Abstract
Objectives
We aimed to investigate the association between BMI and WC and all-cause mortality of Chinese residents in long-term care facilities in Taiwan.
Design
Prospective cohort study.
Setting
Eight long-term care facilities in Taiwan.
Participants
Three hundred and fifty-four residents aged 60 and above (men=156, women=198; median: 78.4 years, range: 60–101years) were recruited during the study period.
Measurements
Anthropometrics and metabolic parameters were measured at baseline. The range of BMI and WC in this study was from 11.6 to 35.3 kg/m2 (mean±SD=21.7±4.2) and from 55.0 to 124.0 cm (mean±SD=82.4±10.9), respectively. Mortality data was from the Department of Health in Taiwan.
Results
During the 5 years of follow-up, there were 219 deaths. After adjusting for age, gender, albumin, Karnofsky performance status scale, hypertension, and diabetes, subjects in the highest quartile of BMI (mean±SD =27.3±2.8 kg/m2) and WC (mean±SD =96.7±7.4cm) had a significantly lower mortality rate than did subjects in the lowest quartile (BMI, mean±SD =16.7±1.7kg/m2; WC, mean±SD =69.6±4.2cm). After further stratification by central obesity status, the subjects in the 2 highest BMI quartiles had a lower mortality rate than those in the lowest BMI quartile, but only in the central obesity group (≥ 90cm in men or ≥ 80cm in women). The adjusted relative risk for all-cause mortality in the highest vs. lowest BMI quartile was 0.17 (95% CI: 0.05–0.57).
Conclusion
BMI and WC were negative predictors for all-cause mortality in Chinese elderly living in long-term care facilities. Subjects with higher WC and BMI had lower all-cause mortality rate.
Keywords: body mass index, waist circumference, elderly, mortality, institutionalized
INTRODUCTION
According to the World Health Organization (WHO), 1.6 billion adults were overweight and at least 400 million adults were obese globally in 20051. Prevalence of obesity increases with the increase in age. In the United States, 73.9 % of men and 68.4 % of women age 60 and above were either overweight or obese (defined as body mass index(BMI) ≥25kg/m2)2. In China, the prevalence of obesity (BMI ≥25kg/m2) among individuals age 20–29 is 21.3%, but it increases to 46.2% among age 60–69. The increased prevalence of obesity represents a great health burden globally.
Many studies have found a U- or a J-shaped association between BMI and mortality among adults3–7. The relationship, however, remains controversial in older populations. Studies found that the influence of obesity on mortality is reduced with increasing age and the optimal weight and waist circumference (WC) for older populations may be higher8–10. In 2007, in a review of 28 studies done by Janssen et al indicated that overweight individuals were not at increased risk for mortality, while obese individuals had only a modest increase in mortality risk11. Moreover, recent studies reported that BMI was actually inversely related to mortality in the older subjects12–13. Baumgartner et al proposed a potential explanation for this paradox, i.e. that total mortality was increased at high body fat mass (FM) and at low fat-free mass (FFM)14–15.
Most studies investigating the relationship between BMI and WC and mortality have been carried out in community-dwelling elderly populations, not in long-term care facilities. The number of people living in long-term care facilities has been rapidly growing in many countries, including Taiwan16. The prevalence of obesity may also increase in long-term care settings. There are only a limited number of studies assessing the relationship between BMI and WC and mortality in residents living in long-term care facilities17–18. Therefore, the aim of this study was to prospectively evaluate the relationship between BMI and all-cause mortality in older Chinese adults living in long-term care facilities in Taiwan. We also used WC (reflecting the degree of central obesity) or calculated resting energy expenditure, REE (reflecting the degree of lean body mass (LBM)) to substitute for BMI in our analyses.
METHODS
Study subjects
The target population was residents living in 8 long-term care facilities which cooperated with the largest tertiary hospital in an urban city in Taiwan during 2002–2003. There were 447 residents living in these 8 facilities during study period. Among these 447 residents, 393 residents aged 60 years old and above were invited to participate in this study. Finally, 354 subjects (men=156, mean age ± SD (standard deviation) = 76.4±7.6 years; women =198, 79.9±7.7 years) agreed to participate in this study were recruited. Deaths were ascertained by computer linkage to the national death registry using ID number. All deaths that occurred between study entry and December 2007 were included. Ethics approval for patient recruitment and data analyses was obtained from the Institutional Review Board.
