Transport of glucocorticoids can be modulated by a changing ratio of cortisol and corticosterone, with evidence of saturable transport in the hypothalamus and pituitary gland.
Abstract
Proper functioning of the hypothalamic-pituitary-adrenal axis depends on the ability of glucocorticoids (GCs), mainly cortisol in humans and corticosterone in rodents, to access brain targets and regulate their own secretion. Being highly lipophilic, GCs have been assumed to passively diffuse through the cell membrane. However, the access of these GCs to the brain may be a more complicated process, because the free movement of molecules into the central nervous system (CNS) is restricted by the presence of the blood-brain and blood-cerebrospinal fluid barriers. GCs do interact with some transporter systems, including the efflux transporter, P-glycoprotein, and members of the organic anion transporter polypeptide (oatp) family, both of which have been found at the blood-CNS barriers. Using an in situ brain/choroid plexus perfusion, P-glycoprotein was shown to not majorly regulate the access of [3H]cortisol and [3H]corticosterone to the choroid plexus or pituitary gland. Interactions of [3H]cortisol and [3H]corticosterone with saturable influx transporters were detected at the hypothalamus, cerebellum, choroid plexus, and pituitary gland. Oatp2 seems to have some role in the influx of [3H]cortisol and [3H]corticosterone to the choroid plexus and the pituitary gland and other transporters, unlikely to be oatp2, may play a very minor role in the access of [3H]cortisol and [3H]corticosterone to the brain, as well as having a significant effect on [3H]glucocorticoid receptor accumulation in the pituitary gland. Overall, these data suggest that the majority of cortisol and corticosterone present in the plasma diffuse into the CNS and that transporters do not play a major role in the accumulation of these GCs in the brain.
Fundamental processes of the body, such as the body's stress response and energy metabolism, are regulated by the hypothalamic-pituitary-adrenal (HPA) axis, and hyperactivity of this axis has been seen in approximately 50% of patients with depression (1). The proper functioning of this axis is dependent on the ability of glucocorticoids (GCs), mainly cortisol in humans and corticosterone in rodents, to access brain targets such as the hypothalamus and regulate their own secretion. Based on their highly lipophilic properties, GCs have been assumed to passively diffuse through the cell membrane, because they must reach their intracellular receptors to exert their actions (2). This notion is also supported by the fact that the lipophilicity of these molecules correlates with their cellular accumulation in artificial phospolipid/cholesterol membranes (3) and that the n-octanol-water partition coefficients correlate to the membrane permeability of these molecules in both hamster fibroblasts and rat hepatoma cells (4).
However, the ability of these GCs to access the brain may be a more complicated process. The free movement of molecules into the majority of brain regions is restricted by the presence of the blood-brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier (BCSFB). The BBB is found at the level of the capillary endothelial cells of cerebral vasculature, whereas the BCSFB is found at the fluid-secreting epithelium of the choroid plexus and at the arachnoid membrane (5). The fenestrated capillaries of the vessels that feed the choroid plexus allow for relatively free movement of molecules into choroid plexus tissue, and some of these molecules may then be transported into CSF. The ventricular distribution of CSF-borne signals occurs by bulk flow, and there is no physical restriction in the movement of molecules from the CSF to the brain; however, movement from CSF to brain is limited (6). Nevertheless, passage through the BCSFB may provide an “easier” route of access to the brain compared with access through the restrictive BBB. Unidirectional brain uptake indexes of [3H]cortisol and [3H]corticosterone in rats, determined 15 sec after a bolus injection, are 1.4 ± 0.3 and 39.0 ± 2%, respectively (7), indicating that corticosterone is able to cross the BBB in situ, although it suggests that cortisol entry across the BBB is more limited. In humans, iv administered cortisol passed from blood into CSF, and intrathecally administered cortisol was detected in blood (8), suggesting that cortisol is not prevented from crossing the BCSFB. Of special note is the differential expression of transporters in capillary endothelium (BBB) and choroidal epithelium (BCSFB) that may contribute to differential access of the same molecules to each barrier compartment (5). Additionally, there are central nervous system (CNS) regions that have nonbarrier type capillaries. These are the circumventricular organs, such as the posterior pituitary gland, and are self-contained structures that are surrounded by physical restrictions, which prevent the free movement of molecules into noncircumventricular organ tissues. It is important to note that fenestrated capillaries also feed both the anterior and posterior pituitary gland, allowing free exchange of blood-borne molecules between blood and tissue. Thus, because CNS tissues have a varied ease of penetration from the peripheral circulation, it is beneficial to conduct a comparative study of regions in which the access to brain is controlled vs. those regions in which the access of molecules is less controlled.
Because the physiochemical properties of GCs suggested that they could quickly cross cellular membranes, it was assumed that they would also easily enter the CNS by passive diffusion. It is now known that GCs also interact with glucose transporters in human cells (9–12) and also the efflux transporter, P-glycoprotein (P-gp) (13–15) in murine cells, and members of the organic anion transporter polypeptide (oatp) family in rat cells (16, 17). These transporters have been identified at the rodent and human BBB and choroid plexuses (18–24). The contribution of these transporters to GC physiology is still unclear, partly because of the different techniques used to examine this issue, as discussed in detail in our previous work (13). For example, experiments using whole-body distribution methods after peripheral injection of radioactive GCs in mice initially suggested that the efflux transporter, P-gp, restricts the access of cortisol and corticosterone to the murine brain (25, 26) and is also implicated in the regulation of the HPA axis (14, 15). However, our recent results obtained from murine brain distribution studies using direct brain perfusion show that P-gp restricts the accumulation of [3H]cortisol in specific brain regions, such as hypothalamus, and does not restrict the access of [3H]corticosterone in any brain regions sampled (13). Indeed, after systemic administration of these [3H]GCs, we also showed the presence of radiolabeled by-products in the plasma, which would make accurate data interpretation in the initial 2001 and 2003 studies difficult (13).
In this study, we extend our investigation of the transport of GCs across the BBB (13) to that of GC transport across the BCSFB and nonbarrier regions in wild-type controls and P-gp-deficient mice. This allowed exploration of the influence of P-gp on CNS delivery via routes other than the BBB. We then explore the interaction of GCs with transporters other than P-gp at the level of both the BBB and BCSFB barriers. The method used is a well-established brain/choroid plexus perfusion technique in anesthetized mice that allows perfusion of both cerebral hemispheres and consequent examination of molecule movement across the BBB and the BCSFB (27).
Materials and Methods
Materials
Radiolabeled substances
[3H]cortisol (74.0 Ci/mmol) and [3H]corticosterone (79.0 Ci/mmol) were purchased from GE Healthcare (Buckinghamshire, UK). [14C]sucrose (0.49 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA).
Unlabeled substances
Cyclodextrin-encapsulated cortisol, corticosterone, and digoxin were purchased from Sigma-Aldrich (Poole, UK). All other materials were purchased from Sigma-Aldrich, unless stated.
Animals
Adult FVB-abcb1a/b(+/+) and FVB-abcb1a/b(−/−) mice were imported from Taconic Farms, Inc. (Germantown, NY), and a breeding colony was established at King's College London under an academic breeding agreement. Genotype was confirmed by PCR analysis (Harlan UK, Ltd. Hillcrest, Belton, UK). All animals were maintained under standard conditions of temperature and lighting and given food and water ad libitum.
Procedures
All procedures are within the guidelines of the Animals (Scientific Procedures) Act, 1986.
