Abstract
Recently it has been recognized that the signal recognition particle (SRP) of Escherichia coli represents a specific targeting device for hydrophobic inner membrane proteins. It has remained unclear, however, whether the bacterial SRP functions in concert with SecA, which is required for the translocation of secretory proteins across the inner membrane. Here, we have analyzed a hybrid protein constructed by fusing the signal anchor sequence of an SRP-dependent inner membrane protein (MtlA) to the mature part of an exclusively SecA-requiring secretory protein (OmpA). We show that the signal anchor sequence of MtlA confers the novel properties onto nascent chains of OmpA of being co-translationally recognized and targeted to SecY by SRP. Once targeted to SecY, ribosome-associated nascent chains of the hybrid protein, however, remain untranslocated unless SecA is present. These results indicate that SRP and SecA cooperate in a sequential, non-overlapping manner in the topogenesis of those membrane proteins which, in addition to a signal anchor sequence, harbor a substantial hydrophilic domain to be translocated into the periplasm.
Keywords: membrane proteins/protein targeting/SecA/signal recognition particle (SRP)/trigger factor
Introduction
Protein export by Gram-negative bacteria encompasses events that lead to the localization of cytosolically synthesized proteins to the inner (plasma) and outer membranes and the intervening periplasmic space. Recent evidence suggests that the bacterial signal recognition particle (SRP) and its receptor (SRP receptor, SR) are necessary and sufficient to target α-helical membrane proteins to, and integrate them into, the inner membrane (MacFarlane and Muller, 1995; de Gier et al., 1996; Ulbrandt et al., 1997; Koch et al., 1999). Differently from this pathway, soluble periplasmic and β-pleated outer membrane proteins of Escherichia coli are translocated across the inner membrane with the help of SecA and SecB, a process that is completely independent of SRP/SR (Behrmann et al., 1998; Koch et al., 1999). These translocated periplasmic and outer membrane proteins are herein collectively called secretory proteins, in keeping with the nomenclature established for eukaryotic cells.
The selection of either the SRP- or the SecA/SecB-dependent targeting route is made at the stage of nascent chains emerging from the ribosome (Beck et al., 2000). The bacterial SRP, consisting of a protein termed Ffh or P48 and the 4.5S RNA, selectively recognizes inner membrane proteins via their long hydrophobic transmembrane segments (Valent et al., 1997; de Gier et al., 1998). In contrast, a similar interaction between SRP and the less hydrophobic and shorter cleavable signal sequences of secretory proteins is prevented by the ribosome-associated chaperone trigger factor, which strongly binds to nascent secretory and cytosolic proteins but not to those of inner membrane proteins. Thus trigger factor sequesters secretory proteins from the SRP-mediated targeting pathway and enables them to interact post-translationally with SecA and SecB (Beck et al., 2000). SRP-dependent integration and SecA/SecB-mediated translocation remain mechanistically distinct processes, even at the level of the membrane where they engage different components of the translocon (Houben et al., 2000; Koch and Muller, 2000; Samuelson et al., 2000). There seems to be no overlap between the SRP-dependent and the SecA/B-dependent targeting pathways when comparing polytopic membrane proteins such as mannitol permease (MtlA) with secretory proteins such as OmpA.
However, the correct topogenesis of a subset of membrane proteins, i.e. membrane proteins with large periplasmic domains, seems to require the coordinated action of both SRP/SR and SecA (Valent et al., 1998; Qi and Bernstein, 1999; Scotti et al., 1999). We therefore deliberately wished to construct a hybrid protein that was a dual substrate for SRP and SecA by transferring an SRP recognition sequence onto an otherwise exclusively SecA/SecB-dependent secretory protein and analyze its requirements for targeting and export. We show here that the resulting chimera has acquired the novel property of being targeted co-translationally by SRP/SR to the inner membrane, but remains dependent on SecA/SecB for translocation across this membrane.
Results
At the ribosome, a hybrid protein of MtlA and OmpA is recognized by both Ffh and trigger factor
As model secretory protein, we used the precursor of the outer membrane protein A (pOmpA) of E.coli, which is first translocated across the inner membrane into the periplasm before it assembles in the outer membrane. Translocation requires SecA, SecB and the proton-motive force, and is paralleled by the proteolytic cleavage of its signal sequence. SRP is not involved (Behrmann et al., 1998), most probably because trigger factor prevents the association of SRP with ribosome–nascent chain complexes (RNCs) of pOmpA. As previously described (Beck et al., 2000), the 125 amino acid N-terminus of pOmpA, when still associated with ribosomes following in vitro synthesis, is cross-linked only to trigger factor (Figure 1A, asterisks in lanes 2, 3, 6 and 7). The reason for the doubling of the cross-links is not known but has been found to be characteristic for in vitro synthesized pOmpA (Beck et al., 2000). No binding of pOmpA-125 to Ffh occurs under these conditions (lanes 4 and 8). In contrast, a 189-residue nascent chain of the inner membrane protein mannitol permease (MtlA) cross-links only to Ffh (x in lanes 14 and 16) due to the presence of a hydrophobic signal anchor sequence. Recognition of MtlA RNCs by trigger factor, if any, is negligible (lanes 11 and 15).
