chronic stretch (4–24 h) increased RhoA activity only. Results are means ± SEM from five independent experiments.
3.2. Time course of stretch-induced activation of SAPKs, p38, and JNK in CFs.
To determine the temporal effects of stretch on SAPKs activation, CFs were stretched from 2 min to 24 h. Results from western blot analysis performed on cell lysates indicate that initially from 5 to 15 min, both p38 and JNK were activated by stretch (Supplementary material online, Figure S1A–C). JNK activity was significantly increased and peaked at 10 min (fold change compared with no stretch; 3.46 ± 0.319, P < 0.01), and gradually diminished to control levels for the remaining portion of the stretch period. p38 activation was biphasic, with initial activation at 2–15 min, followed by a decline and subsequent rise after 2 h, and remained activated until the end of the 24 h study period (fold change compared with no stretch; 1.94 ± 0.085, P < 0.01).
3.3. Rac1 and RhoA differentially regulate Ao gene expression
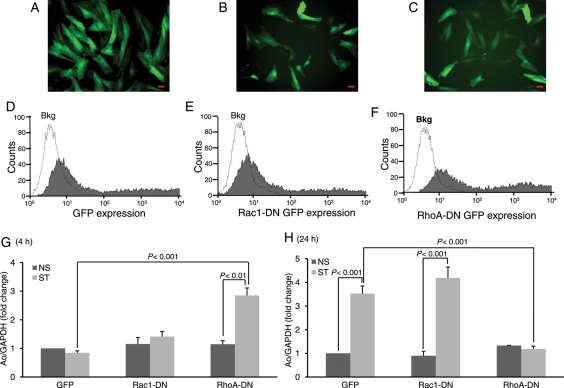
Rho GTPases mediate hypertrophic signals in cardiac myocytes.19,20 However, no studies have determined whether Rho GTPases couple to Ao gene expression in CFs. To test whether Rac1 and/or RhoA activation may be required for stretch-induced regulation of Ao expression, CFs were infected for 24 h with matched titres of recombinant adenoviruses that express either Rac1-DN, RhoA-DN, or GFP (virus control) (Figure 2A–C). Flow cytometry (Figure 2D–F) was used to verify expression of target proteins [mean fluorescence intensity control 3.4 ± 0.59; GFP 1256.3 ± 164.2; Rac1-DN 1536.8 ± 128.59; RhoA-DN 1938.8 ± 178.47] by CFs, prior to mechanical stretch experiments. Intriguingly, during acute stretch (4 h), when Ao gene expression was modestly inhibited, blockade of RhoA function using RhoA-DN resulted in marked expression of Ao in stretched CFs (fold change compared with GFP; 3.39 ± 0.094, P < 0.001). In contrast, expression of Rac1-DN adenovirus had no effect on stretch-induced inhibition of Ao gene expression (Figure 2G). On the other hand, chronic stretch (24 h) significantly upregulated Ao gene expression (fold change compared with NS; 3.39 ± 0.044, P < 0.001) and Rac1-DN expression had no significant effect on stretch-mediated Ao gene expression (Figure 2H). Interestingly, RhoA-DN expression considerably reduced this stretch response. These data indicate that RhoA has an important role in regulating stretch-induced Ao gene expression at both early and later stages of mechanical stretch, whereas Rac1 appears to have only a minimal role in this process (Figure 2H). This is supported by time course results, in which stretch-induced Ao expression corresponded to latter time points (24 h) in which RhoA was markedly increased.
Figure 2.
Rac1 and Rho A differentially regulate Ao gene expression. CFs were transfected with adenoviruses expressing GFP alone (control) or coexpressed with dominant-negative forms of Rac1 (Rac1-DN) or RhoA (RhoA-DN). After 24 h of viral transfection, viral medium was replaced with fresh serum-free medium for stretch experiment. (A–C) Detection of morphological alterations after different viral treatments in CFs. Scale bars =200 µm. (D–F) Results from flow cytometry showing expression of adenovirus-mediated expression of target proteins in CFs. (G and H) After stretch, CFs were harvested and RNA was isolated, expression of Ao was determined. Results are expressed as means ± SEM from five independent experiments. NS, No stretch; ST, Stretch.