In situ brain/choroid plexus perfusion technique
Adult male mice (25–40 wk and 25–46 g) were anesthetized with a medetomidine hydrochloride (2 mg/kg, ip) and ketamine hydrochloride solution (150 mg/kg, ip) and heparinized (100 Units, ip). The body cavity was opened and the left ventricle cannulated with a fine needle (25 gauge) connected to a perfusion circuit. A Watson-Marlow peristaltic pump (323S/RL; Watson-Marlow, Falmouth, UK) was used to perfuse the heart in situ with a modified Krebs-Henseleit mammalian Ringer solution as detailed previously (13), which was warmed (37 C) and oxygenated (95% O2, 5% CO2). With the start of perfusion (5.0 ml/min), the right atrium was sectioned to create an open circuit and allow drainage of the artificial plasma. In all experiments, a 2.5-min predrug perfusion of artificial plasma ensured removal of endogenous GCs from the brain vasculature. [3H]cortisol (3.6 nm) or [3H]corticosterone (3.8 nm), along with [14C]sucrose (vascular space marker, 0.5–1.0 nm), was then administered by a slow-drive syringe pump (model 22; Harvard Apparatus, Kent, UK) into the artificial plasma. After the desired isotope perfusion time period (1, 2.5, 10, and 20 min), a glass capillary was used to take a cisterna magna CSF sample, and the mouse was decapitated. The brain was removed. Samples [frontal cortex, hypothalamus, hippocampus, cerebellum, choroid plexus (IV ventricle), and pituitary gland, comprising anterior and posterior lobes] were dissected and weighed. All brain samples, together with CSF and 100 μl artificial plasma samples, were prepared for liquid scintillation counting as described below. For the multiple time-point perfusions using [3H]cortisol, n = 24–29 abcb1a/b(+/+) mice and n = 27–28 abcb1a/b(−/−) mice were used. For the multiple time-point perfusions using [3H]corticosterone, n = 33–44 abcb1a/b(+/+) and n = 45–48 abcb1a/b(−/−) mice were used due to the variability of the corticosterone accumulation in these tissues. Due to the variability of CSF sampling, n = 17 abcb1a/b(+/+) and n = 11 abcb1a/b(−/−) mice were used for the [3H]cortisol experiments, and n = 28 abcb1a/b(+/+) and n = 29 abcb1a/b(−/−) mice were used for the [3H]corticosterone experiments.
Liquid scintillation counting
All samples were solubilized over approximately 48 h in 0.5 ml of Solvable (PerkinElmer Life and Analytical Sciences, Boston, MA). All samples were vortexed, 3 ml of scintillation counting fluid (Lumasafe; PerkinElmer Life and Analytical Sciences) were then added and the samples vortexed again. The samples were then placed in a Packard TriCarb 2100 or 2900TR (PerkinElmer, Beaconsfield, UK) liquid scintillation counter for estimation of [3H] and [14C] radioactivities. All results were corrected for background radioactivity.
Self-inhibition studies
Single time-point perfusions (20 min) were conducted in FVB-abcb1a/b(+/+) mice, i.e. wild-type controls, with [3H]cortisol in the presence of an excess of unlabeled cortisol (30 or 300 μm) in the artificial plasma, or with [3H]corticosterone in the presence of an excess of unlabeled corticosterone (30 or 300 μm) in the artificial plasma; 30 μm of unlabeled cortisol or unlabeled corticosterone has been used previously to saturate GC receptor (GR) binding in vitro (28–30), and 300 μm of unlabeled cortisol or unlabeled corticosterone was used to ensure total saturation of any transporters present. The 20-min time point was chosen to provide adequate time for any potential transport mechanisms to be saturated. Because the cortisol was available in water-soluble form (encapsulated with cyclodextrin), it was unnecessary to dissolve it in any solvent; however, the corticosterone was dissolved in the recommended solvent, methanol. Control studies were conducted to ensure that the addition of the methanol solvent had no significant effects on the [14C]sucrose or [3H]GC values. Hence, a molar solution was prepared and added to the artificial plasma to achieve a final concentration of either 30 or 300 μm of unlabeled corticosterone. Total solvent concentration in the artificial plasma was 0.05%. For these studies, n = 15–19 FVB-abcb1a/b(+/+), i.e. wild-type controls, were used.
Cross-competition studies
To further investigate the specificity of the possible transport of [3H]GCs, single time-point perfusions (20 min) were conducted in wild-type mice with [3H]cortisol in the presence of an excess of unlabeled corticosterone (30 or 300 μm) or [3H]corticosterone in the presence of an excess of unlabeled cortisol (30 or 300 μm) added to the artificial plasma solution and prepared as previously detailed. For the studies using [3H]cortisol and corticosterone, n = 12–14 wild-type mice were used, and for the studies using [3H]corticosterone and cortisol, n = 20–32 wild-type mice were used due to the variability of corticosterone accumulation.
Transport inhibition studies
Digoxin (25 μm) was used to inhibit oatp2 in wild-type mice. The half-saturation constant (Km) for inhibition of mouse oatp2 being 5.7 μm (21), digoxin is also a substrate of P-gp, the concentration used in these experiments is well above the Km for inhibition of P-gp (14.1 ± 1.6 μm) (31). For these studies, n = 10–14 wild-type mice were used for the [3H]cortisol experiments, and n = 14–26 wild-type mice were used for the [3H]corticosterone experiments.
Expression of results
The concentration of [3H] or [14C] radioactivity in the brain tissue [disintegrations per minute (dpm) g−1] or CSF (dpm ml−1) is expressed as a percentage of that in artificial plasma (dpm ml−1) and is referred to as RTissue%. [14C]sucrose values reflect the vascular space contained in each tissue sample, and these values were used to correct the RTissue values of the radioactive GCs, resulting in RCorrTissue% values termed “vascular space-corrected”. When the [14C]sucrose values did not differ significantly between groups, RCorrTissue% values were used when comparing GR values between strains.
Isolated incubated choroid plexus
An isolated incubated choroid plexus technique was used to examine [3H]cortisol and [3H]corticosterone (as well as [14C]sucrose) accumulation from artificial CSF into choroid plexus tissue (27). Adult FVB-abcb1a/b(+/+) mice were anesthetized and heparinized and perfused with artificial plasma as described above for 4 min to remove blood from the choroid plexus capillaries. After perfusion, the mouse was decapitated and the IVth ventricle choroid plexus removed. The choroid plexus tissue was then incubated in warm (37 C), artificial CSF for 10 min, followed by a 10-min incubation with CSF containing either [3H]cortisol (3.6 nm) and [14C]sucrose (0.5 nm) or [3H]corticosterone (3.8 nm) and [14C]sucrose (1.0 nm). The tissue was then removed and weighed on a Cahn C-33 microbalance. The choroid plexus and CSF samples were then prepared for liquid scintillation counting as described above. The levels of radioactivity in the choroid plexus (dpm g−1) were measured as a ratio of the concentration in the artificial CSF (dpm ml−1). The [3H]GC values within the choroid plexus were corrected for extracellular space by subtracting the [14C]sucrose values.
Self-inhibition studies were also carried out to determine whether [3H]GC distribution into the choroid plexuses was affected by additional drugs or inhibitors and hence indicate the presence of a transporter at this site. Self-inhibition experiments explored the distribution of the [3H]GCs in the presence of unlabeled cortisol or corticosterone as appropriate. The corticosterone was dissolved in methanol and diluted with artificial CSF to achieve a final concentration of 30 or 300 μm in 0.05% methanol. Appropriate methanol controls were also performed. The distribution of the radiolabeled GRs was also explored in the presence of 25 μm of unlabeled digoxin, which was dissolved in warmed CSF.