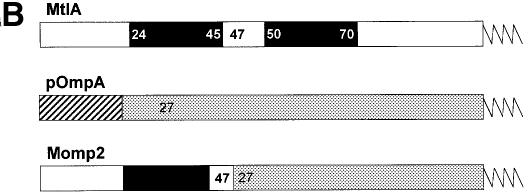
Fig. 1. Nascent chains of Momp2, a hybrid between the polytopic membrane protein MtlA and the secretory protein pOmpA, bind to both Ffh and trigger factor. (A) pOmpA, MtlA and Momp2 were synthesized in vitro by coupled transcription–translation of plasmids pDMB, p717MtlA-B and pMomp2, respectively. The addition of appropriate antisense oligodeoxynucleotides gave rise to a 125 amino acid N-terminal fragment of pOmpA (pOmpA-125), a 189 amino acid fragment of MtlA (MtlA-189) and a 146 amino acid fragment of Momp2 (Momp2-146). Co-synthesis of some full-length products (MtlA, OmpA and Momp2) was observed under these conditions. [35S]methionine-labeled translation products were precipitated with trichloroacetic acid (TCA), separated by SDS–PAGE (7–17% acrylamide) and visualized by phosphoimaging. Where indicated, Ffh (2 ng/µl) was present during synthesis. Cross-linking with DSS was followed by centrifugation through a sucrose cushion in a TLA-100.2 Beckmann rotor at 70 000 r.p.m. for 60 min at 4°C (Behrmann et al., 1998; Beck et al., 2000) and immunprecipitation (IP) using anti-Ffh and anti-trigger factor (TIG) antibodies. Molecular masses are indicated to the right. Cross-linking products with trigger factor (*) and Ffh (x) are indicated. (B) Construction of the hybrid protein Momp2 from MtlA and OmpA. For experimental details, see Materials and methods. Black boxes represent predicted transmembrane segments of MtlA (Sugiyama et al., 1991), with the preceeding and following amino acids each indicated in white. Numbering starts with the first N-terminal amino acid, with that of MtlA sequences given in bold. Only an arbitrarily long N-terminal part of each protein is drawn. The signal sequence of pOmpA is depicted as a hatched area, and the mature sequence as a gray box.
The question arose as to how the cross-linking pattern would change if the first signal anchor sequence of MtlA, which has been identified as a recognition site for Ffh (Beck et al., 2000), was transferred onto OmpA, replacing its authentic cleavable signal sequence. The construction was performed (Figure 1B) such that the first 26 amino acids of pOmpA, including the 21 residue signal sequence and five additional amino acids beyond the signal sequence cleavage site, were replaced by the first 47 amino acids of MtlA. This stretch of MtlA encompasses the 24 residue amphipathic N-terminus followed by the first predicted transmembrane helix and two additional amino acids. As the signal sequence of pOmpA is unlikely to be a major recognition site for trigger factor (Valent et al., 1997), it was predicted that RNCs of the resulting hybrid protein called Momp2 would retain the association of nascent pOmpA with trigger factor but also bind to Ffh. This was in fact the case (Figure 1A, lanes 17–24), with an apparent decrease in trigger factor binding if Ffh was present during synthesis (compare lanes 19 and 23).
SecA is sufficient for translocation of Momp2 in the absence of SRP
What would the requirements be for the export of such a hybrid between a membrane and a secretory protein? The expected topography of Momp2 in the membrane was such that it became anchored by its MtlA-derived signal anchor while the OmpA sequence was translocated across the plasma membrane. To test this, we used an in vitro system allowing the independent assay of SecA/SecB and SRP/SR on translocation and integration of proteins into inside-out membrane vesicles (INVs) (Koch et al., 1999).
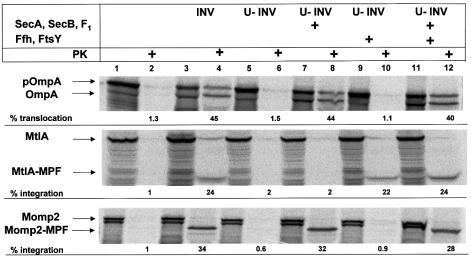
The full-length proteins of pOmpA, MtlA and Momp2 synthesized in vitro in the absence of vesicles were completely susceptible to digestion by proteinase K (Figure 2, lane 2). In contrast, the protease-resistant species obtained in the presence of INVs (lane 4) indicated the known translocation and signal sequence cleavage of pOmpA, the lipid integration of the N-terminal half of MtlA [MtlA-membrane-protected fragment (MPF)] with its cytosolic domain being digested by proteinase K and, in addition, a translocation of Momp2 into INVs. The reduction in size of the membrane-protected fragment of Momp2 (Momp2-MPF) suggests cleavage of the 23 N-terminal amino acids derived from MtlA preceding its first transmembrane segment (cf. Figure 1B).
Fig. 2. Translocation of Momp2 into membrane vesicles is achieved solely by SecA, SecB and the F1-ATPase. pOmpA, MtlA and Momp2 were synthesized in vitro in the presence of the components indicated at the top (INV, E.coli inside-out inner membrane vesicles; U-INV, urea-extracted INV). SecA (80 ng/µl), SecB (40 ng/µl), F1-ATPase (40 ng/µl), Ffh (2 ng/µl) and FtsY (20 ng/µl) were added as specified. Translation products were either precipitated directly with TCA or only after incubation with 0.5 mg/ml proteinase K (PK) for 20 min at 25°C. Indicated are the positions of the precursor (pOmpA) and the signal sequence-free form of OmpA, of full-length MtlA and its fragment resistant towards proteinase K (MtlA-MPF), and of Momp2 and its fragment resistant towards proteinase K (Momp2-MPF).
As shown previously (Koch et al., 1999), pre-treatment of INVs with 6 M urea (U-INVs) abolishes the translocation and integration activities of the membrane vesicles used (Figure 2, lane 6). Whereas restoration of pOmpA translocation into U-INVs required only purified SecA, SecB and the F1-ATPase, and no SRP/SR proteins (Ffh and FtsY), integration of MtlA displayed the reverse dependence (lanes 7–12). Despite possessing an SRP recognition sequence, Momp2 could not be translocated into U-INVs with the help of Ffh and FtsY. Rather, Momp2 proved to behave exactly like the SecA/SecB-dependent pOmpA, indicating that the correct assembly of Momp2 involving translocation of its major part across the plasma membrane required the soluble Sec proteins and could be achieved exclusively by SecA/SecB when SRP/SR were omitted. We reasoned that in vivo, however, SRP being present during synthesis should result in a binding to nascent chains of Momp2 as documented above by cross-linking.
