Abstract
Background. Patients with newly acquired genital herpes simplex virus 2 (HSV-2) infection have virus frequently detected at the genital mucosa. Rates of genital shedding initially decrease over time after infection, but data on long-term viral shedding are lacking.
Methods. For this study, 377 healthy adults with history of symptomatic genital HSV-2 infection collected anogenital swabs for HSV-2 DNA polymerase chain reaction for at least 30 consecutive days.
Results. Time since first genital herpes episode was significantly associated with reduced genital shedding. Total HSV shedding occurred on 33.6% of days in participants <1 year, 20.6% in those 1–9 years, and 16.7% in those ≥10 years from first episode. Subclinical HSV shedding occurred on 26.2% of days among participants <1 year, 13.1% in those 1–9 years, and 9.3% in those ≥10 years from first episode. On days with HSV detection, mean quantity was 4.9 log10 copies/mL for those <1 year, 4.7 log10 copies/mL among those 1–9 years, and 4.6 log10 copies/mL among those ≥10 years since first episode.
Conclusions. Rates of total and subclinical HSV-2 shedding decrease after the first year following the initial clinical episode. However, viral shedding persists at high rates and copy numbers years after infection, and therefore may pose continued risk of HSV-2 transmission to sexual partners.
Herpes simplex virus 2 (HSV-2) is the major cause of genital herpes and one of the most frequent sexually transmitted diseases (STDs) worldwide. HSV-2 establishes life-long infection in humans and is characterized by periods of viral latency and reactivation, manifesting as recurrent genital lesions and viral detection at mucosal sites (also known as “shedding”). Studies of HSV-2 shedding in early genital herpes have demonstrated high HSV-2 shedding rates in the first year of infection, with HSV detected on 9%–40% of all days [1–5]. Nearly half of these days represent subclinical shedding, occurring on the days without genital lesions [6]. Clinical recurrences are also common, with a median rate of 4 recurrences in the first year of infection [7].
The patterns of HSV-2 mucosal shedding after the initial years of infection are less clear. Clinical recurrences among patients followed for several years decreased over time in one study. Most participants had fewer clinical episodes 5 years after their first genital HSV-2 episode; however, nearly 25% of the participants experienced an increase in recurrence rates [7]. HSV-2 shedding may also decrease over time, as 2 studies demonstrated that subclinical shedding rates declined by approximately half after the first year of infection [6, 8]. Despite these observations, detailed data on genital HSV-2 shedding many years after herpes acquisition are limited. Because the long-term natural history of genital herpes affects the risk of transmission, and consequently has psychosocial, clinical management, and public health implications, we sought to describe patterns of genital HSV-2 shedding and recurrences in years remote from the first genital herpes episode.
METHODS
Study population
We evaluated a cohort of healthy adults with a history of symptomatic genital HSV-2 infection enrolled in prospective studies of the natural history of genital herpes at the University of Washington Virology Research Clinic (Seattle, Washington) or the Westover Heights Clinic (Portland, Oregon) from 1992 to 2008. Participants were included in our analysis if they had a known date of their first genital herpes episode, were HSV-2 seropositive, and had at least 30 consecutive days of anogenital swab samples analyzed for HSV by polymerase chain reaction (PCR). No participants were known to be HIV-infected; participants were offered HIV testing if they reported high-risk sexual behavior or requested testing. All participants provided written informed consent, and institutional review boards approved all study protocols.
Procedures
Demographic and clinical data were collected on standardized forms. An experienced research clinician reviewed the clinical signs and symptoms of genital herpes with participants and taught them to keep a diary of genital lesions and symptoms. Antiviral therapy was not used during the sampling session and at least 7 days prior to study entry. Participants were also taught to obtain genital swab specimens for HSV detection as described elsewhere [6, 9]. Depending on the time when they participated in the studies, women were instructed to collect separate genital swabs (from the cervix, vulva, and perianal region) or a mixed swab of the entire anogenital area [10]. Men collected separate genital swabs (from penile skin and perianal area) or a mixed swab of the entire anogenital region [10]. If a lesion was present, participants collected a separate lesion swab. Swabs were placed in PCR transport media and refrigerated until processed for PCR. The reliability of patient sampling methods for detecting HSV DNA from mucosal and skin surfaces has been previously established and has been determined to be comparable or superior to clinician sampling [5, 6].