Data analysis
The data from all the experiments are presented at mean ± sem. The Sigma Stat 2.0 statistical program (SPSS Science Software UK Ltd., Birmingham, UK) was used for all determinations. All data populations were evaluated to ensure normal distribution, and if this criterion was not met, the comparable nonparametric test was used. In the self-inhibition and cross-competition studies, one-way ANOVAs or Kruskal-Wallis analysis on ranks were used to compare [14C]sucrose or [3H]GC values between groups in experiments with more than one control group or more than one concentration. In the multiple-time uptake studies, two-way or one-way ANOVAs were used to compare uptake of radiolabeled drugs between brain regions and between strains as appropriate. Student's t tests were used to compare the [14C]sucrose values and the uptake of [3H]GRs between strains in the transport inhibition studies. In all cases, the level of significance was set at P < 0.05.
Results
In FVB-abcb1a/b(−/−) mice, the absence of P-gp had no significant effect on the [14C]sucrose values in either the choroid plexus or the pituitary gland at any time point (P > 0.05, Student's t tests).
The contribution of P-gp in the distribution of [3H]GRs at the BSCFB and nonbarrier regions [3H]cortisol
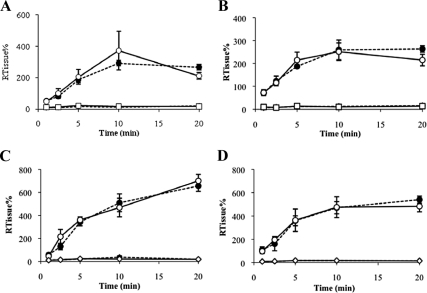
The distribution of [3H]cortisol and [14C]sucrose in FVB-abcb1a/b(+/+) and FVB-abcb1a/b(−/−) mice over time is presented in Fig. 1, A and B. The distribution of [14C]sucrose was significantly lower than the distribution of [3H]cortisol in the choroid plexus (P < 0.001, two-way ANOVA) (Fig. 1A) and pituitary gland (P < 0.001, two-way ANOVA) (Fig. 1B) of both strains. This suggests that cortisol is passing across the barrier and entering the cells. The distribution of [3H]cortisol in the choroid plexus and the pituitary gland were not significantly different between abcb1a/b(−/−) and abcb1a/b(+/+) mice (P > 0.05, two-way ANOVAs). [3H]cortisol was detected in the CSF, being 1.5 ± 0.3% at 1 min and rising to 26.4 ± 9.7% at 20 min, and [3H]cortisol values were not significantly different in abcb1a/b(−/−) mice compared with abcb1a/b(+/+) mice (P = 0.5, two-way ANOVA) (data not shown). Overall, the similarity in distribution between these strains suggests that that P-gp is either not present in these regions or is not functionally relevant.
Fig. 1.
RTissue% values of [3H]cortisol and [14C]sucrose and of [3H]corticosterone and [14C]sucrose in abcb1a/b(+/+) and abcb1a/b(−/−) mice plotted as a function of time using the in situ brain/choroid plexus perfusion technique. [3H]cortisol (3.6 nm) or [3H]corticosterone (3.8 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma. After the desired isotope perfusion time period up to 20 min, the mouse was decapitated, and choroid plexus (A and C), pituitary gland (B and D), and CSF (data not shown) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) and fluid (dpm ml−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]cortisol was not significantly different in any sample between strains [choroid plexus (A) and pituitary gland (B): abcb1a/b(+/+) mice, n=24–29; abcb1a/b (−/−) mice, n = 27–28; CSF: abcb1a/b(+/+) mice, n = 17; abcb1a/b(−/−) mice, n = 11]. The uptake of [3H]corticosterone was not significantly different in any sample between strains [choroid plexus (C) and pituitary gland (D): abcb1a/b(+/+) mice, n = 33–44; abcb1a/b(−/−) mice, n = 45–48; CSF: abcb1a/b(+/+) mice, n = 28; abcb1a/b(−/−) mice, n = 29] and [14C]sucrose values were not influenced by strain. Closed circle (dotted line), [3H]cortisol or [3H]corticosterone [abcb1a/b(+/+) mice]; open circle (solid line), [3H]cortisol or [3H]corticosterone [abcb1a/b(−/−) mice]; closed square (dotted line), [14C]sucrose [abcb1a/b(+/+) mice]; open square (solid line), [14C]sucrose [abcb1a/b(−/−) mice].
[3H]corticosterone
The distribution of [3H]corticosterone and [14C]sucrose in FVB-abcb1a/b(+/+) and FVB-abcb1a/b(−/−) mice over time is presented in Fig. 1, C and D. The distribution of [3H]corticosterone in the choroid plexus and pituitary gland of abcb1a/b(−/−) mice was not significantly different compared with abcb1a/b(+/+) mice at any time point (P > 0.05, Student's t tests). [3H]corticosterone could be detected in the CSF, being 5.1 ± 0.7% at 1 min and rising to 66.7 ± 11.1% at 20 min, in abcb1a/b(+/+) mice, and the distribution of [3H]corticosterone in the CSF was not significantly different between strains (P > 0.05, Student's t tests) (data not shown). Again, this suggests that P-gp may not be present or is not functionally relevant in the CNS accumulation of [3H]corticosterone in these regions.
Self-inhibition and cross-competition studies
The [14C]sucrose values obtained in wild-type [i.e. FVB-abcb1a/b(+/+)] mice were not significantly different in any sample in the absence or presence of unlabeled cortisol or in the presence of unlabeled corticosterone in methanol compared with methanol controls (P > 0.05, one-way ANOVAs). Therefore, the vascular-corrected or extracellular space-corrected [3H]GR values are presented as above.
Self-inhibition [3H]cortisol
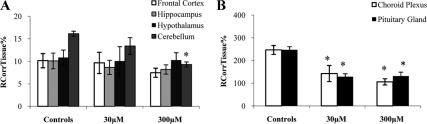
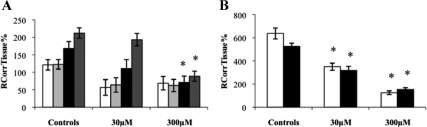
The distribution of [3H]cortisol in the presence and absence of an excess of unlabeled cortisol in wild-type mice is presented in Fig. 2. The distribution of [3H]cortisol in the cerebellum was decreased in the presence of 300 μm, but not 30 μm, of unlabeled cortisol (P < 0.001, one-way ANOVA, vascular space corrected) compared with wild-type controls and was not significantly different in the frontal cortex, hippocampus, or hypothalamus. The distribution of [3H]cortisol in the choroid plexus was decreased in the presence of both 30 and 300 μm of unlabeled cortisol (P = 0.001, one-way ANOVA, extracellular space corrected) compared with controls, as was the uptake of [3H]cortisol in the pituitary gland (P < 0.001, one-way ANOVA, extracellular space corrected). This suggests that a saturable influx mechanism for [3H]cortisol is present in the cerebellum, choroid plexus, and in the pituitary gland.
Fig. 2.
RCorrTissue% (ml × 100 g−1) of [3H]cortisol in the absence and presence of unlabeled cortisol in wild-type mice using in situ brain/choroid plexus perfusion technique. [3H]cortisol (3.6 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma containing unlabeled cortisol (30 or 300 μm). After a perfusion time of 20 min, the mouse was decapitated, and selected brain regions (open white bar, frontal cortex; light gray bar, hippocampus; closed black bar, hypothalamus; dark gray bar, cerebellum) (A) and choroid plexus and pituitary gland (open white bar, choroid plexus; closed black bar, pituitary gland) (B) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]cortisol in the cerebellum was decreased in the presence of 300 μm of unlabeled cortisol (P < 0.001, one-way ANOVA, vascular space corrected), and the uptake of [3H]cortisol in the choroid plexus was decreased in the presence of both 30 and 300 μm of unlabeled cortisol (P = 0.001, one-way ANOVA, extracellular space corrected) compared with controls, as was the uptake of [3H]cortisol in the pituitary gland (P < 0.001, one-way ANOVA, extracellular space corrected) (n = 15–19). *, Significance (P < 0.05).