A secY mutant defective in a functional SecY–SecA interaction allows membrane binding of Momp2 but is impaired in translocation
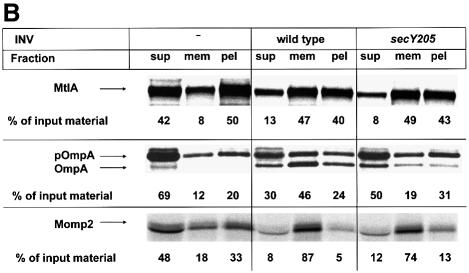
In order to analyze a potential contribution of SRP to the export of Momp2, a different experimental set-up was used. This is depicted in Figure 3, where transport of the three proteins into INVs derived from the secY205 mutant is tested. The secY205 mutation was shown previously (Matsumoto et al., 1997) to impair protein export by interfering with the productive interaction of SecA with SecY, which is the major component of the translocon in the inner E.coli membrane. Consequently, translocation of the SecA-dependent pOmpA and Momp2 into secY205 INVs was found to be diminished drastically compared with wild-type vesicles, whereas the SRP-mediated integration of MtlA, which requires no assistance from SecA, was not affected (Figure 3A, compare lanes 4 and 6). For MtlA, independence of SecA is also reflected by its co-sedimentation with both wild-type and secY205 vesicles. This became visible (Figure 3B) when translation products were separated by sucrose gradient centrifugation to enrich the membrane fraction (mem) from soluble (sup) and pelletable (pel) material. In contrast to MtlA, the major part of OmpA, when synthesized in the presence of secY205 INVs, was recovered from the supernatant fraction due to a defective targeting by SecA to the mutant SecY. In this assay, Momp2 now turned out to behave like a SecA-independent inner membrane protein because it displayed unimpaired binding to the secY205 mutant vesicles.

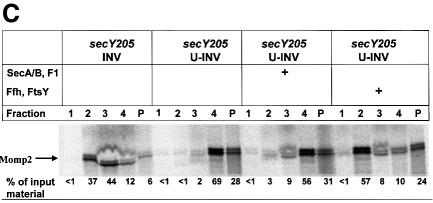
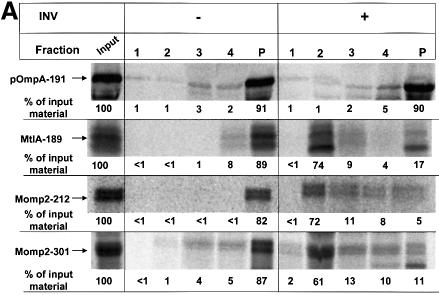
Fig. 3. Translocation of Momp2 requires a functional SecY–SecA interaction but not its membrane association. (A) pOmpA, MtlA and Momp2 were synthesized in vitro in the presence of INV buffer, wild-type INVs or secY205 INVs and the endogenous amounts of SecA, SecB, F1-ATPase, Ffh and FtsY. The percentage of translocation and integration was calculated by quantitation of the radioactivity measured in individual protein bands using phosphoimaging. The indicated percentage of OmpA translocation equals the ratio between radioactivity in the bands of pOmpA and OmpA after and before proteolytic digestion. The percentage of MtlA integration was calculated by the ratio between MtlA-MPF and MtlA, corrected for the loss of [35S]methionine residues during cleavage by proteinase K. The translocation of Momp2 equals the ratio between Momp2-MPF and Momp2. (B) As in (A), but membrane association was analyzed by subfractionation of the translation products on a two-step sucrose gradient. Radioactivity of the three subfractions was quantified and the sum set at 100%. (C) Flotation analyses of full-length Momp2 in the presence of secY205 INVs or secY205 U-INVs. In vitro synthesis of Momp2 was performed in the presence of the indicated components (see Figure 2 for the concentrations used) and the reaction mixture was subsequently separated by a flotation gradient centrifugation. Following centrifugation, 4 × 100 µl fractions were withdrawn from the top of the gradient and, after precipitation with TCA, separated by 15% SDS–PAGE. The pellet fraction was dissolved directly in loading buffer. Fractions 2 and 3 correspond to the membrane fraction.
To show that binding of Momp2 to secY205 INVs reflected a functional interaction that was in fact due to a SecA-independent event, restoration of targeting to urea-extracted secY205 vesicles was examined. As for the two-step sucrose gradient described above, >80% of full-length Momp2 could also be recovered from the secY205 INVs by flotation gradient centrifugation (fractions 2 and 3, representing the membrane fractions of such a gradient) (Figure 3C). On the contrary, urea-treated secY205 INVs did not support membrane association of full-length Momp2 because >90% of Momp2 was recovered from the bottom fractions of the gradient (fractions 4 and P). Association of Momp2 with secY205 U-INVs, however, was almost completely restored by Ffh and FtsY, but not by SecA, SecB and F1-ATPase.
Co-translational targeting of Momp2 nascent chains to the SecYEG translocon
The findings described above suggested that co-sedimentation of Momp2 with secY mutant INVs that are incapable of interacting with SecA was the result of an SRP/SR-dependent targeting of Momp2 to SecY. This is demonstrated directly in Figure 4A. As previously shown, elongation-arrested chains of the inner membrane protein MtlA can be cross-linked to SecY of INVs using the membrane-permeable cross-linker DSS (lane 4, arrowhead). Ribosome-associated Momp2 chains of three different lengths were all found in immediate proximity to SecY when incubated with INVs (lanes 8, 12 and 16), the intensity of the SecY adducts approximately reflecting the degree of synthesis of the nascent chains. As detailed further in Figure 4B, the cross-link between Momp2 nascent chains and SecY was maintained only as long as Momp2 was still bound to the ribosome, indicating a true co-translational targeting.