Laboratory methods
Serum samples were tested for HSV-1 and HSV-2 antibodies using Western blot developed at the University of Washington [11]. Swab samples were evaluated for HSV DNA by quantitative, real-time, fluorescence-based PCR as described elsewhere [12, 13]. Samples with ≥150 copies per mL of HSV DNA were considered positive [14].
Definitions
Time from the first genital herpes episode was defined as the interval between a participant's first reported genital herpes episode and the study sampling session. For participants only recalling the month and year of their first herpes episode, the day was estimated to be the 15th of the month; for those only recalling the year of their first episode, the day and month was estimated to be July 1 to represent the mid-point of the year. A sampling session was defined as the period during which the participant collected genital swabs for at least 30 consecutive days. Clinical recurrences were defined as the presence of any genital lesion consistent with HSV infection noted by the study participant or study clinician. The total HSV genital shedding rate was defined as the number of days on which HSV was detected by PCR from at least 1 genital site, divided by the total number of days with PCR results. Subclinical shedding rates were calculated as for the total HSV genital shedding rate, excluding all days on which genital lesions were observed. Episodes of shedding were defined by consecutive days with positive PCR results and at least 1 day with negative HSV PCR before and after the episode; clinical episodes were defined by consecutive days with genital lesions. Quantity of shedding was measured by HSV copy number as defined by the log10 copies of HSV DNA per mL of PCR buffer. On days with detectable HSV by PCR, the highest amount of HSV DNA from any genital site for that day was used for copy number analysis.
Statistical analysis
Outcomes included total HSV shedding rate, subclinical shedding rate, genital lesion rate, HSV copy number, and episode frequency and duration from time since first genital herpes episode. We examined race, age, gender, sexual preference, age at sexual debut, age at first genital herpes episode, and HSV antibody status as potential covariates. Men were classified as men who have sex with men (MSM) if they reported any sex with men, whereas women were categorized as women who have sex with women (WSW) if they reported exclusively sex with women.
Risk factors for total shedding, subclinical shedding, lesion rates, and episode frequency were evaluated using generalized estimating equations (GEE) with Poisson distribution and log link to account for within-person correlation and for those participants contributing more than one session to the dataset. GEE models with Gaussian distribution were used to compare log copy numbers and episode duration. Univariate predictors with two-sided P values ≤.1 were included in the multivariate models and backward elimination was applied to obtain a final model; two-sided P values ≤.05 were considered significant. Parameterization of time since first genital episode was explored and best fit groupings for shedding analyses were determined to be <1 yr, 1–9 yrs, and ≥10 years. Race was categorized based on best fit groupings as “White” or “Nonwhite”, which included self-identification as “Black”, “Asian”, “Hawaiian/Pacific Islander”, “Other”, or “Mixed” race or ethnicity. All statistical calculations were performed using Stata 9.1 (StataCorp, College Station, TX).
RESULTS
Characteristics of study participants
A total of 377 participants were enrolled in 439 study sessions. The median age was 39 years (range, 20–76 years) and most participants were white (89%). The “nonwhite” group included 29 participants identifying as “Black”, 3 as “Asian”, and 12 as “Mixed” or “Other”. Women comprised 62% of the study population; 45 participants (12%) identified as MSM or WSW (Table 1). Two-hundred-eighteen (58%) were HSV-2 seropositive and HSV-1 seronegative, while 159 (42%) were HSV-1 and HSV-2 seropositive. The median age at sexual debut was 17 years (range, 5–24 years), and the median age at first genital herpes episode was 27 years (range, 8–66 years). The median time since first genital herpes episode and the initiation of the study to evaluate HSV shedding was 7.4 years (range, 0.3–37.8 years).
Table 1.