[3H]corticosterone
The distribution of [3H]corticosterone in the presence and absence of an excess of unlabeled corticosterone in wild-type mice is presented in Fig. 3. The distribution of [3H]corticosterone in hypothalamus was significantly decreased in the presence of 300 μm of unlabeled corticosterone (P = 0.026, Student's t test, vascular space corrected), compared with wild-type controls and was not significantly different in the frontal cortex, hippocampus, or hypothalamus. The distribution of [3H]corticosterone in the choroid plexus was decreased in the presence of both 30 and 300 μm of unlabeled corticosterone (P < 0.001, one-way ANOVAs, extracellular space corrected) compared with controls, as was the uptake of [3H] corticosterone in the pituitary gland (P < 0.001, one-way ANOVA, extracellular space corrected). This suggests that a saturable influx mechanism for [3H]corticosterone is present in the hypothalamus, choroid plexus, and in the pituitary gland.
Fig. 3.
RCorrTissue% (ml × 100 g−1) of [3H]corticosterone in the absence and presence of unlabeled corticosterone in wild-type mice using in situ brain/choroid plexus perfusion technique. [3H]corticosterone (3.8 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma containing unlabeled corticosterone (30 or 300 μm), dissolved in methanol. After a perfusion time of 20 min, the mouse was decapitated, and selected brain regions (open white bar, frontal cortex; light gray bar, hippocampus; closed black bar, hypothalamus; dark gray bar, cerebellum) (A) and choroid plexus and pituitary gland (open white bar, choroid plexus; closed black bar, pituitary gland) (B) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]corticosterone in hypothalamus was significantly decreased in the presence of 300 μm of unlabeled corticosterone (P = 0.026, Student's t test, vascular space corrected), but no other brain regions were affected. The uptake of [3H]corticosterone in the choroid plexus and in the pituitary gland was significantly decreased in the presence of both concentrations of unlabeled corticosterone compared with methanol controls (both P < 0.001, one-way ANOVAs, corrected for extracellular space) (n = 15). *, Significant difference (P < 0.05) in the [3H]corticosterone values compared to methanol controls.
Cross-competition studies [3H]cortisol + corticosterone
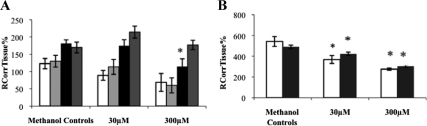
The distribution of [3H]cortisol in the presence and absence of an excess of unlabeled corticosterone in wild-type mice is presented in Fig. 4. The distribution of [3H]cortisol in the hypothalamus and in the cerebellum was significantly increased in the presence of 300 μm of unlabeled corticosterone (P = 0.013 and P = 0.020, respectively, Bonferroni t test, after one-way ANOVA, vascular space corrected) compared with methanol controls. This suggests that a saturable efflux mechanism for [3H]cortisol that can be affected by corticosterone is present in these regions. The distribution of [3H]cortisol in the pituitary gland was significantly decreased in the presence of 300 μm of unlabeled corticosterone compared with methanol controls (P = 0.024, Kruskal-Wallis ANOVA on ranks), suggesting that a saturable influx mechanism for [3H]cortisol that can be affected by corticosterone is present in the pituitary gland.
Fig. 4.
RCorrTissue% (ml × 100 g−1) of [3H]cortisol in the absence and presence of unlabeled corticosterone in wild-type mice using in situ brain/choroid plexus perfusion technique. [3H]cortisol (3.6 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma containing unlabeled corticosterone (30 or 300 μm), dissolved in methanol. After a perfusion time of 20 min, the mouse was decapitated, and selected brain regions (open white bar, frontal cortex; light gray bar, hippocampus; closed black bar, hypothalamus; dark gray bar, cerebellum) (A) and choroid plexus and pituitary gland (open white bar, choroid plexus; closed black bar, pituitary gland) (B) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]cortisol in the hypothalamus and in the cerebellum was significantly increased in the presence of 300 μm of unlabeled corticosterone (P = 0.013 and P = 0.020, respectively, Bonferroni t test, after one-way ANOVA, vascular space corrected) compared with methanol controls. The uptake of [3H]cortisol in the pituitary gland was significantly decreased in the presence of 300 μm of unlabeled corticosterone compared with methanol controls (P = 0.024, Kruskal-Wallis ANOVA on ranks), (n = 12–14). *, Significant difference (P < 0.05) compared with methanol controls.
[3H]corticosterone + cortisol
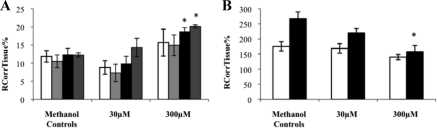
The distribution of [3H]corticosterone in the presence and absence of an excess of unlabeled cortisol in wild-type mice is presented in Fig. 5. The distribution of [3H]corticosterone in the hypothalamus and cerebellum was significantly decreased in the presence of 300 μm of unlabeled cortisol compared with wild-type controls (P = 0.017 and P = 0.002, respectively, one-way ANOVA, vascular space corrected). The distribution of [3H]corticosterone in the choroid plexus and the pituitary gland was significantly decreased in the presence of both concentrations of unlabeled cortisol compared with wild-type controls (both P < 0.001, one-way ANOVA, extracellular space corrected). These results suggest that a saturable influx mechanism for [3H]corticosterone that can be affected by cortisol is present in these regions.
Fig. 5.
RCorrTissue% (ml × 100 g−1) of [3H]corticosterone in the absence and presence of unlabeled cortisol in wild-type mice using in situ brain/choroid plexus perfusion technique. [3H]corticosterone (3.8 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma containing unlabeled cortisol (30 or 300 μm). After a perfusion time of 20 min, the mouse was decapitated, and selected brain regions (open white bar, frontal cortex; light gray bar, hippocampus; closed black bar, hypothalamus; dark gray bar, cerebellum) (A) and choroid plexus and pituitary gland (open white bar, choroid plexus; closed black bar, pituitary gland) (B) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]corticosterone in the hypothalamus and cerebellum was significantly decreased in the presence of 300 μm of unlabeled cortisol compared with wild-type controls (P = 0.017 and P = 0.002, respectively, one-way ANOVA, vascular space corrected), and the uptake of [3H]corticosterone in the choroid plexus and the pituitary gland was also significantly decreased in the presence of both concentrations of unlabeled cortisol compared with wild-type controls (both P < 0.001, one-way ANOVA, corrected for extracellular space) (n = 20–32). *, Significant difference (P < 0.05) when compared with controls.
Unlabeled digoxin
We specifically investigated the contribution of oatp2, using its inhibitor, digoxin. Oatp2 has been detected in choroidal epithelium, likely localized to the apical membrane (or subapically) (19, 20) and is also found in murine brain capilliaries (21, 22), where it bidirectionally transports substrates (32). Also, oatp2 has been shown to efflux the neuroactive steroid dehydroepiandrosterone sulfate (33), thus oatp2 was hypothesized to be the transporter possibly detected in these self-inhibition and cross-competition studies in wild-type mice.