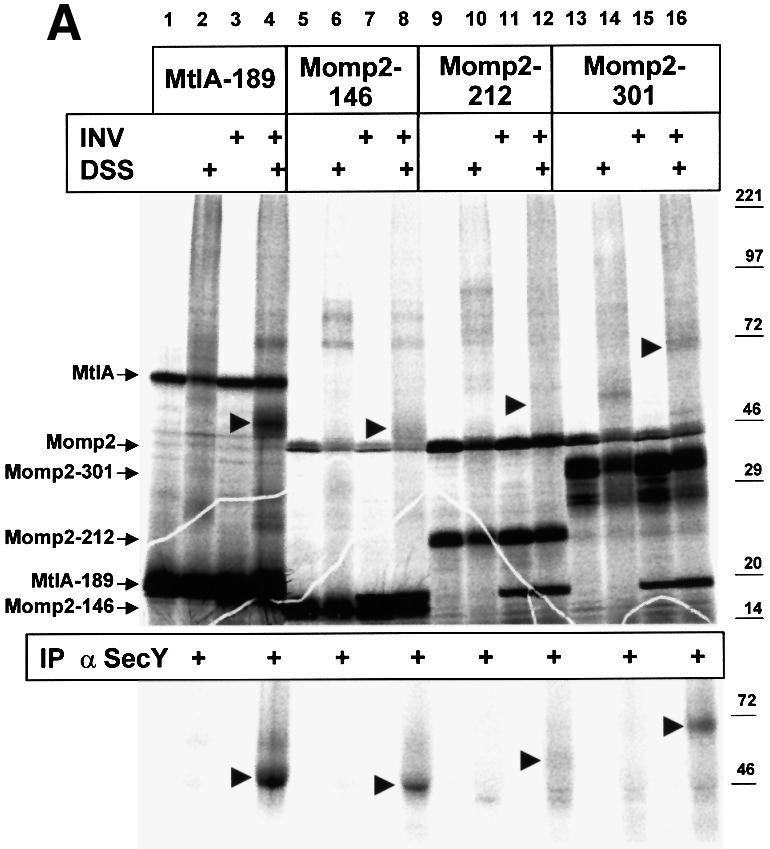
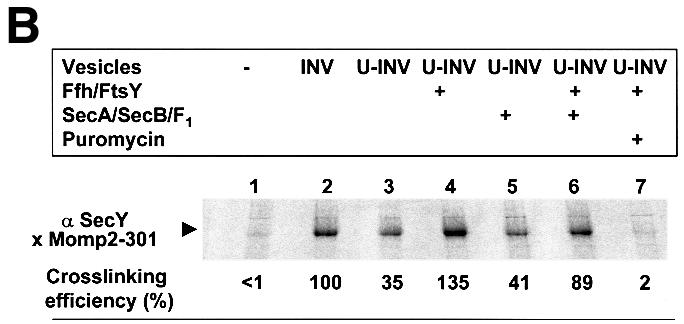
Fig. 4. Co-translational targeting of Momp2 RNCs to SecY, which is mediated by SRP. (A) Nascent chains of MtlA and Momp2 of the indicated lengths were synthesized in vitro (cf. Figure 1) in the presence of the endogenous amounts of SecA, SecB, F1-ATPase, Ffh and FtsY. When indicated, inside-out inner membrane vesicles (INVs) were present during synthesis and samples were treated post-translationally with DSS. Immunoprecipitation of the samples in the even-numbered lanes using anti-SecY polyclonal antibodies is shown below. (B) RNCs of Momp2-301 were synthesized in vitro in the presence of the indicated components. Nascent chains in lane 7 were treated with 0.8 mM puromycin for 10 min at 37°C before immunoprecipitation.
As expected, co-translational targeting of Momp2 to SecY of INVs varied with the amount of SRP/SR present. Figure 4B illustrates that the amount of co-immunoprecipitated Momp2–SecY adduct (lane 2) decreased upon pre-treatment of INVs by urea to remove membrane-bound SRP/SR and SecA (lane 3). Surprisingly, it was not abolished under these conditions, suggesting an SRP/SR-independent targeting of Momp2 RNCs to SecY much like that previously described for eukaryotic pre-prolactin RNCs and Sec61α (Neuhof et al., 1998). Beyond this basal level, binding of Momp2 RNCs to SecY could be stimulated only by Ffh and FtsY, but not by SecA/SecB (lanes 3–6). Importantly, no Momp2–SecY adduct was obtained after dissociating RNCs by puromycin (lane 7), proving the co-translational nature of this targeting event.
Characterization of SRP-dependent targeting and SecA-dependent translocation as distinct steps in the translocation of Momp2
The data shown so far suggest that the topogenesis of Momp2 requires the coordinated action of both the SRP components and SecA, with SRP presumably involved specifically in targeting whereas SecA is probably required for translocating the OmpA domain across the membrane.
By flotation analyses, we were able to address directly the requirements for the individual steps of Momp2 translocation. As shown in Figure 5A, when synthesized in the absence or in the presence of INVs, ∼90% of RNCs of the secretory protein pOmpA stayed in the bottom fraction (fraction P) of the flotation gradient. This is due to the fact that secretory E.coli proteins are not targeted to the plasma membrane as nascent chains (Behrmann et al., 1998), consistent with their post-translational mode of translocation. For a membrane protein such as MtlA synthesized in the presence of INVs, however, >80% of the nascent chains were recovered from the membrane fraction (fractions 2 and 3) of the gradient. Momp2 RNCs of two different lengths fractionated in this flotation gradient like MtlA, which confirms the data in Figure 4 showing that the targeting of this hybrid protein also occurs in a co-translational manner.
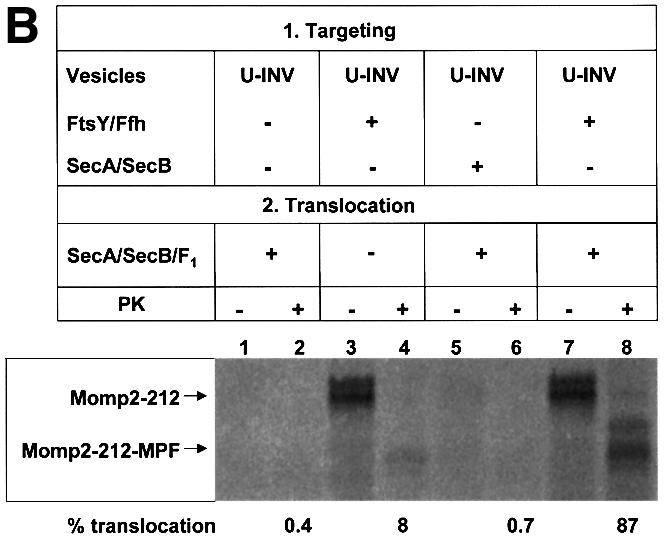
Fig. 5. SRP-dependent targeting and SecA-dependent translocation are distinct steps in the translocation of Momp2 nascent chains. (A) Flotation analyses of ribosome-associated nascent chains (RNCs) of OmpA, MtlA and Momp2 in the absence or presence of membrane vesicles (INV). After in vitro synthesis in the presence of SecA, SecB, Ffh and FtsY, RNCs were incubated with INV buffer or INVs for 15 min at 37°C. For experimental details, see Materials and methods and Figure 3. (B) Momp2 RNCs were synthesized in the presence of urea-treated INVs (U-INV). FtsY, Ffh, SecA and SecB were present during synthesis as indicated. After flotation as specified in (A), the INV-containing fraction 2 of each gradient was withdrawn and incubated for 15 min at 37°C in the presence of puromycin to release the ribosome. SecA, SecB and F1-ATPase were present during this incubation. The reaction mixtures were subsequently split in half; one half was precipitated directly with TCA to identify the membrane-associated nascent chains of Momp2, and the other was first digested with proteinase K (PK) before precipitation with TCA to test for complete translocation.