Anthropometric index
Trained staff measured the weight, height, and WC. WC is taken at the midway point between the inferior margin of the last rib and the iliac crest in a horizontal plane. BMI is calculated as weight (kg) divided by height squared (m2). BMI and WC were divided into quartiles by genders as follows. BMI quartiles I–IV: <18.5, 18.5–20.5, 20.6–23.6, >23.6 kg/m2 in men; <19.4, 19.4–21.4, 21.5–25.0, >25.0 kg/m2 in women; WC quartiles I–IV: <73.6, 73.6–81.0, 81.1–87.4, > 87.4 cm in men; <75.5, 75.5–81.5, 81.6–89.1, > 89.1 cm in women. BMI was also divided into four levels according to the obesity definition using Taiwan19 and WHO for Asians criteria20.
Biomedical markers
Blood pressure (BP) was measured by the same trained staff on the right arm using an appropriately sized cuff and a standard mercury sphygmomanometer in a seated position. A venous blood sample was taken after a 12-hr fast for determination of plasma glucose, albumin, and lipid profile using a biochemical autoanalyzer (Beckman Cou, Fullerton, CA, USA) at the Clinical Laboratory Department of China Medical University Hospital.
Resting energy expenditure (REE)
REE was calculated according to Liu’s predictive equation for Chinese21 as shown below: REE (kcal/day) = 13.88 × (weight in kg) + 4.16 × (height in cm) − 3.43 × (age in year) − 112.4 × (gender) + 54.34, where gender is 0 for men and 1 for women.
REE were divided into quartiles by genders as follow: I–IV: <1078, 1078–1201, 1202–1381, > 1208 kcal/day in men; <835, 836–939, 940–1041, > 1041 kcal/day in women.
Questionnaire, diabetes, hypertension, and Karnofsky performance status scale (KPS)
Each participant completed a structured questionnaire. Medical records were reviewed by physicians. Smoking history was divided into 3 classes as follows: never, former, and current. Diabetes was defined as: (1) fasting glucose ≥ 126 mg/dL and/or (2) diabetes history and on oral hypoglycemic agents or insulin treatment. Hypertension was defined as: (1) systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg and/or (2) hypertension history and on anti-hypertensive drugs. KPS is an assessment tool used to assist physicians and caretakers in measuring a patient’s ability to carry out activities of daily living. KPS runs from 0–100, where 0 is death and 100 is perfect health 22. KPS below 60 was defined as subjects who require some assistance22.
Statistical analysis
Data are presented as the mean (SD) for continuous variables. Log transformation was used for variables with significant deviation from normal distribution, assessed by the Kolmogorov–Smirnov test before further analyses. Analysis of variance (ANOVA) test was used for comparing mean values of continuous variables across BMI quartiles. Student’s t test for unpaired data was used to compare mean values between two groups. Proportions and categorical variables were presented as percentage and tested by the χ2 test and by the two-tailed Fisher’s exact method when appropriate. Cox proportional hazard regression analyses adjusted for potential confounders were used to estimate the relative risks (RRs) for all-cause mortality. Survival curves adjusted for other covariates were drawn for different BMI quartiles23–24. All statistical tests were 2-sided at the 0.05 significance level. These statistical analyses were performed using the PC version of SPSS statistical software (13th version, SPSS Inc., Chicago, IL, USA).
RESULTS
During the 5-years of follow-up, there were 219 deaths. The overall 5-year mortality rate was 208.6 per 1000 person-years. The baseline characteristics of the population sample according to BMI quartiles are listed in Table 1. Compared to the lowest quartile of BMI, the higher quartiles of BMI tended to have greater weight, BMI, REE, WC, fasting glucose, total cholesterol (TCHOL), triglyceride (TG), albumin, prevalence of high KPS and diabetes, but lower age and high-density-lipoprotein cholesterol (HDL-C).
Table 1.