Demographic and Clinical Characteristics of Study Cohort (n = 377 participants; 439 sessions)
| Time since first genital herpes episode |
||||
| <1 yr | 1-9 yrs | ≥10 yrs | ||
| Characteristic | Entire cohort | (n=78) | (n=172) | (n=189) |
| Age, years, median(range) a | 39 (20–76) | 29 (20–62) | 31 (20–76) | 47 (2068) |
| Gender (N, %) | ||||
| Male | 145 (38%) | 20 (26%) | 50 (29%) | 99 (52%) |
| Female | 232 (62%) | 58 (74%) | 122 (71%) | 90 (48%) |
| Race | ||||
| White | 336 (89%) | 71 (91%) | 154 (90%) | 169 (89%) |
| Nonwhite | 40 (11%) | 7 (9%) | 17 (10%) | 20 (11%) |
| Age at first sex, yrs, median (range) | 17 (5–24) | 17 (9–23) | 17 (5–23) | 17 (5–23) |
| Sexual preference | ||||
| Heterosexual | 236 (63%) | 70 (90%) | 123 (71%) | 85 (45%) |
| Homosexual (MSM, WSW) | 45 (12%) | 6 (8%) | 25 (15%) | 25 (13%) |
| Missing | 96 (25%) | 2 (2%) | 24 (14%) | 79 (42%) |
| HSV antibody status | ||||
| HSV-2 only | 218 (58%) | 57 (73%) | 101 (59%) | 96 (51%) |
| HSV-1 & HSV-2 | 159 (42%) | 21 (27%) | 71 (41%) | 93 (49%) |
| Age at first genital herpes episode, yrs, median(range) | 27 (8–66) | 28 (19–62) | 27 (18–66) | 26 (8–52) |
| Sessions observed per participant | ||||
| 1 session | 377 (100%) | 77 (99%) | 139 (81%) | 161 (85%) |
| 2 sessions | 54 (14%) | 1 (1%) | 32 (18%) | 21 (11%) |
| 3 or more sessions | 8 (2%) | – | 1 (1%) | 7 (4%) |
| Episode frequency, mean no., annualized | ||||
| Total genital shedding | 14.4 | 15.3 | 13.5 | 14.9 |
| Subclinical shedding | 11.6 | 12.6 | 10.6 | 12.3 |
| Genital lesion | 8.7 | 7.6 | 8.2 | 9.6 |
| Episode length, mean no. of days | ||||
| Total genital shedding | 4.6 | 5.4 | 4.5 | 4.3 |
| Subclinical shedding | 2.6 | 2.5 | 2.8 | 2.6 |
| Genital lesion | 7.4 | 10.4 | 7.2 | 6.5 |
NOTE. Data are no. (%) of participants, unless otherwise indicated.
Refers to age at time of study session; Summarizes age for all study sessions (n = 439), including age at each session for participants contributing multiple sessions.
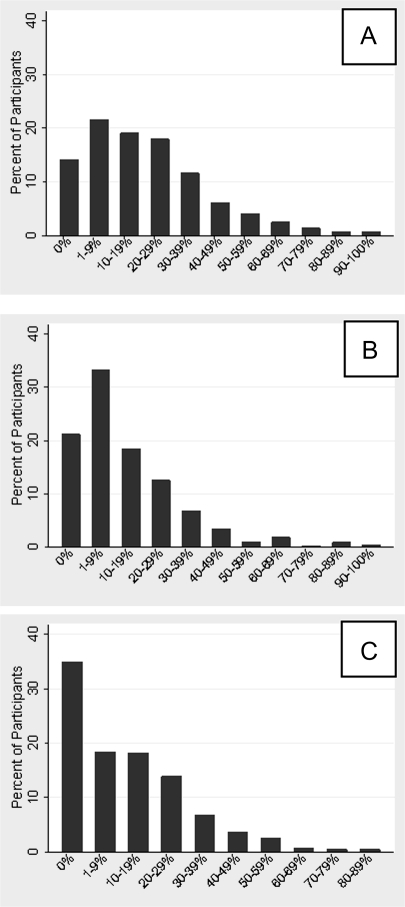
The study participants collected genital swabs for a median of 64 days per session (range, 30–180 days). Fifty-four participants (14%) contributed 2 study sessions; 8 participants (2%) contributed 3 or more sessions. A total of 25,752 days with genital swabs were included in the analysis. HSV was detected at least once among 324 (86%) participants, 302 (81%) had at least 1 day of viral shedding on a day without lesions, and 253 (68%) had at least 1 day with a genital lesion (Figure 1). To determine whether daily genital swabbing increased the risk of viral shedding, we compared the rate of shedding on the first day of the sampling session to subsequent days. As shown in Figure S1 (which is available in the supplementary material online), no difference was detected between the rate of shedding on the first day versus subsequent days of the study.
Figure 1.
Distribution of percentage of days with (A) HSV shedding, (B) Subclinical HSV shedding, and (C) Genital lesions.