Although cross-linking had revealed contacts between Momp2 RNCs and SecY, even in the absence of SRP/SR (Figure 4B), these clearly were not of a stable nature as they did not withstand gradient centrifugation (Figure 5B). In the presence of U-INVs, nascent Momp2 chains did not co-fractionate with the vesicles (lane 1, showing material collected from the interface between the 1.25 and 0.25 M sucrose steps). However, if Momp2 RNCs were allowed to bind to U-INVs in the presence of Ffh and FtsY before centrifugation, membrane targeting was restored (lane 3). When gradient-isolated U-INVs bearing SRP/SR-targeted Momp2 RNCs were treated with puromycin to release the Momp2 chains from the ribosomes, only a minor fraction of them became resistant toward proteinase K (lane 4). Obviously SRP-dependent targeting per se did not result in a subsequent translocation into the lumen of the vesicles. For this to occur, SecA/B had to be added during incubation with puromycin, which increased the amount of protease-resistant material to almost 90% (lane 8). If SecA and SecB were present during synthesis of Momp2 in the presence of U-INVs but in the absence of FtsY and Ffh, no stable membrane targeting of Momp2 nascent chains was observed (lane 5). Taken together, these data demonstrate that Momp2 RNCs are first co-translationally targeted to the membrane by SRP/SR before an interaction with SecA is required to allow translocation across the membrane.
Discussion
Recent evidence indicates that in E.coli, largely hydrophobic membrane proteins are recognized co-translationally by SRP. In contrast, nascent chains of secretory proteins are prevented from binding to SRP by trigger factor, allowing a post-translational SecA/SecB-mediated translocation across the cytoplasmic membrane (Beck et al., 2000). We show here that the signal anchor sequence of an inner membrane protein (MtlA) confers recognition by SRP onto the nascent chains of a secretory protein (pOmpA), which otherwise are recognized only by trigger factor. These results demonstrate that for the engagement of the SRP pathway, a single, highly hydrophobic transmembrane domain is sufficient, rather than a combination of structural features, as has been proposed previously (Newitt et al., 1999). This is in full agreement with previous results demonstrating that the signal anchor sequence is the primary recognition site for SRP (Valent et al., 1997; de Gier et al., 1998; Beck et al., 2000). In addition to being recognized by SRP, RNCs of the resulting hybrid protein Momp2 also interact with trigger factor. Because trigger factor was found not to bind to MtlA sequences (Beck et al., 2000), its recognition of Momp2 RNCs must involve the mature part of OmpA. Consistent with this conclusion, trigger factor also binds to cytosolic proteins, and a cleavable signal sequence was ruled out as a major docking site for trigger factor (Valent et al., 1997). Momp2, therefore, is a dual substrate for SRP and trigger factor whose RNCs interact precisely as predicted from the previous analyses of RNCs of bona fide membrane and secretory proteins.
SRP bound to Momp2 RNCs turned out to be the dominating determinant for the subsequent targeting steps. First, binding of trigger factor becomes weaker in the presence of Ffh and FtsY. Secondly, by interacting with Ffh, Momp2 RNCs acquired the ability to be targeted co-translationally to the SecYE translocon, a property that pOmpA RNCs do not possess (Behrmann et al., 1998; this study). The SRP-dependent co-translational targeting of Momp2 is indicated by three lines of evidence: (i) in contrast to pOmpA, in vitro synthesized full-length Momp2 protein associates with secY205 mutant vesicles in an SRP-dependent fashion, although the muta tion abolishes a functional SecA–SecY interaction; (ii) Momp2 RNCs cross-link to SecY and this interaction depends absolutely on the presence of ribosomes and is stimulated by Ffh/FtsY but not by SecA/SecB; and (iii) in contrast to pOmpA, nascent chains of Momp2 stably associate with membrane vesicles so that the membrane-targeted RNCs can be isolated by flotation centrifugation, and again this binding of Momp2 RNCs to membrane vesicles is strictly dependent on Ffh and FtsY. These results clearly indicate that the bacterial SRP, much like its eukaryotic counterpart, functions as a co-translational targeting device rather than as an unspecific chaperone as previously suggested (Hartl and Wiedmann, 1993; Duong et al., 1997). Although we show here that in the absence of SRP/SR, SecA/SecB are sufficient to translocate Momp2 into INVs, the targeting function of SecA/SecB appears to be a strict post-translational event, as membrane association of Momp2 RNC can only be achieved by SRP/SR but not by SecA/SecB. The strong binding of trigger factor to Momp2 RNC in the absence of SRP presumably prevents a SecA interaction at the ribosome, much as has been demonstrated for OmpA RNC (Beck et al., 2000). Collectively, our results suggest that due to the dominant role of SRP in recognizing signal anchor-type sequences at the ribosome, proteins like Momp2 are also targeted co-translationally to the SecYE translocon in vivo, although this has not yet been demonstrated directly.