[3H]cortisol
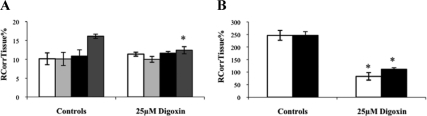
The distribution of [3H]cortisol was significantly decreased in the cerebellum, the choroid plexus, and the pituitary gland in the presence of unlabeled digoxin compared with wild-type controls (P = 0.004, P < 0.001, and P < 0.001, respectively, Student's t test, vascular/extracellular space corrected) (Fig. 6). All other regions were not affected.
Fig. 6.
RCorrTissue% (ml × 100 g−1) of [3H]cortisol in the absence and presence of unlabeled digoxin in wild-type mice using in situ brain/choroid plexus perfusion technique. [3H]cortisol (3.6 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma containing unlabeled digoxin (25 μm). After a perfusion time of 20 min, the mouse was decapitated, and selected brain regions (open white bar, frontal cortex; light gray bar, hippocampus; closed black bar, hypothalamus; dark gray bar, cerebellum) (A) and choroid plexus and pituitary gland (open white bar, choroid plexus; closed black bar, pituitary gland) (B) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]cortisol was significantly decreased in the cerebellum, choroid plexus, and pituitary gland in the presence of unlabeled digoxin compared with wild-type controls (P = 0.004, P < 0.001, and P < 0.001, respectively, Student's t test, corrected for vascular or extracellular space) (n = 10–14). *, Significance (P < 0.05) compared with controls.
[3H]corticosterone
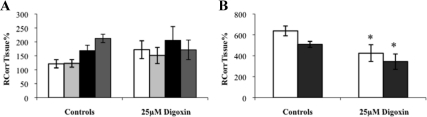
The distribution of [3H]corticosterone was not significantly different in the absence or presence of unlabeled digoxin in any of the brain regions (P > 0.05, Student's t tests, vascular space corrected) (Fig. 7A). However, the distribution of [3H]corticosterone was significantly decreased in the choroid plexus and pituitary gland in the presence of unlabeled digoxin compared with wild-type controls (P = 0.019 and P = 0.020, respectively, Student's t tests) (Fig. 7B).
Fig. 7.
RCorrTissue% (ml × 100 g−1) of [3H]corticosterone in the absence and presence of unlabeled digoxin in wild-type mice using in situ brain/choroid plexus perfusion technique. [3H]cortisol (3.8 nm), along with [14C]sucrose (vascular space marker; 0.5–1.0 nm), was administered by a slow-drive syringe pump into the artificial plasma containing unlabeled digoxin (25 μm). After a perfusion time of 20 min, the mouse was decapitated, and selected brain regions (open white bar, frontal cortex; light gray bar, hippocampus; closed black bar, hypothalamus; dark gray bar, cerebellum) (A) and choroid plexus and pituitary gland (open white bar, choroid plexus; closed black bar, pituitary gland) (B) were sampled. The concentration of [3H] or [14C] radioactivity in the tissues (dpm g−1) is expressed as a percentage of that in the artificial plasma (dpm ml−1). The uptake of [3H]corticosterone was not significantly different in the absence or presence of unlabeled digoxin in any of the brain regions (P > 0.05, Student's t tests, vascular space corrected). However, the uptake of [3H]corticosterone was significantly decreased in the choroid plexus and pituitary gland in the presence of unlabeled digoxin compared with wild-type controls (P = 0.019 and P = 0.020, respectively, Student's t tests, corrected for extracellular space) (n = 14–26).*, Significance (P < 0.05) compared with controls.
Isolated incubated choroid plexus
This method focuses on the apical (i.e. CSF-facing) choroid plexus membrane that is opposite to the basal (i.e. blood-facing) membrane that is the focus of in situ brain/choroid plexus perfusion studies, although the influence of transport processes at the basal membrane cannot be eliminated completely. The distribution of [3H]cortisol into choroid plexus tissue from artificial CSF was unaffected by the presence of 300 μm cortisol and was significantly increased in the presence of unlabeled digoxin (25 μm) (P = 0.04, Student's t test, extracellular space corrected) (Fig. 8), and the distribution of [3H]corticosterone was reduced in the presence of an excess of unlabeled 300 μm corticosterone (P = 0.014, Student's t test, extracellular space corrected) (Fig. 8) but unaffected by digoxin. This suggests that there are multiple transporters that interact with GCs present at the choroid plexus.
Fig. 8.
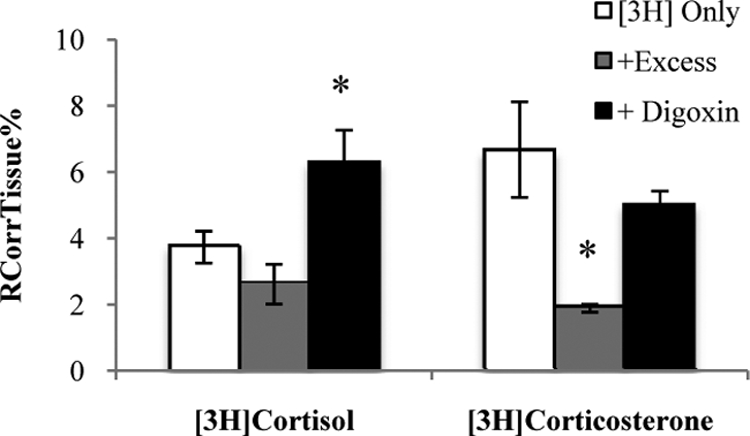
RCorrTissue% (ml × 100 g−1) of [3H]cortisol and [3H]corticosterone in the absence and presence of unlabeled respective GR in wild-type mice using the isolated incubated choroid plexus technique. Isolated IVth ventricle choroid plexuses obtained from adult FVB mice were incubated in warm (37 C) artificial CSF for 10 min, followed by a 10-min incubation in either [3H]cortisol (3.6 nm) and [14C]sucrose (0.5 nm) or [3H]corticosterone (3.8 nm) and [14C]sucrose (1 nm). After perfusion, the mouse was decapitated and the choroid plexus isolated. The concentration of [3H] or [14C] radioactivity in the choroid plexus tissue (dpm g−1) is expressed as a percentage of that in the artificial CSF (dpm ml−1). The uptake of [3H]cortisol into choroid plexus tissue was increased in the presence of unlabeled digoxin (25 μm) (P = 0.04, Student's t test, corrected for extracellular space), and the uptake of [3H]corticosterone was reduced in the presence of an excess of unlabeled corticosterone (P = 0.014, Student's t test, extracellular space corrected). Open white bar, [3H]GR only; light gray bar, [3H]GR with 300 μm of same unlabeled GR; closed black bar, [3H]GR with 25 μm of digoxin.
Discussion
The present study allowed exploration of the influence of P-gp on CNS delivery via routes other than the BBB, as well as investigating the possible transport of GCs by other transporters at the BBB, BCSFB, and nonbarrier regions. Interactions of [3H]cortisol and [3H]corticosterone with saturable influx transporters were detected at the hypothalamus, cerebellum, choroid plexus, and pituitary gland; the influx transporter for [3H]cortisol detected in the cerebellum was sensitive to digoxin; and the influx transporters for [3H]cortisol and for [3H]corticosterone detected in the choroid plexus and in the pituitary were sensitive to digoxin. We also obtained evidence of an efflux transporter for [3H]cortisol at the hypothalamus, which may be P-gp (13) and at the cerebellum, which is likely not P-gp, because the absence of P-gp had no effect in this region in our previous work (13). This study illustrates some of the mechanisms that could regulate the HPA axis in humans in times of stress. Although it is difficult to know if these interactions would be present in humans, who produce both cortisol and corticosterone, these data indicate that the presence of both of these hormones in the bloodstream and their changing plasma concentrations could produce changes in the accumulation of GCs in the CNS, thus providing additional regulation to the HPA axis.