Total HSV genital shedding
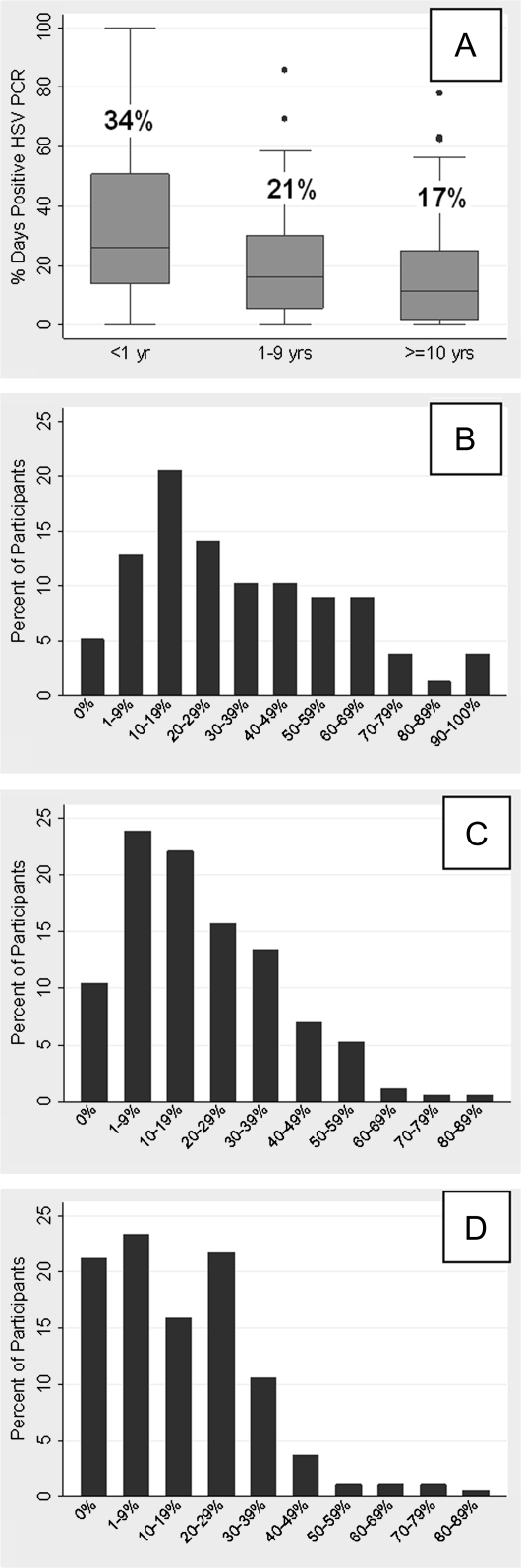
The overall percentage of days with genital HSV shedding was 21.2% (5447 of 25,752 days). The time since first clinical episode of genital HSV-2 was significantly associated with reduced genital shedding. HSV shedding occurred on 33.6% of days among participants <1 year from their first herpes episode, 20.6% of days among those 1–9 years (incidence rate ratio [IRR], .71 [95% confidence interval {CI}, .59–.85]; P < .001), and 16.7% of days among those ≥10 years from first herpes episode (IRR, .52 [95% CI, .42–.64]; P < .001). These differences in shedding rate remained significant in multivariate analysis among those 1–9 years (IRR, .72 [95% CI, .60–.86]; P < .001) and those ≥10 years (IRR, .57 [95% CI, .45–.72]; P < .001), after adjusting for age and race (Table 2 and Figure 2).
Table 2.
Risk Factors for Herpes Simplex Virus (HSV) Genital Shedding or Clinical Recurrence
| Total HSV shedding |
Subclinical HSV shedding |
Genital lesions |
||||
| Risk factor | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
| Time since first genital herpes episodea | ||||||
| 1-9 years vs <1 year | .71 (.59–.85);P <.001 | .72 (.60–.86); P<.001 | .59 (.47–.74); P<.001 | .59 (.47–.75); P<.001 | .87 (.66–1.15); P=.33 | .90 (.69–1.17); P=.44 |
| 10 or more years vs <1 year | .52 (.42–.64); P<.001 | .57 (.45–.72):P<.001 | .38 (.29–.50); P<.001 | .41 (.30–.56); P<.001 | .74 (.55–.99); P=.05 | .87 (.62–1.21); P=.41 |
| Racea: Nonwhite vs white | .52 (.35–.77); P=.001 | .54 (.37–.78); P=.001 | .64 (.41–1.01);P=.05 | .67 (.44–1.01);P=.05 | .45 (.27–.78);P=.004 | .46 (.27–.79);P=.005 |
| Age,a year | .98 (.97–.99); P<.001 | .99 (.98–1.00); P=.20 | .98 (.97–.99);P<.001 | .99 (.98–1.00);P=.36 | .98 (.97–.99);P=.01 | .99 (.98–1.00);P=.06 |
| Gender: Female vs Male | 1.1 (.89–1.29); P=.47 | – | 1.15 (.90–1.47); P=.27 | – | 1.04 (.89–1.29); P=.75 | – |
| Homosexual (MSM, WSW) vs heterosexual | .81 (.60–1.1); P=.17 | – | .75 (.51–1.10) P=.15 | – | .82 (.55–1.21); P=.32 | – |
| Age at first sex:>=18 vs <18 | 1.0 (.85–1.29);P=.68 | – | 1.16 (.89–1.53):P=.25 | – | .92 (.70–1.21); P=.55 | – |
| HSV antibody status: HSV-1 and HSV-2 vs HSV-2 only | .98 (.81–1.18);P=.82 | – | .92 (.72–1.17):P=.50 | – | .93 (.72–1.18); P=.54 | – |
NOTE. Data are incidence rate ratio (95% confidence interval); P-value unless otherwise indicated.