Although Ffh and FtsY thus play a dominant role in the targeting of Momp2 RNCs to the SecYE translocon, they fail to support the translocation of the periplasmic domain of Momp2, i.e. the OmpA moiety, across the membrane. This step necessitates the function of SecA, which strongly suggests that in vivo, Momp2 would be a dual substrate for both SRP/SR and SecA/SecB. These requirements are clearly different from those encountered at the endoplasmic reticulum (ER) of mammalian cells. There, a secretory protein once targeted to the membrane by means of SRP/SR is believed to be secreted into the lumen of the ER solely by the ongoing polypeptide elongation at the ribosome (Matlack et al., 1998). It remains to be seen why bacteria require SecA as an indispensable motor protein probably for any translocated polypeptide chain.
Our results not only identify individual functions of SRP/SR and SecA/SecB but they also show their concerted action in the topogenesis of dual substrates such as Momp2. The mechanisms of targeting and translocation elucidated here for the in vitro engineered hybrid protein Momp2 are also very likely to reflect the topogenesis of similarly structured proteins occurring in a living E.coli cell. In fact, several membrane-anchored proteins, which like Momp2 are composed of one or even multiple transmembrane domains in addition to extended periplasmic loops, have also been shown to require SecA for insertion into the membrane (Kihara and Ito, 1998; Valent et al., 1998; Qi and Bernstein, 1999; Tian et al., 2000), with SecA probably involved in a late step in the topogenesis of these membrane proteins (Scotti et al., 1999). Here, we directly demonstrate a sequential mode of membrane insertion, involving co-translational targeting by SRP/SR and a subsequent SecA-dependent translocation, which results in a stable, protease-protected insertion into the membrane. The results presented here, therefore, suggest that the question of whether an inner membrane protein of E.coli requires only SRP/SR for integration or additionally SecA/SecB is only a matter of its composition.
Interestingly, whereas Momp2 RNCs can be cross-linked at low efficiency to SecY even in the absence of SRP/SR, this interaction does not withstand centrifugation, indicating that the SRP-independent targeting to SecY does not lead to a functional interaction. On the contrary, Momp2 RNCs targeted to INVs by SRP/SR can be translocated from the bound state following isolation and addition of SecA/SecB. Precedents for an SRP-independent non-functional binding to the translocon of the ER in vitro have in fact been reported (Jungnickel and Rapoport, 1995). This has been attributed to a high ratio of RNCs relative to non-translating ribosomes and a rather large number of accessible translocons in the experimental set-up used (Neuhof et al., 1998; Raden and Gilmore, 1998).
A direct association between ribosomes and SecY has been demonstrated recently in E.coli (Prinz et al., 2000). As in eukaryotic cells, a tight seal between the ribosome and the translocon is most likely required to prevent small molecules from passing through the aqueous SecY channel (Matlack et al., 1998; Johnson and van Waes, 1999). For bacterial membrane proteins depending only on SRP/SR for their proper insertion, it is conceivable that this tight seal, once formed, is maintained during the ongoing polypeptide synthesis, just like in mammalian cells (Crowley et al., 1993, 1994). For membrane proteins like Momp2, which require SecA in addition to the SRP/SR, a tight and stable ribosome–translocon seal is mechanistically difficult to imagine, since the motor activity of SecA requires its insertion and deinsertion into the SecY channel (Economou and Wickner, 1994). It should be emphasized that the experiments shown here demonstrate that SecA is able to translocate Momp2 RNCs after they have been targeted to the membrane in an SRP/SR-dependent manner, i.e. after the ribosome–SecY contact has been formed. Furthermore, the translocation can be accomplished with SecA added from the outside and does not depend on SecA already bound to the translocon prior to the targeting step. Thus, one has to envisage that the ribosome–translocon contact in a bacterial cell is dynamic enough to maintain accessibility of the nascent chain to even large proteins such as SecA.
Based on the data described above, we conclude that the export of Momp2 as an example of membrane-anchored periplasmic E.coli proteins proceeds in three distinct steps (Figure 6). In a first step, the signal anchor sequence of Momp2 is recognized co-translationally by SRP. The subsequent interaction of the SRP–RNC with the SRP receptor (FtsY) leads to a co-translational targeting of the RNC to the SecYE complex (Figure 6B). For these initial steps, SecA is dispensable. SecA is, however, essential for the last step of Momp2 translocation, i.e. for translocating the hydrophilic OmpA moiety accross the membrane.
Fig. 6. Model of the export of Momp2 as an example of membrane-anchored periplasmic E.coli proteins. (A) Ribosome-associated nascent chains of Momp2 are recognized co-translationally by SRP (Ffh and 4.5S RNA) via their signal anchor sequence. For simplicity, the experimentally verified interaction of nascent Momp2 with trigger factor is not depicted. SecA does not associate with Momp2 at this stage. (B) Following interaction between SRP and its receptor (FtsY), nascent Momp2 is targeted co-translationally to SecY. Again, this step does not require SecA. SecA, therefore, is not an indispensable constituent of the SecY translocon. (C) After the initial insertion into the translocon, SecA subsequently binds to Momp2 in order to promote translocation of the hydrophilic OmpA moiety across the membrane. It is not clear whether the presumed direct contact between ribosome and SecY is loosened at this stage and whether SecA binds first to the polypeptide chain or to SecY. SecG, which is not required for the insertion of inner membrane proteins into the SecY translocon (Koch and Müller, 2000), has been omitted despite its unquestioned involvement in the SecA-dependent translocation step.