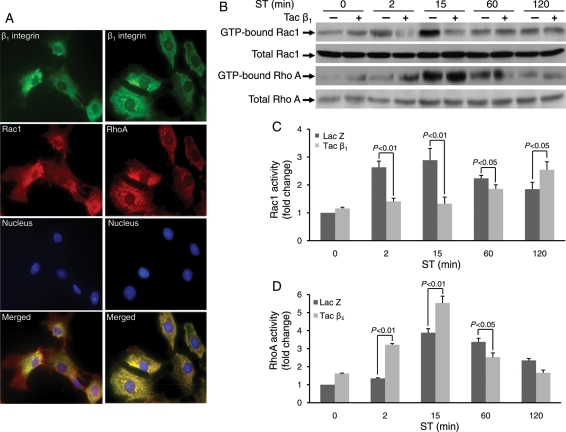
3.4. β1 integrin is an upstream regulator of Rac1 and RhoA
β1 integrin has been shown to function as an important mechanotransducer in cardiac tissue.12,21 Immunostaining of CFs revealed that β1 integrin staining overlaps with that of Rac1 and RhoA (Figure 3A), suggesting that β1 integrin may be an upstream activator of these Rho GTPases. To test the possible role of β1 integrin in mediating Rac1 and RhoA activation, CFs were infected with chimeric β1 integrin [extracellular domain replaced by the extracellular domain of the interleukin-2 receptor (Tac)] that functions as a dominant-negative (gift from Dr Robert Ross, VA San Diego Healthcare System, CA, USA) or LacZ adenovirus (virus control). Rac1 and RhoA activities were measured in virus-transfected cells using GST-pull-down assays.18 Blockade of β1 integrin decreased stretch-induced activation of Rac1 at 2, 15, and 60 min stretch (fold reduction compared with respective LacZ; 2 min, 0.535 ± 0.053, P < 0.01; 15 min, 0.459 ± 0.093, P < 0.01; and 60 min 0.187 ± 0.019, P < 0.05), but increased at later time points (Figure 3B and C). In contrast, blockade of β1 integrin increased stretch-induced RhoA activity until 15 min (fold change compared with LacZ; 2 min, 2.38 ± 0.016, P < 0.01; 15 min, 1.42 ± 0.079, P < 0.01) (Figure 3B and D). At later time points, RhoA activity was decreased.
Figure 3.
β1 integrin is an upstream regulator of Rac1 and RhoA. (A) Immunofluorescent staining for β1 integrin, Rac1 and RhoA. CFs were stained with antibodies against β1 integrin, Rac1, and RhoA. Nuclei were immunostained using DAPI. As a negative control, the cell samples were incubated without primary antibody. Appropriate Alexa-488 (green) or Alexa-594 (red) -conjugated secondary antibodies were used to visualize the specific proteins by fluorescent microscopy (60X oil). Scale bars =10 µm. (B–D) CFs were infected with chimeric integrin subunits [extracellular domains replaced by the extracellular domains of the interleukin-2 receptor (Tac)] that function as dominant-negatives and LacZ as a virus control. Rac1 and RhoA activities were measured using GST-pull-down assays. (B and C) Blockade of β1 integrin decreased stretch-induced activation of Rac1 at 2, 15, and 60 min, but no effect at later time points. (B and D) In contrast, blockade of β1 integrin increased stretch-induced RhoA up to 15 min. Bar graphs show fold changes in Rac1 and RhoA activities after Tac β1 adenovirus expression compared with LacZ control virus. Values are means ± SEM from five independent experiments.