In our examination of the role of P-gp at the BCSFB (i.e. choroid plexus) and in an HPA axis-related nonbarrier region (i.e. pituitary gland) using transporter-deficient mice, no functional P-gp activity was detected. In support of our data, a recent article investigating P-gp at the BCSFB revealed a lower level of P-gp expression in the rat and human choroid plexuses compared with brain microvessels using immunohistochemical analysis with the P-gp-specific murine antibody C219 (5), which suggests a reduced contribution of P-gp in the transport of its substrates across the BCSFB. P-gp has been detected in porcine and rodent choroid plexuses previously (24, 34), and evidence of P-gp activity at the choroid plexus has been seen (35). To further investigate the activity of P-gp at the BCSF barrier, 99mTc-sestamibi, a nonmetabolized pharmaceutical that is known to be transported by both human P-gp and multidrug resistance associated protein 1 (24), was injected into the mouse tail vein. The radioactivity associated with serum, blood, and CSF of 99mTc-sestamibi in abcd1a/1b(+/+) and abcd1a/b(−/−) mice was measured, and the CSF-to-serum concentrations were not significantly different in abcd1a/b(+/+) and abcd1a/b(−/−) mice (24). Together, these findings support our results and provide evidence that P-gp exerts a negligible effect in the distribution of [3H]cortisol and [3H]corticosterone at the BCSFB. Because P-gp has also been detected in rat and human pituitary cells (36, 37), it can be concluded that although P-gp is present and functioning in the murine choroid plexus and in the pituitary gland, P-gp does not significantly affect the accumulation of [3H]cortisol or [3H]corticosterone in these regions.
The presence of [3H]cortisol and [3H]corticosterone in the CSF, representative of nonprotein-bound molecule, also supports the hypothesis that these GCs are not prevented from crossing the BCSFB by P-gp. These data are consistent with our previous work that showed that P-gp does not greatly affect the access of [3H]cortisol and [3H]corticosterone to the murine brain, although efflux of [3H]cortisol by P-gp was detected in the hypothalamus (13). This also indicates that it is necessary to investigate the role of transporters specifically in the regions of interest when a question is posed about the role of transport in CNS accumulation, because the differential expression of transporters in various brain regions and also at the blood-CNS barriers may significantly affect accumulation.
Influx transport at the BBB
There was only limited evidence that [3H]cortisol and [3H]corticosterone were being transported inward at the BBB. Only in the cerebellum was the distribution of [3H]cortisol reduced in the presence of 300 μm, but not 30 μm, of unlabeled cortisol, but this was not seen in the frontal cortex, hippocampus, or cerebellum, in the presence of unlabeled cortisol. The distribution of [3H]corticosterone was only reduced in the hypothalamus in the presence of 300 μm, but not 30 μm, of unlabeled corticosterone, but this was not seen in the frontal cortex, hippocampus, or cerebellum, in the presence of unlabeled corticosterone. The distribution of [3H]corticosterone was also significantly reduced in the hypothalamus and cerebellum in the presence of 300 μm, but not 30 μm, of unlabeled cortisol. Interestingly, these data suggest that an influx mechanism is in place in the brain, which may transport both [3H]cortisol and [3H]corticosterone. Although an influx mechanism is present, it must have a relatively large Km (greater than the physiological concentrations of GCs), because a quite large concentration of either cortisol or corticosterone (>30 μm) is needed to detect any transporter activity. Furthermore it makes only a relatively small contribution to the overall brain distribution of these GCs. Possibly, the decrease in the distribution of [3H]corticosterone in the hypothalamus could also indicate saturation of the mineralocorticoid receptor (MR) and GR, present in this brain regions, because both labeled and unlabeled forms of corticosterone would bind to these receptors. Because the unlabeled corticosterone is present in an extremely high concentration, it is likely that the overall concentration of [3H]corticosterone that could be detected in these regions would be reduced. The 30 μm concentration of unlabeled cortisol or corticosterone was previously used to saturate binding of GR in vitro (28–30) but does not guarantee binding of saturation sites in situ. However, it can reasonably be assumed that the 300 μm would ensure total saturation of the MR and GR in these regions, as well as transporters, that are present. The brain/choroid plexus perfusion method used in this present study is designed to detect transport, thus the likelihood is that this decrease that is seen in the distribution of [3H]cortisol and [3H]corticosterone in the presence of unlabeled GCs is indicative of influx. Because this influx mechanism is present in the hypothalamus, it may reveal a very interesting facilitative mechanism in HPA axis regulation, most important in humans, because they produce both corticosterone and cortisol, with the plasma concentration of cortisol 10–20 times higher than the concentration of corticosterone (38).
Interestingly, the distribution of [3H]cortisol in the hypothalamus and cerebellum was actually increased in the presence of 300 μm of unlabeled corticosterone, but the distribution was not changed in any other brain region. This increase indicated the presence of an efflux mechanism and the identification of an efflux mechanism in the hypothalamus is consistent with the previously published multiple time-point perfusion data that identified efflux by P-gp in this region (13), although it cannot be confirmed that the mechanism seen in these cross-competition studies is P-gp. Our previous multiple time-point perfusion data obtained in mice did not detect efflux by P-gp in the cerebellum (13), which suggests that 1) another efflux transporter that can efflux [3H]cortisol may be present in this region, 2) P-gp is present in low levels in the cerebellum, or 3) P-gp has a very minor role in the accumulation of [3H]cortisol. The most interesting is that the [3H]cortisol self-inhibition studies conducted did not identify any efflux of [3H]cortisol in the presence of an excess of unlabeled cortisol. This could be due to differences in the affinity of this transporter for cortisol and corticosterone and /or suggests that there is inhibition of a bidirectional transporter or indicates the presence of separate influx and efflux transporters. Potentially, this difference could implicate P-gp, because corticosterone has been identified as a suspected inhibitor of P-gp, rather than a substrate (39). If corticosterone is in fact inhibiting P-gp, this could explain why an increase in [3H]cortisol accumulation could be seen with the combination of cortisol and corticosterone and not with cortisol alone. If what is seen here is actually inhibition of P-gp, it would also suggest that P-gp does not have a major role in the CNS accumulation of [3H]cortisol, because no efflux was detected with any other GC combination or in any other brain region under investigation, supporting the hypothesis that although P-gp can transport [3H]cortisol, P-gp does not have a major regulatory influence on the entry of [3H]cortisol or [3H]corticosterone to the brain, as explored in our previous work (13).
Influx transport at the BCSFB and in a nonbarrier region
A much more robust effect of the influx transport of [3H]cortisol and [3H]corticosterone was seen in the choroid plexus, the site of the BCSFB, and the pituitary gland, a CNS region that is not controlled by a blood-CNS barrier. The distribution of [3H]cortisol and the distribution of [3H]corticosterone in both these regions was reduced in the presence of both concentrations of unlabeled GCs in almost all the combinations. This would indicate that an influx mechanism is present in these regions, and it is interesting that this influx mechanism can affect the accumulation of [3H]GCs in these areas, because the lipophilicity of these molecules and the presence of fewer tight junctions (in choroid plexus) and fenestrated capillaries (in the choroid plexus and in the pituitary gland) would allow more free movement than in BBB-regulated regions. The presence of an influx mechanism at the pituitary gland is particularly interesting, because it could indicate a small facilitative mechanism in HPA axis regulation. This finding, coupled with the lack of an effect of P-gp detected in the pituitary gland, would lend weight to the idea that the pituitary gland is an extremely important point of regulation for the HPA axis.