Materials and methods
Plasmids and construction of Momp2
T7-dependent expression of MtlA and OmpA was performed using the plasmids p717MtlA-B (Beck et al., 2000) and pDMB (Behrmann et al., 1998), respectively. To generate the hybrid protein Momp2, a SpeI cleavage site was first introduced into ompA, changing asparagine to threonine and threonine to serine at amino acid positions 26 and 27 of pOmpA. This was achieved using a two-step PCR method (Dilsiz and Crabbe, 1994) involving the mutagenic primer OmpA66 (5′-CCGAAAGATACTAGTGGTACACTGG-3′; the altered nucleotides representing the SpeI cleavage site are underlined), as well as the OmpA168 primer (5′-CCAGTTGGTTTTCATGGGTC-3′) and a T7 RNA polymerase promoter primer (Promega). The resulting plasmid is pOmpA-Spe. The same strategy was used to introduce a SpeI site into mtlA, leaving threonine at position 47 and changing leucine to serine at position 48 of MtlA. This was done analogously, employing primers MtlA 129 (5′-CCGAACGAGACTAGTGCGAAGCTGG-3′) and MtlA 282 (5′-GCATGTCTGCGCCGACGATAAC-3′). The resulting plasmid p717MtlA-Spe2 was linearized at a single KpnI site located 3′ of mtlA, blunt-ended and subsequently cut with SpeI to remove the mtlA sequences except for the first 47 amino acids. This 3.7 kb fragment was then ligated onto the 1 kb fragment of pOmpA-Spe obtained after cleavage with PstI, blunt-ending and subsequent cleavage with SpeI. The newly constructed plasmid containing the hybrid gene outlined in Figure 1B was termed pMomp2. Sequence analysis confirmed the expected nucleotide sequence of the hybrid gene.
In vitro synthesis and treatments of full-length proteins and RNCs
The composition of the reconstituted transcription/translation system of E.coli and the purification of its components, the preparation of INVs, urea extraction of INVs, the analysis of membrane association by a two-step sucrose gradient and the proteinase protection assay employed in this study have been described previously (Behrmann et al., 1998; Koch et al., 1999). For cross-linking experiments, triethanolamine acetate was replaced by HEPES-NaOH. Synthesis of nascent chains was achieved as described previously (Beck et al., 2000) using the β5-oligo (Behrmann et al., 1998) for pOmpA-125 and Momp2-146; the β8-oligo (Behrmann et al., 1998) for pOmpA-191 and Momp2-212; and the oligonucleotide 5′-GGAGATCAGGTAATCAACAAC-3′ at a concentration of 120 µg/ml for Momp2-301. RNCs of MtlA-189 oligonucleotides were synthesized as described (Beck et al., 2000). For release of the ribosome, puromycin was added to the reaction mixture at a final concentration of 0.8 mM with a further incubation for 15 min at 37°C.
Chemical cross-linking using DSS (Pierce) was performed as described previously (Beck et al., 2000). If not continued by immunoprecipitation, samples were precipitated with 5% TCA and prepared for SDS–PAGE. Immunoprecipitation was performed on 4-fold (and, in the case of anti SecY antibodies, 15-fold) scaled up reactions using polyclonal rabbit antibodies, covalently linked to protein A–Sepharose matrix (Beck et al., 2000).
Flotation analysis of full-length proteins and RNCs
After in vitro synthesis in the presence of Ffh (2 ng/µl), FtsY (20 ng/µl), SecA (80 ng/µl), SecB (40 ng/µl) and F1-ATPase (40 ng/µl), when indicated, membrane binding of full-length proteins and RNCs was assayed by vesicle flotation (Millmann and Andrews, 1999). The reaction mixture was adjusted to 1.6 M sucrose (final volume 100 µl) and overlaid with 200 µl of 1.25 M sucrose and 100 µl of 0.25 M sucrose, each prepared in 50 mM triethanolamine acetate, 8 mM magnesium acetate, 70 mM potassium acetate and 1 mM dithiothreitol (DTT). Following centrifugation in a TLA-100.2 rotor (Beckmann) at 100 000 r.p.m. for 90 min, the gradient was fractionated into 100 µl aliquots, which were subsequently analyzed by SDS–PAGE. For subsequent translocation assays of RNCs, only fraction 2 representing the major membrane fraction was withdrawn and incubated further for 15 min at 37°C in the presence of 0.8 mM puromycin, 2.5 mM ATP, 8 mM creatine phosphate, 40 µg/ml creatine phosphokinase and 2 mM DTT. SecA, SecB and F1-ATPase at the above-mentioned concentrations were present during this incubation where indicated. Half of this reaction mixture was treated subsequently with proteinase K (0.5 mg/ml for 20 min at 25°C) before addition of TCA, while the second half was precipitated directly with 10% TCA.
Acknowledgments
Acknowledgements
We gratefully acknowledge E.Schaffitzel and Drs E.Deuerling and B.Bukau for providing anti-trigger factor antibodies. This work was supported by a grant from the Sonderforschungsbereich 388 and the Fonds der Chemischen Industrie. M.Müller was also supported by a grant from the European Union (QLK3-CT-1999-00917).