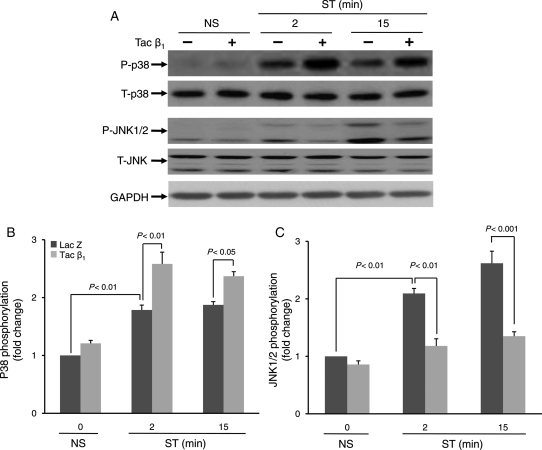
3.5. β1 integrin couples to stretch-induced activation of SAPKs, p38, and JNK
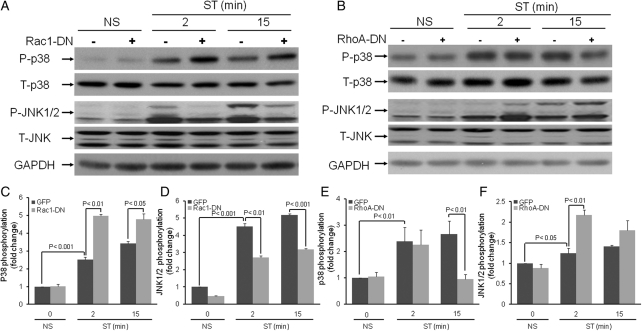
Previous studies suggest that JNK and p38 are activated in the left ventricle during pressure overload-induced cardiac remodelling.22 In cardiac myocytes, the rapid activation of MAP kinases in response to mechanical stimulation has been well documented.23,24 Though less investigated, activation of MAP kinases has also been observed in CFs subjected to short durations of mechanical stretch.8 In the present study, we determined whether β1 integrin may couple to stretch-induced activation of p38 and JNK. Blockade of β1 integrin attenuated the stretch-induced phosphorylation of JNK (fold reduction compared with respective LacZ; 2 min 0.56 ± 0.01, P < 0.01; 15 min 0.51 ± 0.06, P < 0.001) (Figure 4A and C). In contrast, basal as well as stretch-induced p38 phosphorylation was significantly activated in Tac β1-treated cells (fold change compared with LacZ control; 2 min 1.45 ± 0.058, P < 0.01; 15 min 1.26 ± 0.011, P < 0.05) (Figure 4A and B).
Figure 4.
β1 integrin couples to stretch-induced activation of stress-activated protein kinases. Prior to stretching, CFs were infected with adenovirus (24 h) which resulted in expression of Tac β1 or control protein (Lac Z). (A) After 2 and 15 min of stretch (ST) or no stretch (NS), phosphorylation levels of stress-activated protein kinases, p38 and JNK1/2, were determined. (B and C) Bar graphs show fold changes in stress-activated protein kinase (p38 and JNK1/2) phosphorylation after Tac β1 expression compared with control virus. Blockade of β1 integrin significantly increased both basal and stretch-induced p38 activity, however, decreased stretch-induced JNK1/2 activation. Values are means ± SEM from four independent experiments.