Baseline subjects’ characteristics according to BMI quartiles by genders.
| BMI quartile | P | ||||
|---|---|---|---|---|---|
| Characteristic | I(n=87) | II(n=87) | III(n=92) | IV(n=88) | Value |
| Men [n (%)]* | 38 (43.7) | 39 (44.8) | 40 (43.5) | 39 (44.3) | 0.998 |
| Age (years)† | 79.6±8.2 | 80.0±7.6 | 77.5±7.7 | 76.4±7.4 | 0.006 |
| Height (cm)†, ‡ | 152.4±7.5 | 153.3±7.5 | 152.6±8.7 | 153.4±7.5 | 0.743 |
| Weight (kg)† | 38.8±5.1 | 47.1±4.2 | 52.8±5.9 | 64.3±8.2 | <0.001 |
| BMI (kg/m2, range)† | 16.7±1.7 (M:11.9–18.3; F:11.6–19.3) | 20.0±0.8 (M:18.5–20.5; F:19.4–21.4) | 22.6±1.1 (M:20.6–23.6; F:21.5–25.0) | 27.3±2.8 (M:23.7–34.0; F:25.1–35.3) | <0.001 |
| REE (kcal/day)† | 891±148 | 1010±138 | 1092±172 | 1260±177 | <0.001 |
| WC (cm)† | 71.8±5.9 | 78.7±6.4 | 84.3±6.2 | 94.1±9.6 | <0.001 |
| Systolic BP (mmHg)†, ‡ | 125.0±14.2 | 125.9±14.5 | 124.7±15.1 | 124.1±14.4 | 0.869 |
| Diastolic BP (mmHg)†, ‡ | 75.0±10.4 | 73.6±10.0 | 76.7±11.5 | 73.6±10.2 | 0.177 |
| TCHOL(mg/dL)† | 171.8±39.7 | 164.6±38.7 | 174.0±45.2 | 185.9±43.7 | 0.010 |
| TG (mg/dL)†, ‡ | 83.8±70.3 | 98.2±66.7 | 103.8±49.1 | 164.8±341.3 | <0.001 |
| HDL-C(mg/dL)† | 55.6±16.2 | 52.6±12.8 | 49.9±12.1 | 48.2±12.5 | 0.002 |
| Albumin (g/dL)†,‡ | 3.03±0.46 | 3.16±0.44 | 3.24±0.42 | 3.33±0.44 | <0.001 |
| KPS > 60% [n (%)]*,§ | 9 (10.8) | 13 (16.3) | 19 (22.4) | 28 (32.6) | <0.001 |
| Diabetes [n (%)]* | 15 (17.2) | 23 (26.4) | 32 (34.8) | 42 (47.7) | <0.001 |
| Hypertension [n (%)]* | 44 (50.6) | 46 (52.9) | 57 (62.0) | 57 (64.8) | 0.163 |
| Smoking [n (%)]*,¶ | 0.146 | ||||
| Current | 0(0) | 2 (2.5) | 2 (2.4) | 4 (4.7) | |
| Former | 6 (7.2) | 13 (16.3) | 15 (17.6) | 15 (17.6) | |
| Never | 77 (92.8) | 65 (81.3) | 68 (80.0) | 66 (77.6) | |
Abbreviation: BMI, body mass index; REE, resting energy expenditure; BP, blood pressure; TCHOL, total cholesterol; TG, triglycerides; HDL-C, high-density-lipoprotein cholesterol; KPS, Karnofsky performance status scale.
Pearson chi-square test was used for categorical data. Data were presented with n (%).
ANOVA test was used for comparing mean values of continuous variables between groups. Data were presented as mean ± SD.
Statistics are tested using the log-transformed values.
No.=334.
No.=333.
Table 2 shows the baseline characteristics of the survivors and those who died. The survivors were younger and had greater WC and albumin.
Table 2.
Baseline subjects’ characteristics by survival status and all-cause deaths
| Survivors (n=135) | All-cause deaths (n=219) | P Value | |
|---|---|---|---|
| Men [n (%)]* | 56 (41.5) | 100 (45.7) | 0.442 |
| Age (years)† | 76.8±7.8 | 79.3±7.7 | 0.004 |
| Height (cm)†, ‡ | 153.0±6.9 | 152.8±8.3 | 0.796 |
| Weight (kg)† | 52.2±11.4 | 50.0±10.8 | 0.065 |
| BMI (kg/m2)† | 22.3±4.4 | 21.4±4.1 | 0.055 |
| REE (kcal/day)† | 1086±208 | 1050±207 | 0.120 |
| WC (cm)† | 84.0±12.2 | 81.4±9.9 | 0.038 |
| Systolic BP (mmHg)†, ‡ | 124.5±14.7 | 125.2±14.4 | 0.659 |
| Diastolic BP (mmHg)†, ‡ | 74.2±9.5 | 75.1±11.2 | 0.534 |
| TCHOL(mg/dL)† | 177.0±45.5 | 172.2±40.5 | 0.303 |
| TG (mg/dL)†, ‡ | 108.8±74.9 | 114.8±220.1 | 0.428 |
| HDL-C(mg/dL)† | 51.3±13.3 | 51.7±14.0 | 0.767 |
| Albumin (g/dL)†, ‡ | 3.26±0.43 | 3.15±0.46 | 0.028 |
| KPS > 60% [n (%)]*, ¶ | 27 (21.6) | 42 (20.1) | 0.742 |
| Diabetes [n (%)]* | 38 (28.1) | 74 (33.8) | 0.268 |
| Hypertension [n (%)]* | 78 (57.8) | 126 (57.5) | 0.964 |
| Smoking [n (%)]*, # | 0.992 | ||
| Current | 3 (2.4) | 5 (2.4) | |
| Former | 18 (14.4) | 31 (14.9) | |
| Never | 104 (83.2) | 172 (82.7) |
Abbreviation: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TCHOL, total cholesterol; TG, triglycerides; HDL-C, high-density-lipoprotein cholesterol; REE, resting energy expenditure; KPS, Karnofsky performance status scale.