References
- Beck K., Wu,L.F., Brunner,J. and Muller,M. (2000) Discrimination between SRP- and SecA/SecB-dependent substrates involves selective recognition of nascent chains by SRP and trigger factor. EMBO J., 19, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M., Koch,H.G., Hengelage,T., Wieseler,B., Hoffschulte,H.K. and Muller,M. (1998) Requirements for the translocation of elongation-arrested, ribosome-associated OmpA across the plasma membrane of Escherichia coli. J. Biol. Chem., 273, 13898–13904. [DOI] [PubMed] [Google Scholar]
- Crowley K.S., Reinhart,G.D. and Johnson,A.E. (1993) The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell, 73, 1101–1115. [DOI] [PubMed] [Google Scholar]
- Crowley K.S., Liao,S., Worrel.,V.E., Reinhart,G.D. and Johnson,A.E. (1994) Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell, 78, 461–471. [DOI] [PubMed] [Google Scholar]
- de Gier J.W., Mansournia,P., Valent,Q.A., Phillips,G.J., Luirink,J. and von Heijne,G. (1996) Assembly of a cytoplasmic membrane protein in Escherichia coli is dependent on the signal recognition particle. FEBS Lett., 399, 307–309. [DOI] [PubMed] [Google Scholar]
- de Gier J.W., Scotti,P.A., Saaf,A., Valent,Q.A., Kuhn,A., Luirink,J. and von Heijne,G. (1998) Differential use of the signal recognition particle translocase targeting pathway for inner membrane protein assembly in Escherichia coli. Proc. Natl Acad. Sci. USA, 95, 14646–14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilsiz N. and Crabbe,M.J.C. (1994) A high yield modification of mutation by overlap extension using three primers. Anal. Biochem., 222, 510–511. [DOI] [PubMed] [Google Scholar]
- Duong F., Eichler,J., Price,A., Leonard,M.R. and Wickner,W. (1997) Biogenesis of the Gram-negative bacterial envelope. Cell, 91, 567–573. [DOI] [PubMed] [Google Scholar]
- Economou A. and Wickner,W. (1994) SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell, 78, 835–843. [DOI] [PubMed] [Google Scholar]
- Hartl F.-U. and Wiedmann, M (1993) A signal recognition particle in Escherichia coli? Curr. Biol., 3, 86–89. [DOI] [PubMed] [Google Scholar]
- Houben E.N.G., Scotti,P.A., Valent,Q.A., Brunner,J., de Gier,J.-W.L., Oudega,B. and Luirink,J. (2000) Nascent Lep inserts into the Escherichia coli inner membrane in the vicinity of YidC, SecY and SecA. FEBS Lett., 476, 229–233. [DOI] [PubMed] [Google Scholar]
- Johnson A.E. and van Waes,M.A. (1999) The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol., 15, 799–842. [DOI] [PubMed] [Google Scholar]
- Jungnickel B. and Rapoport,T.A. (1995) A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell, 82, 261–270. [DOI] [PubMed] [Google Scholar]
- Kihara A. and Ito,K. (1998) Translocation, folding and stability of the HflKC complex with signal anchor topogenic sequences. J. Biol. Chem., 273, 29770–29775. [DOI] [PubMed] [Google Scholar]
- Koch H.G. and Müller,M. (2000) Dissecting the translocase and integrase functions of the Escherichia coli SecYEG translocon. J. Cell Biol., 150, 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H.G., Hengelage,T., Neumann-Haefelin,C., MacFarlane,J., Hoffschulte,H.K., Schimz,K.L., Mechler,B. and Muller,M. (1999) In vitro studies with purified components reveal signal recognition particle (SRP) and SecA/SecB as constituents of two independent protein-targeting pathways of Escherichia coli. Mol. Biol. Cell, 10, 2163–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane J. and Muller,M. (1995) The functional integration of a polytopic membrane protein of Escherichia coli is dependent on the bacterial signal-recognition particle. Eur. J. Biochem., 233, 766–771. [DOI] [PubMed] [Google Scholar]
- Matlack K.E.S., Mothes,W. and Rapoport,T.A. (1998) Protein translocation: tunnel vision. Cell, 92, 381–390. [DOI] [PubMed] [Google Scholar]
- Matsumoto G., Yoshihisa,T. and Ito,K. (1997) SecY and SecA interact to allow SecA insertion and protein translocation across the Escherichia coli plasma membrane. EMBO J., 16, 6384–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millmann J.S. and Andrews,D.W. (1999) A site specific, membrane-dependent cleavage event defines the membrane binding domain of FtsY. J. Biol. Chem., 274, 33227–33234. [DOI] [PubMed] [Google Scholar]
- Neuhof A., Rolls,M.M., Jungnickel,B., Kalies,K.U. and Rapoport,T.A. (1998) Binding of signal recognition particle gives ribosome–nascent chain complexes a competitive advantage in endoplasmic reticulum membrane interaction. Mol. Biol. Cell, 9, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newitt J.A., Ulbrandt,N.D. and Bernstein,H.D. (1999) The structure of multiple polypeptide domains determines the signal recognition particle targeting requirement of Escherichia coli inner membrane proteins. J. Bacteriol., 191, 4561–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz A., Behrens,C., Rapaport,T.A., Hartmann,E. and Kalies,K.U. (2000) Evolutionarily conserved binding of ribosomes to the translocation channel via the large ribosomal RNA. EMBO J., 19, 1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H.Y. and Bernstein,H.D. (1999) SecA is required for the insertion of inner membrane proteins targeted by the Escherichia coli signal recognition particle. J. Biol. Chem., 274, 8993–8997. [DOI] [PubMed] [Google Scholar]
- Raden D. and Gilmore,R. (1998) Signal recognition particle-dependent targeting of ribosomes to the rough endoplasmic reticulum in the absence and presence of the nascent polypeptide associated complex. Mol. Biol. Cell, 8, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J.C., Chen,M., Jiang,F., Möller,I., Wiedmann,M., Kuhn,A., Phillips,G.J. and Dalbey,R.E. (2000) YidC mediates membrane protein insertion in bacteria. Nature, 406, 637–641. [DOI] [PubMed] [Google Scholar]
- Scotti P.A. Valent,Q.A., Manting,E.H., Urbanus,M.L., Driessen,A.J.M., Oudega,B. and Luirink, J (1999) SecA is not required for signal recognition particle-mediated targeting and initial membrane insertion of a nascent inner membrane protein. J. Biol. Chem., 274, 29883–29888. [DOI] [PubMed] [Google Scholar]
- Sugiyama J.E., Mahmoodian,S. and Jacobson,G.R. (1991) Membrane topology analysis of Escherichia coli mannitol permease by using a nested-deletion method to create mtlA–phoA fusions. Proc. Natl Acad. Sci. USA, 88, 9603–9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Boyd,D. and Beckwith,J. (2000) A mutant hunt for defects in membrane protein assembly yields mutations affecting the bacterial signal recognition particle and Sec machinery. Proc. Natl Acad. Sci. USA, 97, 4730–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrandt N.D., Newitt,J.A. and Bernstein,H.D. (1997) The E.coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell, 88, 187–196. [DOI] [PubMed] [Google Scholar]
- Valent Q.A., Degier,J.W.L., von Heijne,G., Kendall,D.A., ten Hagen-Jongman,C.M., Oudega,B. and Luirink,J. (1997) Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol. Microbiol., 25, 53–64. [DOI] [PubMed] [Google Scholar]
- Valent Q.A., Scotti,P.A., High,S., de Gier,J.W., von Heijne,G., Lentzen,G., Wintermeyer,W., Oudega,B. and Luirink,J. (1998) The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J., 17, 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]