3.6. Rac1 and RhoA couple to Ao expression through SAPKs
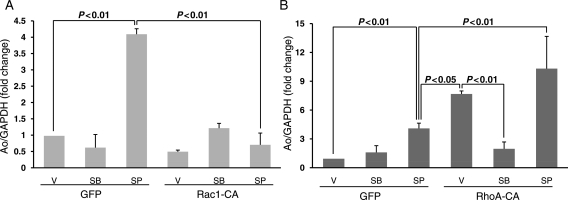
The above results demonstrate that β1 integrin plays a predominant role in at least the initial activation of Rac1, RhoA, and SAPKs. To determine whether Rac1 and RhoA couple to SAPKs cascades, CFs infected with Rac1-DN and RhoA-DN were stretched for 2 and 15 min, and SAPKs activities were measured by western blot analysis. Results revealed that Rac1 blockade increased p38 phosphorylation (fold change compared with respective GFP control 2 min, 1.98 ± 0.02, P < 0.01; 15 min, 1.40 ± 0.08, P < 0.05), but reduced JNK phosphorylation (fold reduction compared with respective GFP control; 2 min 0.60 ± 0.04, P < 0.01; 15 min, 0.61 ± 0.024, P < 0.001) at both time points (Figure 5A–C). In contrast, RhoA blockade reduced stretch-induced p38 phosphorylation by almost three-fold and increased JNK (fold change compared with GFP control; 2 min, 1.75 ± 0.03, P < 0.01) activity (Figure 5D–F). To further explore the importance of JNK and p38 as downstream effectors of Rac1 and RhoA in the regulation of Ao gene expression, CFs were infected with adenovirus expressing constitutively active Rac1 (Rac1-CA) and RhoA (RhoA-CA) in the absence of mechanical stretch (Figure 6). Ao gene expression was suppressed in CFs expressing Rac1-CA (Figure 6A) and significantly increased Ao gene expression in cells expressing RhoA-CA (Figure 6B). Although pharmacological inhibition of JNK1/2 (20 µM SP-60125; Calbiochem) significantly increased Ao gene expression in CFs expressing GFP (Figure 6A) and RhoA-CA (Figure 6B), JNK1/2 blockade failed to prevent inhibition of Ao gene expression in cells expressing Rac1-CA (Figure 6A). These results suggest that Rac1 can also inhibit Ao gene expression via a JNK-independent pathway. Treatment of CFs with p38 pharmacological inhibitor, completely abolished the stimulatory effects on Rho-CA on Ao gene expression (Figure 6B), suggesting that p38 is the primary downstream effector. These results suggest that Rac1 is an upstream activator of JNK, whereas RhoA is an upstream activator of p38.
Figure 5.
Rac1 and RhoA couple to stretch-induced activation of stress-activated protein kinases. Prior to stretching, CFs were infected with adenovirus (24 h) which resulted in expression of Rac1-DN, RhoA-DN, or control protein (GFP). (A and D) After 2 and 15 min of stretch (ST) or no stretch (NS), phosphorylation levels of p38 and JNK1/2 were determined. (B and C) Bar graphs show fold changes in p38 and JNK1/2 phosphorylation after Rac1-DN expression compared to control virus. Expression of Rac1-DN significantly increased both basal and stretch-induced p38 activity, however, stretch-induced JNK1/2 activation was decreased. (E and F) Bar graphs show fold changes in JNK1/2 and p38 phosphorylation after RhoA-DN expression compared with control virus. Blockade of RhoA signalling by RhoA-DN expression significantly inhibited stretch-induced p38 activation at 15 min. However, Rho-DN expression increased stretch-induced JNK1/2 (both 2 and 15 min) activation. Values are means ± SEM from five independent experiments.
Figure 6.
Role of JNK and p38 on Rac1 and RhoA-mediated regulation of angiotensinogen gene expression. CFs were treated for 24 h with adenoviruses expressing constitutively active Rac1 (Rac1-CA) (A) and RhoA (RhoA-CA) (B). Cells were then treated for 1 h with p38 inhibitor (10 µM SB-203580) and JNK1/2 inhibitor (20 µM SP-60125) and cells were harvested for RNA. Expression of Ao mRNA was determined by real-time RT-PCR as described in the Methods. Both RhoA overexpression and JNK1/2 inhibition markedly increased the basal Ao gene expression. RhoA-CA-mediated basal activation of Ao gene expression was significantly reduced by p38 blockade. However, JNK1/2 inhibition-mediated basal activation of Ao is completely abrogated by overexpression of Rac1. Results are means ± SEM from four independent experiments. SB, SB-203580; SP, SP-60125; V, vehicle (0.05% DMSO).