The contribution of oatp2
The addition of digoxin, an inhibitor of oatp2, does suggest that oatp2 is the transport mechanism responsible for the differences in [3H]GC uptake in the choroid plexus and pituitary gland. The presence of digoxin was also able to reduce the distribution of [3H]cortisol in the cerebellum, which suggests that oatp2 may also play a role in the access of [3H]cortisol to this brain region. Previous research has indicated interactions of steroid hormones with oat/oatp transporters in rat and bovine cells (16, 33, 40, 41), of which GRs are classed, although this is likely the first study to show that these transporters may influence the access of GRs to murine CNS regions. However, not all of the transport mechanisms that were detected in the studies can be identified as mediated by oatp2. The broadly selective inhibitor probenecid used by Steffgen et al. (41) may have effects on the oat, oatp, or multidrug resistance associated protein transporter families, also making these transporters candidates for those mechanisms seen here. Furthermore, GRs have been shown to modulate or inhibit the transport of known substrates of glucose transporters in rodent and human cells (9–12) and some OCT transporters in rat and human cells (42, 43).
GR access to the CNS
We have identified region-specific transport mechanisms for influx and efflux of GCs. The noted influences of transporters on this movement are suggestive that some of the accumulation of [3H]cortisol and [3H]corticosterone is mediated by saturable transporters. However, much of this evidence was found after the use of high concentrations of unlabeled drugs, likely indicating that these interactions have a minimal physiological function. Nevertheless, the interactions found between cortisol and corticosterone are very interesting, and although it is difficult to interpret these implications for humans, because mice do not produce cortisol, it could be an illustration of some of the mechanisms that are in place to regulate the HPA axis in times of stress. It is very interesting that the hypothalamus, a key region that controls the function of the HPA axis, has been shown to have saturable influx mechanisms for both cortisol and corticosterone and a corticosterone-sensitive efflux mechanism for cortisol. As previously stated, the majority of these identified mechanisms required a concentration of GC that far exceeds the physiological concentration of GCs in either mice or humans. However, these data indicate that the presence of both hormones and subsequent interactions with transporters can produce minor changes in the accumulation of GCs in CNS structures.
Overall, these data suggest that the majority of cortisol and corticosterone present in the plasma diffuse into the CNS and that transporters do not play a major role in the accumulation of these GCs in the brain. It is a necessity of the in situ brain/choroid plexus perfusion that the concentration in the plasma is delivered at a steady state. However, it is a limitation of this study that all of our perfusion experiments were conducted at a single concentration, and experiments investigating the accumulation of corticosterone, as well as cortisol, at differing concentrations would yield valuable data. In an in vivo system, the presence of corticosterone binding globulin in the plasma and the action of 11β-hydroxysteroid dehydrogenases in the brain (44) will affect the concentrations of free GCs available to bind to MR and GR in the CNS. In addition, the variability that we found with the accumulation of corticosterone may be due to the pulsatile release of corticosterone, which is released in an ultradian rhythm (45). This would mean that the concentration of corticosterone already present in the brains of these mice will be varied dependent on the time at which the perfusion was performed and thus the availability of the, mostly, GR changing the radioactive signal able to be detected in those regions. Washout studies would also provide clarification to the question of retention of GCs in these tissues, but it is unlikely that the retention times of GCs by those tissues highly expressing MR or GR would differ from those tissues that do not. As plasma corticosterone levels in humans are only 5% of the circulating cortisol concentrations, but the concentrations of corticosterone in brain are 30% of brain cortisol (26), this would indicate that there is differential entry of these hormones, possibly by some of the mechanisms identified here, and changes in their accumulation could be an underlying problem in HPA axis regulation in humans during stress (46).
In conclusion, P-gp does not majorly regulate the access of [3H]cortisol and [3H]corticosterone to the choroid plexus or pituitary gland, although the combination of high concentrations of cortisol and corticosterone in the blood may change the access of GCs to the brain. The transporter oatp2 seems to have some role in the influx of [3H]cortisol and [3H]corticosterone to the choroid plexus, and the pituitary gland and other transporters, unlikely to be oatp2, may play a very minor role in the access of [3H]cortisol and [3H]corticosterone to the brain, through both the BBB and BCSFB, as well as having a significant effect on [3H]GC accumulation in a nonbarrier region.
Acknowledgments
This work was supported by a United Kingdom Medical Research Council Clinician Scientist Fellowship G108/603 (to C.M.P.) and by The Wellcome Trust Multiuser Equipment Grant 080268 (to S.A.T.). Elements of this work have been presented by B.L.M. at the 2007 Society for Neuroscience Meeting.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BBB
- Blood-brain barrier
- BCSFB
- blood-CSF barrier
- CSF
- cerebrospinal fluid
- dpm
- disintegrations per minute
- GC
- glucocorticoid
- GR
- glucocorticoid receptor
- HPA
- hypothalamic-pituitary-adrenal
- Km
- Michaelis-Menten constant
- MR
- mineralocorticoid receptor
- oatp
- organic anion transporter polypeptide
- P-gp
- P-glycoprotein.
References
- 1. Pariante CM, Lightman SL. 2008. The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468 [DOI] [PubMed] [Google Scholar]
- 2. Holsboer F. 2000. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501 [DOI] [PubMed] [Google Scholar]
- 3. Snart RS, Wilson MJ. 1967. Uptake of steroid hormones into artificial phospolipid/cholesterol membranes. Nature 215:964. [DOI] [PubMed] [Google Scholar]
- 4. Giorgi EP, Stein WD. 1981. The transport of steroids into animal cells in culture. Endocrinology 108:688–697 [DOI] [PubMed] [Google Scholar]
- 5. Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C, Ghersi-Egea JF. 2008. Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol 510:497–507 [DOI] [PubMed] [Google Scholar]
- 6. Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. 2008. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardridge WM, Mietus LJ. 1979. Transport of steroid hormones through the rat blood-brain barrier. J Clin Invest 64:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy BE, Cosgrove JB, McIlquham MC, Pattee CJ. 1967. Adrenal corticoid levels in human cerebrospinal fluid. Can Med Assoc J 97:13–17 [PMC free article] [PubMed] [Google Scholar]
- 9. Lacko L, Wittke B, Geck P. 1975. Interaction of steroids with the transport system of glucose in human erythrocytes. J Cell Physiol 86:673–680 [DOI] [PubMed] [Google Scholar]
- 10. Horner HC, Packan DR, Sapolsky RM. 1990. Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology 52:57–64 [DOI] [PubMed] [Google Scholar]
- 11. Virgin CE, Jr, Ha TP, Packan DR, Tombaugh GC, Yang SH, Horner HC, Sapolsky RM. 1991. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem 57:1422–1428 [DOI] [PubMed] [Google Scholar]
- 12. de Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish S, Golomb J, Volkow N, Daisley K, Orentreich N, McEwen B. 1997. Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer's disease. J Clin Endocrinol Metab 82:3251–3259 [DOI] [PubMed] [Google Scholar]