Pearson chi-square test was used for categorical data. Data were presented with n (%).
Student's t-test for unpaired data was used for the comparison of mean values between groups. Data were presented as mean ± SD.
Statistics are tested using the log-transformed values.
No.=334.
No.=333
Table 3 shows all-cause mortality rate and RRs according to different BMI, REE, and WC quartiles, in unadjusted and adjusted analyses. The subjects in the lowest quartile of BMI, WC, and REE had the highest mortality rate (Table 3). Compared to the lowest quartile of BMI, REE, and WC, using Cox proportional hazard regression analyses, the unadjusted RRs for all-cause mortality were significantly decreased in highest BMI, REE, and WC quartiles (all p values < 0.05, Table 3). Knowing that age, nutritional status (such as albumin), lipid profiles (such as TCHOL, TG, HDL-C), diabetes, hypertension, and performance status (such as KPS) were risk factors for all-cause mortality, we further adjusted for these potential confounders. Compared to the lowest quartile, the adjusted RRs for all-cause mortality were significantly decreased in the highest quartile of BMI, REE, or WC (all p values< 0.05, Table 3). Compared to the lowest quartile of BMI, REE, and WC, the adjusted RRs for the highest quartile of BMI, REE, or WC were 0.62(95% confidence interval [CI]: 0.40,0.96), 0.61(0.38,0.99), or 0.58(0.37,0.91), respectively. We also found that BMI categories using Taiwan and/or WHO for Asians criteria were inversely related to mortality (Table 3).
Table 3.
Unadjusted and adjusted relative risks of all-cause mortality by baseline BMI, WC, and REE quartiles (district models) using the Cox proportional hazards regression analyses.
| Variables, quartile (No.) | Deaths (No.) | 5-year mortality rate per 1000 persons-years | Unadjusted all-cause mortality relative risk (95% CI) | Adjusted all-cause mortality relative risk (95% CI)*, †† |
|---|---|---|---|---|
| BMI_quartiles† | ||||
| I (87) | 58 | 256.6 | 1.00(Reference) | 1.00(Reference) |
| II (87) | 53 | 210.9 | 0.82(0.57–1.19) | 0.73(0.49–1.08) |
| III (92) | 59 | 213.6 | 0.83(0.57–1.19) | 0.79(0.53–1.17) |
| IV (88) | 49 | 165.5 | 0.64(0.44–0.93)** | 0.62(0.40–0.96)** |
| BMI_Asia‡ | ||||
| I (75) | 52 | 274.0 | 1.00(Reference) | 1.00(Reference) |
| II (158) | 101 | 224.8 | 0.82(0.59–1.14) | 0.75(0.53–1.07) |
| III (44) | 22 | 141.3 | 0.51(0.31–0.84)*** | 0.54(0.32–0.92)** |
| IV (77) | 44 | 172.7 | 0.63(0.42–0.94)** | 0.60(0.38–0.95)** |
| BMI_TW§ | ||||
| I (75) | 52 | 274.0 | 1.00(Reference) | 1.00(Reference) |
| II (181) | 114 | 216.6 | 0.79(0.57–1.09) | 0.74(0.53–1.05) |
| III (62) | 35 | 168.5 | 0.61(0.40–0.94)*** | 0.60(0.37–0.96)** |
| IV (36) | 18 | 143.0 | 0.52(0.30–0.89)*** | 0.50(0.28–0.89)** |
| REE¶ | ||||
| I (88) | 61 | 276.1 | 1.00(Reference) | 1.00(Reference) |
| II (89) | 56 | 222.8 | 0.80(0.56–1.16) | 0.78(0.53–1.17) |
| III (89) | 54 | 189.7 | 0.68(0.47–0.98)** | 0.73(0.49–1.10) |
| IV (88) | 48 | 164.1 | 0.59(0.40–0.86)*** | 0.61(0.38–0.99)** |
| WC# | ||||
| I (83) | 57 | 258.9 | 1.00(Reference) | 1.00(Reference) |
| II (88) | 50 | 192.8 | 0.74(0.51–1.09) | 0.69(0.45–1.06) |
| III (83) | 58 | 238.5 | 0.91(0.63–1.32) | 0.93(0.62–1.39) |
| IV (86) | 46 | 159.1 | 0.61(0.42–0.90)** | 0.58(0.37–0.91)** |
Abbreviation: CI, confidence interval; BMI, body mass index; REE, resting energy expenditure; KPS, Karnofsky performance status scale; TCHOL, total cholesterol; TG, triglycerides; HDL-C, high-density-lipoprotein cholesterol.