4. Discussion
Rho GTPases participate in cardiac remodelling and fibrosis in the mechanically overloaded myocardium. In the present study, we explored the regulatory effects of Rac1 and RhoA on Ao gene expression in mechanically stretched CFs. Results of this work demonstrated that stretch-induced regulation of Ao gene expression in CFs is primarily mediated by RhoA. Our results also indicate that with chronic stretch, p38 (stress-activated protein kinase) is a downstream target for RhoA and during this time, RhoA activation is independent of β1 integrin. The role of integrins in the activation and translocation of SAPK to the nucleus is well established in other cells.25 Moreover, we have reported the involvement of SAPKs (p38 and JNK) in regulation of Ao gene expression in both cardiac myocytes and fibroblasts.14 However, the role of upstream activators of SAPK as well as downstream effectors of β1integrin is not clearly known in CFs. A recent study has demonstrated that inhibition of Rho signalling by Clostridium difficle toxin B (Rac1 and RhoA inhibitor) or C3 exoenzyme (RhoA inhibitor) inhibited stretch-induced activation of both p38 and JNK in cardiac myocytes.26 In the present study, we demonstrated p38 and JNK in CFs to be regulated by RhoA and Rac1, respectively. As expected, stretch-induced early activation of JNK was significantly inhibited by expression of Rac1-DN, indicating that Rac1 is an upstream activator of JNK-signalling. This is consistent with previous work showing that endothelin-1 (hypertrophic stimulus)-induced activation of JNK was attenuated by toxin B.27 Interestingly, stretch-induced p38 activity was enhanced in CFs expressing dominant-negative Rac1, indicating that the Rac1/JNK pathway is a negative regulator of p38.
In contrast to Rac1, previous studies suggest that RhoA is a mediator of hypertrophic responses in the myocardium.28,29 In a recent study, inhibition of the RhoA effector ROCK, using the ROCK inhibitor GSK 576371, was found to prevent left ventricular hypertrophy and reduce collagen deposition, which were accompanied by improved diastolic function in pressure overload-induced cardiac hypertrophy in the rat.17 In the diabetic mouse heart, which has reduced ventricular performance, RhoA has been shown to activate p38, which is also activated in the failing myocardium.30,31 It has been demonstrated that p38 inhibition improves cardiac function and attenuates cardiac remodelling following myocardial infarction.32 Recent studies also suggest that p38 and JNK may engage in negative cross-talk during heart failure.22 Our results also suggest that regulatory interactions between p38 and JNK are time-dependent. Stretch initially activated both p38 and JNK (2–15 min), however, with prolonged stretch there was a loss in JNK activity (30 min–24 h). In contrast, p38 remained active until 24 h. It is possible that prolonged activation of p38 results in inactivation of JNK via early immediate gene expression33 and/or release of autocrine factors.34
Stretch-induced differential regulation of Rho GTPases and hence JNK and p38, may be due to differential regulation of RhoA and Rac1 by one or more upstream regulators. Earlier studies have demonstrated that chronic stretch induces β1 integrin expression in cardiac myocytes.35 The present study indicates that 2–15 min following mechanical stretch, β1 integrin was an important regulator of Rac1 and RhoA activity, whereas β1 integrin-independent signalling mechanisms were important afterwards. Mechanosensing and autocrine regulators of Rac1 and RhoA following prolonged (>30 min) stretch remain to be explored in CFs and other cell systems.