Significant variables used in multivariate analysis bolded.
Figure 2.
Percentage of total days with positive HSV PCR sample by time from first genital herpes episode. A, Entire cohort (overall percentage of days indicated above box plot for each group); B, Participants <1 yr since first episode; C, Participants 1–9 year since first episode; D, Participants ≥10 years since first episode.
Nonwhite race was also significantly associated with less shedding compared with white race (IRR, .52 [95% CI, .35–.77]; P = .001) in univariate analysis and after adjusting for time since first episode and age (IRR, .54 [95% CI, .37–.78]; P = .001) (Table 2). Subjects of nonwhite race had higher shedding rates than whites during first year of infection, but lower rates in subsequent years (P = .02); however, this observation was based on having only 7 nonwhite persons in the <1 year group, and therefore only main effects were included in the final model. HSV-1 serostatus was not a predictor of total shedding in univariate or multivariate analysis, nor did we find an interaction between HSV-1 infection and race. No other variables, including gender, age at acquisition, or sexual preference, were found to be significant predictors of shedding in multivariate analysis.
Subclinical HSV genital shedding
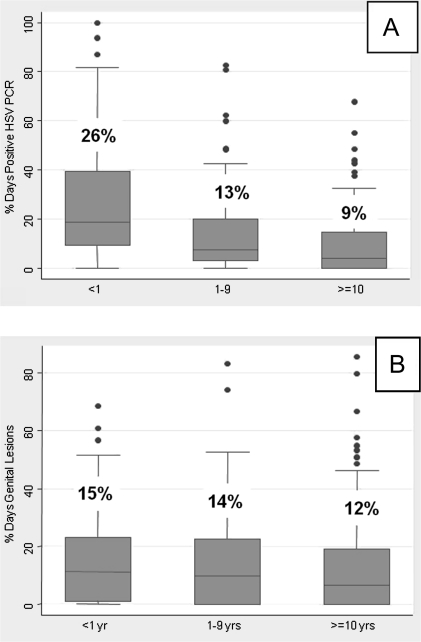
The overall percentage of days with subclinical HSV shedding was 13.7% (3014 of 22,037 days). The time since the first genital herpes episode was significantly associated with reduced subclinical shedding. Subclinical HSV shedding occurred on 26.2% of days among participants <1 year from first herpes episode, 13.1% of days among those 1–9 years (IRR, .59 [95% CI, .47–.74]; P < .001), and 9.3% of days among those ≥10 years from first herpes episode (IRR, .38 [95% CI, .29–.50]; P < .001). These differences remained significant among those 1–9 years (IRR, .59 [95% CI, .47–.75]; P < .001) and those ≥10 years from first episode (IRR, .41 [95% CI, .30–.56]; P < .001) after adjusting for age and race (Table 2 and Figure 3).
Figure 3.
Percentage of days with positive HSV PCR sample by time from first genital herpes episode. A, Subclinical positive days; B, Genital lesion days; Overall percentage of days indicated above box plot for each group.
Nonwhite race was also associated with less subclinical shedding compared with white race near statistical significance (IRR, .64 [95% CI, .41–1.01]; P = .057) in univariate analysis and after adjusting for time since first episode and age (IRR, .67 [95% CI, .44–1.01]; P = .057) (Table 2). No other variables, including HSV-1 serostatus, were found to be significant predictors of shedding in multivariate analysis.