- 13. Mason BL, Pariante CM, Thomas SA. 2008. A revised role for p-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild type and abcb1a/b-deficient mice. Endocrinology 149:5244–5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yau JLW, Noble J, Thomas S, Kerwin R, Morgan PE, Lightman S, Seckl JR, Pariante CM. 2007. The antidepressant desipramine requires the ABCB (mdr1)-type p-glycoprotein to upregulate the glucocorticoid receptor in mice. Neuropsychopharmacology 32:1–10 [DOI] [PubMed] [Google Scholar]
- 15. Müller MB, Keck ME, Binder EB, Kresse AE, Hagenmeyer TP, Landgraf R, Holsboer F, Uhr M. 2003. ABCB1 (MDR1)-type p-glycoproteins at the blood-brain barrier modulate the activity of the hypothalamic-pituitary-adrenocortical system: implications for affective disorder. Neuropsychopharmacology 28:1991–1999 [DOI] [PubMed] [Google Scholar]
- 16. Bossuyt X, Müller M, Hagenbuch B, Meier PJ. 1996. Polyspecific drug and steroid clearance by an organic anion transporter of mammalian liver. J Pharmacol Exp Ther 276:891–896 [PubMed] [Google Scholar]
- 17. Béery E, Middel P, Bahn A, Willenberg HS, Hagos Y, Koepsell H, Bornstein SR, Müller GA, Burckhardt G, Steffgen J. 2003. Molecular evidence of organic ion transporters in the rat adrenal cortex with adrenocorticotropin-regulated zonal expression. Endocrinology 144:4519–4526 [DOI] [PubMed] [Google Scholar]
- 18. Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. 2000. Organic anion-transporting polypeptides mediate transport of opioid peptides across the blood-brain barrier. J Pharmacol Exp Ther 294:73–79 [PubMed] [Google Scholar]
- 19. Gao B, Meier PJ. 2001. Organic anion transport across the choroid plexus. Microsc Res Tech 52:60–64 [DOI] [PubMed] [Google Scholar]
- 20. Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H, Yawo H. 1998. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem 273:22395–22401 [DOI] [PubMed] [Google Scholar]
- 21. van Montfoort JE, Schmid TE, Adler ID, Meier PJ, Hagenbuch B. 2002. Functional characterization of the mouse organic-anion-transporting polypeptide 2. Biochem Biophys Acta 1564:183–188 [DOI] [PubMed] [Google Scholar]
- 22. Ohtsuki S, Takizawa T, Takanaga H, Hori S, Hosoya K, Terasaki T. 2004. Localization of organic anion transporting polypeptide 3 (oatp3) in mouse brain parachymal and capillary endothelial cells. J Neurochem 90:743–749 [DOI] [PubMed] [Google Scholar]
- 23. Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Declèves X. 2007. Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res 1134:1–11 [DOI] [PubMed] [Google Scholar]
- 24. Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. 1999. Choroid plexus epithelial expression of MDR1 P-glycoprotein and multidrug resistance-associated preotein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA 96:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER. 2001. Multidrug resistance p-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142:2686–2694 [DOI] [PubMed] [Google Scholar]
- 26. Uhr M, Holsboer F, Müller MB. 2002. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b p-glycoproteins. J Neuroendocrinol 14:753–759 [DOI] [PubMed] [Google Scholar]
- 27. Sanderson L, Khan A, Thomas S. 2007. Distribution of suramin, an antitrypanosomal drug, across the blood-brain and blood-cerebrospinal fluid interfaces in wild-type and P-glycoprotein transporter-deficient mice. Antimicrob Agents Chemother 51:3136–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pariante CM, Kim RB, Makoff A, Kerwin RW. 2003. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulation membrane steroid transporters. Br J Pharmacol 139:1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pariante CM, Hye A, Williamson R, Makoff A, Lovestone S, Kerwin RW. 2003. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology 28:1553–1561 [DOI] [PubMed] [Google Scholar]
- 30. Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. 2004. Do antidepressants regulate how cortisol affects the brain? 2003 Curt Richter award paper. Psychoneuroendocrinology 29:423–447 [DOI] [PubMed] [Google Scholar]
- 31. Ito S, Koren G, Harper PA, Silverman M. 1993. Energy-dependent transport of digoxin across renal tubular cell monolayers (LLC-PK1). Can J Physiol Pharmacol 71:40–47 [DOI] [PubMed] [Google Scholar]
- 32. Li L, Meier PJ, Ballatori N. 2000. Oatp2 mediates bidirectional organic solute transport: a role for intracellular gluthathione. Mol Pharmacol 58:335–340 [DOI] [PubMed] [Google Scholar]
- 33. Asaba H, Hosoya K, Takanaga H, Ohtsuki S, Tamura E, Takizawa T, Terasaki T. 2000. Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J Neurochem 75:1907–1916 [DOI] [PubMed] [Google Scholar]
- 34. Baehr C, Reichel V, Fricker G. 2006. Choroid plexus epithethial monolayers—a cell culture model from porcine brain. Cerebrospinal Fluid Res 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kassem NA, Deane R, Segal MB, Chen R, Preston JE. 2007. Thyroxine (T4) transfer from CSF to choroid plexus and ventricular brain regions in rabbit: contributory role of P-glycoprotein and organic anion transporting polypeptides. Brain Res 1181:44–50 [DOI] [PubMed] [Google Scholar]
- 36. Ritz V, Marwitz J, Sieder S, Ziemann C, Hirsch-Ernst KI, Quentin I, Steinfelder HJ. 1999. Contribution of mdr1b-type p-glycoprotein to okadaic acid resistance in rat pituitary GH3 cells. Naunyn Schmiedebergs Arch Pharmacol 360:116–121 [DOI] [PubMed] [Google Scholar]
- 37. Tachibana O, Yamashima T, Yamashita J, Takabatake Y. 1994. Immunohistochemical expression of human chorionic gonadotropin and p-glycoprotein in human pituitary glands and craniopharyngiomas. J Neurosurg 80:79–84 [DOI] [PubMed] [Google Scholar]
- 38. Raubenheimer PJ, Young EA, Andrew R, Seckl JR. 2006. The role of corticosterone in hypothalamic-pituitary-adrenal axis feedback. Clin Endocrinol (Oxf) 65:22–26 [DOI] [PubMed] [Google Scholar]
- 39. Orlowski S, Mir LM, Belehradek J, Jr, Garrigos M. 1996. Effects of steroids and verapamil on P-glycoprotein ATPase activity: progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non-exclusive modulators. Biochem J 317:515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. 1999. Molecular cloning and characterization of a multispecific organic anion transporter from rat brain. J Biol Chem 274:13675–13680 [DOI] [PubMed] [Google Scholar]
- 41. Steffgen J, Rohrbach S, Beery E, Ersoy D, Jarry H, Metten M, Bornstein SR, Müller GA, Burckhardt G. 1999. Demonstration of a probenecid inhibitable anion exchanger involved in the release of cortisol and camp and in the uptake of p-amionhippurate in bovine adrenocortical cells. Cell Physiol Biochem 9:72–80 [DOI] [PubMed] [Google Scholar]
- 42. Arndt P, Volk C, Gorboulev V, Budiman T, Popp C, Ulzheimer-Teuber I, Akhoundova A, Koppatz S, Bamberg E, Nagel G, Koepsell H. 2001. Interactions of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. Am J Physiol Renal Physiol 281:F454–F468 [DOI] [PubMed] [Google Scholar]
- 43. Horvath G, Mendes ES, Schmid N, Schmid A, Conner GE, Salathe M, Wanner A. 2007. The effect of corticosteroids on the disposal of long-acting β2 agonists by airway smooth muscle cells. J Allergy Clin Immunol 120:1103–1109 [DOI] [PubMed] [Google Scholar]
- 44. Holmes MC, Seckl JR. 2006. The role of 11β-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol 248:9–14 [DOI] [PubMed] [Google Scholar]
- 45. Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. 2008. The significance of glucocorticoid pulsatility. Eur J Pharmacol 583:255–262 [DOI] [PubMed] [Google Scholar]
- 46. de Kloet ER, Derijk RH, Meijer OC. 2007. Therapy insight: is there an imbalance response of mineralcorticoid and glucocorticoid receptors in depression? Nat Clin Pract Endocrinol Metab 3:168–179 [DOI] [PubMed] [Google Scholar]