In contrast to our previous study performed in pure cultures of cardiac myocytes,12 blockade of β1 integrin inhibited JNK activation and stimulated p38. The possible cell type differences in these responses may be due to differences in coupling of β1 integrin to Rho GTPases and/or coupling between Rho GTPases to SAPK. It has been reported that 4% stretch caused rapid activation of JNK, and this activation is mediated by integrin receptors in CFs.8 Moreover, extracellular matrix components exert a significant role on the stretch-induced activation of JNK in this model, although p38 activity was unaffected. In contrast to the above studies, expression of Tac β1 inhibited the stretch-induced JNK activation in our model, however, p38 activity was further improved under this treatment. The differential response of β1 integrin in our stretch model may be due to differences in the extracellular matrix and intensity of stretch (20% as in our model). This result further confirms our hypothesis that β1 integrin, either by activating Rac1 or inhibiting RhoA, can modulate both JNK and p38 activities.
The activities of JNK and p38 are important in the regulation of Ao in the myocardium. Because Rac1 and RhoA differentially regulate JNK with regard to p38, we sought to understand the roles of Rac1 and RhoA on stretch-induced activation of Ao by expressing Rac1-DN and RhoA-DN in CFs. Intriguingly, a remarkable inhibition of stretch-induced activation of Ao gene expression was observed in RhoA-DN-treated CFs. However, CFs infected with Rac1-DN further sustained the stretch-induced Ao gene expression. This result also suggests that the initial inhibition of Ao by stretch (2–4 h) may be due to activation of Rac1, while Rac1 is concomitantly activating JNK. Although JNK appears to be an important downstream target of Rac1 and important negative regulator of Ao expression, the inability of JNK1/2 inhibitor to block downregulation of Ao expression in CFs expressing constitutively active Rac1 suggests that a JNK-independent inhibitory pathway is also operational. This is consistent with a recent study in which Rac1 was shown to directly phosphorylate the transcriptional repressor B-cell lymphoma 6 (BCL-6) proto-oncogene, independent of Rac1-induced Jun N-terminal kinase activation.36 Interestingly, one of the downstream targets of BCL-6 is p50 nuclear factor-kappaB1,36 which stimulates Ao gene transcription in the liver.37 In contrast to Rac1, the stimulatory effects of RhoA on Ao gene expression in CFs appeared to be solely dependent on p38 activation. Pursuant to our current knowledge, this is the first study to suggest that Rac1 and RhoA regulate Ao gene expression in CFs.
The role of integrins on myocardial functions and progression of heart disease is still an issue of debate. Recently, several investigators have suggested that β1 integrin can promote mechanical stretch-induced cardiac hypertrophy and remodelling,38,39 however, other in vivo and in vitro studies suggest that β1 integrin inhibits these responses.40,41 In a recent study, moderate spontaneous cardiac hypertrophy associated with systolic and diastolic dysfunction was reported in β3 integrin knockout mice, and these defects were worsened by thoracic aortic constriction.42 Defects in β1 integrin resulting in myocardial dysfunction after myocardial infarct also suggest that it is protective. In the present study, the inhibitory effects of β1 integrin on the RhoA-p38 signalling cascade and stimulatory effects of β1 integrin on Rac1-JNK signalling also suggest a protective role of β1 integrin during chronic and acute stretch conditions, respectively in CFs. Although results from the present study provide evidence for the role of Rho GTPases in mechanical stretch-induced Ao gene expression, it will be important to identify the downstream effector systems responsible for mediating the regulatory effects of Rac1 and RhoA on Ao transcription and/or mRNA stability in CFs.
In conclusion, considerable progress has been made regarding the role of Rac1 and RhoA in cardiac myocyte signalling and hypertrophy in the past decade. The implication of these findings is that activation of RhoA/p38 signalling pathways mediate stretch-induced activation of Ao gene expression in CFs and could, therefore, contribute to the hypertrophic response observed in the haemodynamically overloaded myocardium. Thus, our findings provide new insights into how mechanotransduction may alter Ao levels, which can maintain normal cardiac physiology or initiate pathological responses.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (5R01-HL068838–6); and Scott and White Hospital.