Genital lesions
The overall percentage of days with genital lesions was 13.6% (3810 of 28,087 days). Lesions occurred on 15.5% of days among participants <1 year from their first genital herpes episode, 14.0% of days among those 1–9 years, and 12.3% of days among those ≥10 years from first herpes episode. The percentage of days with genital lesions was reduced in persons who were 10 or more years since initial genital HSV-2 episode compared with those within 1 year of first episode (IRR, .74 [95% CI, .55–.99]; P = .05) in univariate analysis, but the reduction was attenuated after adjusting for age and race in multivariate analysis (Table 2 and Figure 3).
Nonwhite race was associated with fewer genital lesions compared with white race (IRR, .45 [95% CI, .27–.78]; P = .005) in univariate analysis and after adjusting for time since first episode and age (IRR, .46 [95% CI, .27–.79]; P = .005) (Table 2). No other variables, including HSV-1 serostatus, were found to be significant predictors of genital lesions in multivariate analysis.
HSV DNA copy number
The quantity of HSV DNA detected by PCR was slightly but significantly lower among those more remote from their first genital HSV-2 episode compared with those within the first year of their initial episode. Of the 5477 days on which HSV-2 was detected on any genital swab, the mean quantity was 4.9 log10 copies/mL (range, 3.0–7.6 log10 copies/mL) for those within 1 year of first episode of genital HSV-2, 4.7 log10 copies/mL (range, 2.3–7.2 log10 copies/mL) among those 1–9 years, and 4.6 log10 copies/mL (range, 2.3–7.0 log10 copies/mL) for those ≥10 years since first episode. The quantity differed significantly between those 10 or more years compared with <1 year since first herpes episode (coef −.31 [95% CI, −.60 to −.02]; P = .04), but was not significant for those 1–9 years compared with <1 year (coef −.17 [95% CI, −.46 to .12]; P = .26).
Of the 3014 days on which HSV was detected on days without lesions, the mean quantity was 4.5 log10 copies/mL (range, 2.5–7.2 log10 copies/mL) for those <1 year of first episode, 4.2 log10 copies/mL (range, 2.3–6.6 log10 copies/mL) among those 1–9 years, and 4.1 log10 copies/mL (range, 2.2–7.0 log10 copies/mL) for those ≥10 years since first episode. Compared with those <1 year since first herpes episode, the quantity differed significantly between both those 1–9 years (coef −.32 [95% CI, −.64, −.02]; P = .05) and those 10 or more years since first episode (coef −.47 [95% CI, −.81, −.14]; P = .006).
Episode frequency and duration
Next, we investigated whether the decline in shedding rates over time was attributable to reduced frequency or shorter duration of episodes. The mean frequency of episodes over a 1-year period for the entire cohort was 14.4 episodes of total HSV-2 shedding, 11.6 episodes of subclinical shedding, and 8.7 episodes with genital lesions. Episode frequency did not change significantly over time since first herpes episode.
The duration of clinical recurrences decreased after the first year following the first genital HSV-2 episode. The mean recurrence length was 10.4 days (range, 1–32 days) among participants <1 year from their first herpes episode, 7.2 days (range, 1–23 days) among those 1–9 years (coef −3.2 [95% CI, −6.2 to −.21]; P = .04), and 6.5 days (range, 1–24 days) among those ≥10 years from first herpes episode (coef −4.0 [95% CI, −6.8 to −1.06]; P = .007). These decreases remained significant among those 1–9 years (coef −3.2 [95% CI, −6.2 to −.18]; P = .04), and those ≥10 years from first herpes episode (coef −4.0 [95% CI, −6.9 to −1.03]; P = .008) after adjusting for race. In contrast to clinical recurrences, the duration of total and subclinical episodes did not change significantly by year since initial HSV-2 episode. The mean duration of any viral shedding episode was 4.6 days, and mean subclinical episode length was 2.6 days for the entire cohort.
Race was found to be significantly associated with both frequency and duration of recurrences. Rates of total shedding episodes were over 25% lower for non-white compared with white race (IRR, .74 [95% CI, .55–.98]; P = .03) after adjusting for time since first episode of genital HSV-2. Nonwhite race was also associated with a mean 1.2 day shorter duration of any shedding episode (95% CI, −2.2 to −.07; P = .036) and 3.3 day shorter duration of recurrences as compared with white race (95% CI, −5.0 to −1.6; P < .001) after adjusting for time since first episode (data not shown). No significant differences in length of subclinical episodes were found between white and nonwhite groups, but the numbers were small. No other variables, including age, gender, sexual preference, age at sexual debut, age at first genital herpes episode, or HSV antibody status, were found to be significant predictors of episode frequency or duration in the analyses.