Analyses adjusting for age, gender, albumin, KPS, TCHOL, TG, HDL-C, hypertension, and diabetes
BMI was divided into quartiles by genders: <18.5, 18.5–20.5, 20.6–23.6, >23.6 kg/m2 in men; <19.4, 19.4–21.4, 21.5–25.0, >25.0 kg/m2 in women
BMI was divided using WHO for Asians criteria as below: I (underweight): <18.5, II (normal weight): 18.5–22.9, III (Overweight): 23.0–24.9, IV (Obesity): ≥ 25.0 kg/m2
BMI was divided using Taiwan’s criteria as below: I (underweight): <18.5, II (normal weight): 18.5–23.9, III (Overweight): 24.0–26.9, IV (Obesity): ≥ 27.0 kg/m2
REE was divided into quartiles by genders: <1078, 1078–1201, 1202–1381, > 1208 kcal/day in men; <835, 836–939, 940–1041, > 1041 kcal/day in women
WC was divided into quartiles by genders: <73.6, 73.6–81.0, 81.1–87.4, > 87.4 cm in men; <75.5, 75.5–81.5, 81.6–89.1, > 89.1 cm in women
P value < 0.05.
P value < 0.01.
No.=334
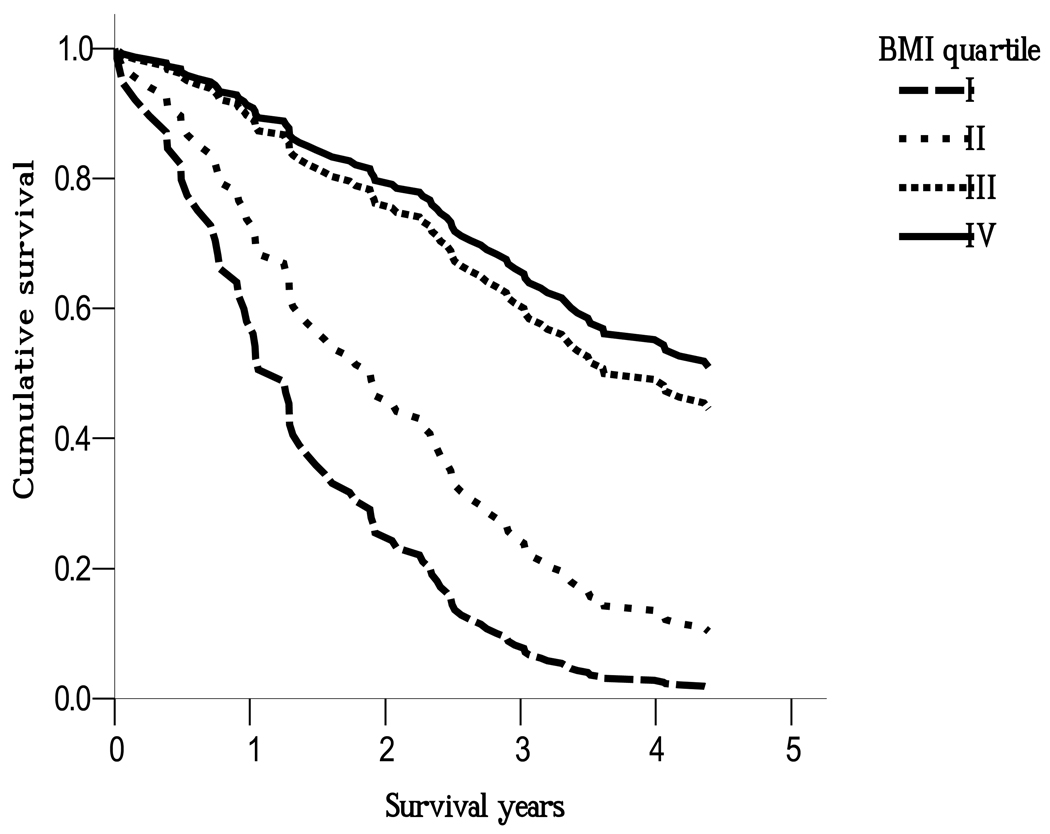
The correlation between BMI and WC was high (Pearson correlation=0.789, p< 0.001) and the interaction between WC and BMI quartiles was significant (p < 0.001), so we further stratified WC into 2 categories according to the central obesity definition of WHO for Asians 20. Figure 1 shows the association between BMI quartiles and mortality in the central obesity group (≥ 90 cm in men or ≥ 80cm in women). Among the central obesity group, compared to subjects in the lowest BMI group (quartile I), the adjusted RRs among subjects in BMI quartiles II, III, IV were 0.56(0.17,1.82), 0.20(0.06,0.65), 0.17(0.05,0.57), respectively. Among the non-central obesity group, the adjusted RRs were 0.59(0.37,0.93), 0.95(0.56,1.60), and 0.93(0.47,1.86), respectively. We also stratified BMI into 2 categories according to the general obesity definition by WHO for Asians (BMI ≥ 25kg/m2 was defined as general obesity). Among the general obesity group, compared to subjects in the lowest WC group (quartile I), the adjusted RRs among subjects in WC quartiles II, III, IV were 2.07(0.15, 28.8), 0.41(0.04,4.59), and 0.35(0.04,3.51), respectively. Among non-general obesity group, the adjusted RRs were 0.60(0.38,0.94), 0.93(0.61,1.42), and 0.58(0.32,1.05), respectively(Figure not shown).
Figure 1.
The association between BMI quartile and risk of all-cause mortality in the central obesity defined by WHO for Asians using the Cox proportional hazard regression analyses after adjusting for age, gender, albumin, Karnofsky performance status scale, total cholesterol, triglycerides, high-density lipoprotein cholesterol, hypertension, and diabetes. As compared to lowest BMI group (quartile I), the adjusted relative risks were 0.56 (95% CI: 0.17, 1.82), 0.20 (0.06, 0.65), 0.17 (0.05, 0.57) among BMI quartile II, III, IV, respectively.