References
- 1.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85:643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- 2.Thienelt CD, Weinberg EO, Bartunek J, Lorell BH. Load-induced growth responses in isolated adult rat hearts. Role of the AT1 receptor. Circulation. 1997;95:2677–2683. doi: 10.1161/01.cir.95.12.2677. [DOI] [PubMed] [Google Scholar]
- 3.Rockman HA, Wachhorst SP, Mao L, Ross J., Jr ANG II receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am J Physiol. 1994;266:H2468–H2475. doi: 10.1152/ajpheart.1994.266.6.H2468. [DOI] [PubMed] [Google Scholar]
- 4.Baker KM, Booz GW, Dostal DE. Cardiac actions of angiotensin II: role of an intracardiac renin-angiotensin system. Annu Rev Physiol. 1992;54:227–241. doi: 10.1146/annurev.ph.54.030192.001303. doi:10.1146/annurev.ph.54.030192.001303. [DOI] [PubMed] [Google Scholar]
- 5.Shivakumar K, Dostal DE, Boheler K, Baker KM, Lakatta EG. Differential response of cardiac fibroblasts from young adult and senescent rats to ANG II. Am J Physiol Heart Circ Physiol. 2003;284:H1454–H1459. doi: 10.1152/ajpheart.00766.2002. [DOI] [PubMed] [Google Scholar]
- 6.Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, et al. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–310. doi: 10.1172/JCI1026. doi:10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Pozo MA, Schwartz MA. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. doi: 10.1016/j.tcb.2007.03.001. doi:10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Laforest S, Milanini J, Parat F, Thimonier J, Lehmann M. Evidences that beta1 integrin and Rac1 are involved in the overriding effect of laminin on myelin-associated glycoprotein inhibitory activity on neuronal cells. Mol Cell Neurosci. 2005;30:418–428. doi: 10.1016/j.mcn.2005.08.006. doi:10.1016/j.mcn.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. doi:10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 12.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, et al. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol. 2007;43:137–147. doi: 10.1016/j.yjmcc.2007.05.012. doi:10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kacimi R, Gerdes AM. Alterations in G protein and MAP kinase signaling pathways during cardiac remodeling in hypertension and heart failure. Hypertension. 2003;41:968–977. doi: 10.1161/01.HYP.0000062465.60601.CC. doi:10.1161/01.HYP.0000062465.60601.CC. [DOI] [PubMed] [Google Scholar]
- 14.Lal H, Verma SK, Golden HB, Foster DM, Smith M, Dostal DE. Stretch-induced regulation of angiotensinogen gene expression in cardiac myocytes and fibroblasts: opposing roles of JNK1/2 and p38alpha MAP kinases. J Mol Cell Cardiol. 2008;45:770–778. doi: 10.1016/j.yjmcc.2008.09.121. doi:10.1016/j.yjmcc.2008.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. doi:10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, et al. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol. 2003;35:59–70. doi: 10.1016/s0022-2828(02)00278-x. doi:10.1016/S0022-2828(02)00278-X. [DOI] [PubMed] [Google Scholar]
- 17.Phrommintikul A, Tran L, Kompa A, Wang B, Adrahtas A, Cantwell D, et al. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H1804–H1814. doi: 10.1152/ajpheart.01078.2007. doi:10.1152/ajpheart.01078.2007. [DOI] [PubMed] [Google Scholar]
- 18.Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, et al. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. doi:10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clerk A, Sugden PH. Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ Res. 2000;86:1019–1023. doi: 10.1161/01.res.86.10.1019. [DOI] [PubMed] [Google Scholar]
- 20.Aoki H, Izumo S, Sadoshima J. Angiotensin II activates RhoA in cardiac myocytes: a critical role of RhoA in angiotensin II-induced premyofibril formation. Circ Res. 1998;82:666–676. doi: 10.1161/01.res.82.6.666. [DOI] [PubMed] [Google Scholar]