DISCUSSION
Our study is the first to our knowledge to describe the natural history of viral shedding in HSV-2 seropositive persons more than 10 years since first clinical episode of genital herpes, and it reveals several important observations about long-term HSV-2 reactivation. Rates of total and subclinical HSV-2 shedding and genital HSV-2 lesions decrease after the first year following the first genital herpes episode. However, most persons still shed HSV-2 either clinically or subclinically at high rates for years after infection. We also observed that the quantity of HSV appears to decrease slightly over time, but mean DNA copy numbers remain within a half-log of DNA copy numbers present in the year following the first genital HSV-2 episode. Importantly, the mean copy numbers in our study remain well above 3 log10 copies/mL in all time groups, which is likely a sufficient quantity to transmit infection [15]. These high rates and quantity of HSV shedding observed in years remote from infection suggest that patients with long-standing genital HSV-2 infection pose a continued risk of HSV-2 transmission to sexual partners.
Together, these data support the hypothesis that a virologic “set point” may be established in genital herpes infections, in which the rate and quantity of virus shed decrease following the first year of infection but ultimately establish a pattern of persistent and stable reactivation. Studies of other herpesviruses, particularly Epstein-Barr Virus (EBV), have shown that virus levels reach an equilibrium and maintain a constant level in host cells after acute infection [16]. Reductions in HSV shedding might be explained by the development of a more efficient host immune response that leads to improved clearance of virus [17], since repeated detection of virus is likely the result of frequent reactivations rather than persistence of infectious material in the mucous membranes [18, 19]. The amount of virus released from neurons may also decrease over time, as the concentration of latent HSV DNA present in ganglia has been shown to decline with time in animal models [20, 21]. Either of these explanations could also account for the shorter duration of clinical episodes observed in years following the initial clinical episode. Additional studies of HSV immunology and neuronal viral load in humans are needed to verify these hypotheses.
Our finding that nonwhite race was associated with lower shedding rates, lower clinical recurrence rates, and shorter clinical durations compared with white race is intriguing. Differences in HSV-2 seropositivity by race and ethnicity have been observed in the National Health and Nutrition Examination Survey (NHANES), in which blacks were found to have higher rates of HSV-2 infection as compared with other racial groups [22]. A recent study also reported that white race was associated with greater likelihood of HSV-2 viremia during primary genital infection [23]. Differences in HSV-2 shedding among racial groups have been attributed to differences in HSV-1 acquisition, since rates of HSV-1 infection are higher among nonwhite populations, and prior HSV-1 infection attenuates HSV-2 severity close to the time of HSV-2 acquisition [24, 25]. However, in our study, HSV-1 seropositivity had no impact on the HSV-2 set point of long-term shedding or recurrence rates. It is possible that genetic or immunologic differences among racial or ethnic groups may affect the ability to control HSV-2 infection. Racial differences may also have important implications for the epidemiology of HSV-2 in Africa and may in part explain the high rates of herpes infections in African countries. As our study only included 11% nonwhite subjects, additional prospective studies with greater numbers of nonwhite participants, including natural history studies of genital herpes in Africa, are needed to elucidate this observation.
Our study is limited to adults with a history of symptomatic genital herpes, so its findings may not be generalizable to those with serologic evidence of HSV-2 infection but no clinical history. Our study is also based on the time from the first clinical diagnosis and may not accurately reflect the time from HSV-2 infection. In some persons, the first recognized episode of genital herpes occurs months or years following HSV-2 acquisition [26, 27]. This possible misclassification of time since genital herpes acquisition would result in a conservative misclassification, however, and would diminish the apparent decrease in HSV-2 shedding observed after the first year of infection. From a clinical perspective these limitations may not be important, since most counseling and treatment efforts are focused on patients with symptomatic genital herpes and are guided by clinical symptoms. Our sample may also be biased toward those with more severe disease, although these participants took part in studies of the natural history of genital herpes and were not enrolled based on the severity of their clinical symptoms. The length of sampling sessions did vary among participants, but session length was determined by study protocol rather than symptoms, so participants with more severe disease are not over-represented.
In summary, our study demonstrates that while HSV-2 reactivation and HSV-2 shedding quantity decrease after the first year following the first genital herpes episode, HSV-2 shedding frequency, clinical recurrences, and HSV-2 shedding quantity remain at high levels for many years following infection. These findings have important implications for the long-term management of genital HSV and may warrant strategies such as long-term use of antiviral medications for clinical suppression and continued condom use to reduce transmission to partners.