DISCUSSION
We have demonstrated that BMI and WC are inversely related to all-cause mortality in older Chinese adults living in long-term care facilities in Taiwan. We also have found that residents with central obesity and higher BMI have a lower mortality than residents with central obesity and lower BMI. Even after adjusting for potential confounders, the results are similar. As the world is aging and the number of residents living in long-term care facilities is increasing, our findings are important for health care and policy makers affecting the elderly living in long-term care facilities.
The association between BMI and mortality has been identified as U- or J- shape among younger or middle-aged adults 3–4. This association in the elderly, however, is still controversial. A meta-analysis done by Janssen et al found that BMI was inversely associated with mortality risk in elderly Asians 11. Compared to middle-aged adults, previous studies found that the optimal BMI among the elderly shifted upwards 3, 25. The optimal range of BMI proposed among the elderly varied among studies, but ranged from approximately 25–27 kg/m2 in Chinese 3 and 25–30 kg/m2 in Caucasians 25. The results of our study are similar to those of most but not all studies done in Asians 26–28. For example, a study done among Korean elderly 4 found that elderly subjects with underweight but not obesity had higher all-cause mortality compared to subjects with normal weight. However, another study done by Gu, in China, showed that obese (BMI ≥30 kg/m2) elderly subjects had higher total mortality compared to subjects with BMI between 24–24.9 kg/m2 3. Compared to this latter study, the subjects in our study had a lower mean BMI (BMI quartile IV mean(SD) was 27.3(2.8) kg/m2 in our study vs. 32.3(2.6) kg/m2 in the obese group of Gu’s study). Actually, elderly subjects with BMI ≥25 and <30 kg/m2 did not show a significant increase in all-cause mortality compared to subjects with BMI between 24–24.9 kg/m2 in Gu’s study. Age may be also a factor that could explain these differences. Studies done in Caucasians found that, in relatively younger elderly (age from 60 to 75 years), increased BMI was associated with an increase of all-cause mortality, whereas in older elderly (age over 75 years) higher BMI protected from mortality 27. The mean age in our study subjects was 78.4 years and our results are consistent with those of other studies of older elderly 27.