- 21.Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381–390. doi: 10.1016/j.cardiores.2004.04.027. doi:10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Kyoi S, Otani H, Matsuhisa S, Akita Y, Tatsumi K, Enoki C, et al. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc Res. 2006;69:888–898. doi: 10.1016/j.cardiores.2005.11.015. doi:10.1016/j.cardiores.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. Embo J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, et al. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest. 1995;96:438–446. doi: 10.1172/JCI118054. doi:10.1172/JCI118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:21691–21694. doi: 10.1074/jbc.271.36.21691. doi:10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, et al. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol. 2005;202:536–553. doi: 10.1002/jcp.20151. doi:10.1002/jcp.20151. [DOI] [PubMed] [Google Scholar]
- 27.Clerk A, Pham FH, Fuller SJ, Sahai E, Aktories K, Marais R, et al. Regulation of mitogen-activated protein kinases in cardiac myocytes through the small G protein Rac1. Mol Cell Biol. 2001;21:1173–1184. doi: 10.1128/MCB.21.4.1173-1184.2001. doi:10.1128/MCB.21.4.1173-1184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. doi:10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 29.Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. doi: 10.1016/j.tips.2005.12.002. doi:10.1016/j.tips.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, et al. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–R802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 31.Roussel E, Gaudreau M, Plante E, Drolet MC, Breault C, Couet J, et al. Early responses of the left ventricle to pressure overload in Wistar rats. Life Sci. 2008;82:265–272. doi: 10.1016/j.lfs.2007.11.008. doi:10.1016/j.lfs.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 32.See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. doi:10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Cullingford TE, Markou T, Fuller SJ, Giraldo A, Pikkarainen S, Zoumpoulidou G, et al. Temporal regulation of expression of immediate early and second phase transcripts by endothelin-1 in cardiomyocytes. Genome Biol. 2008;9:R32. doi: 10.1186/gb-2008-9-2-r32. doi:10.1186/gb-2008-9-2-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuzawa J, Booz GW, Hunt RA, Shimizu N, Karoor V, Baker KM, et al. Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3 : an autocrine loop for hypertrophy. Hypertension. 2000;35:1191–1196. doi: 10.1161/01.hyp.35.6.1191. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SJ, Truskey GA, Kraus WE. Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. Am J Physiol Cell Physiol. 2007;292:C2057–C2069. doi: 10.1152/ajpcell.00493.2006. doi:10.1152/ajpcell.00493.2006. [DOI] [PubMed] [Google Scholar]
- 36.Barros P, Jordan P, Matos P. Rac1 signaling modulates BCL-6-mediated repression of gene transcription. Mol Cell Biol. 2009;29:4156–4166. doi: 10.1128/MCB.01813-08. doi:10.1128/MCB.01813-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 38.Umar S, van der Valk EJ, Schalij MJ, van der Wall EE, Atsma DE, van der Laarse A. Integrin stimulation-induced hypertrophy in neonatal rat cardiomyocytes is NO-dependent. Mol Cell Biochem. 2009;320:75–84. doi: 10.1007/s11010-008-9900-8. doi:10.1007/s11010-008-9900-8. [DOI] [PubMed] [Google Scholar]
- 39.Willey CD, Palanisamy AP, Johnston RK, Mani SK, Shiraishi H, Tuxworth WJ, et al. STAT3 activation in pressure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci. 2008;4:184–199. doi: 10.7150/ijbs.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurthy P, Subramanian V, Singh M, Singh K. Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension. 2007;49:865–872. doi: 10.1161/01.HYP.0000258703.36986.13. doi:10.1161/01.HYP.0000258703.36986.13. [DOI] [PubMed] [Google Scholar]
- 41.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. doi:10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren J, Avery J, Zhao H, Schneider JG, Ross FP, Muslin AJ. Beta3 integrin deficiency promotes cardiac hypertrophy and inflammation. J Mol Cell Cardiol. 2007;42:367–377. doi: 10.1016/j.yjmcc.2006.11.002. doi:10.1016/j.yjmcc.2006.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.