Supplementary Data
Supplementary data are available at http://www.oxfordjournals.org/our_journals/jid/online.
Funding
This study was supported in part by National Institutes of Health grant A1-30731 and K24 A1-071113, and a grant from GlaxoSmithKline. The funding sources played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
References
- 1.Gupta R, Wald A, Krantz E, et al. Valacyclovir acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 2.Fife K, Warren T, Ferrera D, et al. Effect of valacyclovir on viral shedding in immunocompetent patients with recurrent herpes simplex virus 2 genital herpes: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81:1321–7. doi: 10.4065/81.10.1321. [DOI] [PubMed] [Google Scholar]
- 3.Leone P, Warren T, Hamed K, Fife K, Wald A. Famciclovir reduces viral mucosal shedding in HSV-seropositive persons. Sex Transm Dis. 2007;34:900–7. doi: 10.1097/OLQ.0b013e318063c749. [DOI] [PubMed] [Google Scholar]
- 4.Mark K, Corey L, Meng T, et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J Infect Dis. 2007;195:1324–31. doi: 10.1086/513276. [DOI] [PubMed] [Google Scholar]
- 5.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J Clin Invest. 1997;99:1092–7. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical symptomatic genital herpes infections. N Engl J Med. 1995;333:770–5. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti J, Zeh J, Corey L. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med. 1999;131:14–20. doi: 10.7326/0003-4819-131-1-199907060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Wald A, Zeh J, Selke S, Warren T, Ashley R, Corey L. Genital shedding of herpes simplex virus among men. J Infect Dis. 2002;186:S34–9. doi: 10.1086/342969. [DOI] [PubMed] [Google Scholar]
- 9.Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Hobson A, Wald A, Wright N, Corey L. Evaluation of a quantitative competitive PCR assay for measuring herpes simplex virus DNA content in genital tract secretions. J Clin Microbiol. 1997;35:548–52. doi: 10.1128/jcm.35.3.548-552.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryncarz AJ, Goddard J, Wald A, Huang ML, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37:1941–7. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margaret A, Wald A, Huang M, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin mucosa. J Clin Microbiol. 2007;45:1618–20. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cone RW, Hobson AC, Brown Z, et al. Frequent detection of genital herpes simplex virus DNA by polymerase chain reaction among pregnant women. JAMA. 1994;272:792–6. [PubMed] [Google Scholar]
- 16.Kahn G, Miyashita E, Yang B, Babcock G, Thorley-Lawson D. Is EBV persistence in vivo a model for B cell homeostasis? Immunity. 1996;5:173–9. doi: 10.1016/s1074-7613(00)80493-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Koelle DM, Cao J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–9. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffer JT, Abu-Raddad L, Mark KE, et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med. 2009;1:7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanberry LR, Kern ER, Richards JT, Overall JC., Jr. Recurrent genital herpes simplex virus infection in guinea pigs. Intervirology. 1985;24:226–31. doi: 10.1159/000149647. [DOI] [PubMed] [Google Scholar]
- 21.Stanberry LR. Pathogenesis of herpes simplex virus infection and animal models for its study. Curr Top Microbiol Immunol. 1992;179:15–30. doi: 10.1007/978-3-642-77247-4_2. [DOI] [PubMed] [Google Scholar]
- 22.Wald A. Herpes simplex virus type 2 transmission: risk factors virus shedding. Herpes. 2004;11:130A–7A. [PubMed] [Google Scholar]
- 23.Johnston C, Margaret A, Selke S, Remington M, Corey L, Wald A. Herpes simplex virus viremia during primary genital infection. J Infect Dis. 2008;198:31–4. doi: 10.1086/588676. [DOI] [PubMed] [Google Scholar]
- 24.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, complications. Ann Intern Med. 1983;98:958–72. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 25.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–8. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein DI, Lovett MA, Bryson YJ. Serologic analysis of first-episode nonprimary genital herpes simplex virus infection. Presence of type 2 antibody in acute serum samples. Am J Med. 1984;77:1055–60. doi: 10.1016/0002-9343(84)90188-8. [DOI] [PubMed] [Google Scholar]
- 27.Diamond C, Selke S, Ashley R, Benedetti J, Corey L. Clinical course of patients with serologic evidence of recurrent genital herpes presenting with signs symptoms of first episode disease. Sex Transm Dis. 1999;26:221–5. doi: 10.1097/00007435-199904000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.