Another aspect represents body composition. BMI and WC are the most common anthropometric indices used for predicting effects of obesity. However, the associations between BMI and body fat and fat distribution were weaker in the elderly than in the younger or middle-aged adults 11, 29 and previous studies found that excess body fat may have less influence on mortality in the elderly than in the younger or middle-aged adults 11. A negative association between LBM and all-cause mortality has been observed in prospective studies 7, 30. We used calculated REE as a surrogate for LBM and also found that REE is negatively associated with all-cause mortality in our population. It is reasonable to speculate that the subjects in the higher BMI quartiles in our study had greater LBM which may have offered some protection against mortality. This supports the hypothesis that the preservation of LBM may have greater importance than excess fatness with regard to survival in older elderly.
Studies on the association between all-cause mortality and WC among the elderly are scanty and controversial. Janssen et al found that WC was a positive predictor of mortality 31, but Price and Heitmann found that WC was not associated with all-cause mortality in older persons 30, 32. Ours, however, may be the first study to show that WC is a negative predictor for all-cause mortality in the elderly. In Figure 1, we also found that high BMI was protective among those with central obesity, but not among those without central obesity. In other words, the association between BMI and mortality was stronger among the centrally obese. There are some possible explanations. First, it is the selective survival theory which states that obese individuals with susceptibility to the adverse effects may have already died at a younger age 11. Thus, elderly individuals with less susceptibility to the adverse effects are left. Second, our finding may be due to differences in population’s race i.e. being specific to elderly Chinese. Body composition differs in Caucasians vs. Asians, i.e. for a given BMI, Asians have greater body fat accumulation than Caucasian adults 33. Aging-induced adipose tissue increase differs among races. Wu found that, with increasing age, Asian women have more truncal fat than African-American or Caucasian women 34. Study on dialysis patients where obese had lower mortality than normal weight patients 35, the authors proposed that, in these sick individuals, the adipose tissue may secrete protective cytokines and other hormonal products. This hypothesis, that body fat may actually be protective, merits further study in elderly Chinese. Third, our finding may be due to our unique study population living in long-term care facilities, not living in the community. There are some possible differences between these two populations which may influence the association between obesity and mortality. Malnutrition (such as low serum albumin) and poor performance status (such as low KPS) increased the risk of all-cause mortality in the instituted elderly 36. We adjusted for these potential confounders into models in Table 3, and the inverse association still persisted. Chronic inflammation could also play an important role in the increased risk of all-cause mortality in the elderly. If we further adjusted for C-reactive protein into models in Table 3, the results were similar (data not shown). Older subjects living in long-term care facilities tend to have more chronic diseases or undiagnosed diseases than subjects living in the community. Chronic diseases (such as diabetes and hypertension) were the major causes of death in the elderly, so we adjusted for these diseases in our models. From adjusted models in Table 3, we found that the inverse association between mortality and BMI and WC still persisted after these adjustments but was weaker. Meanwhile, malnutrition and cachexia are common in institutional settings. In the context of cachexia, an “obesity paradox” has been observed, such that obese persons have a survival advantage37–38. This may be related to the large amount of unintentional weight loss experienced by residents of long-term care facilities and the possibility that physiologic reserve provided by fat is important for survival. Therefore, subjects with normal weight at baseline may have been previously overweight or obese but they may have lost weight due to undiagnosed disease 39. To clarify this potential influence, we excluded those who died during the first 6 months of follow-up and the results remained the same. Finally, smoking decreases body weight and increases fat accumulation, so smoking may have influenced the association 11. In our study, from Table 1 and 2, we found that smoking status was not a potential confounder. When we further adjusted for smoking status in the adjusted models in Table 3, the associations between BMI/WC/REE and mortality were still not changed. Although studies done by Bigaard and Heitmann found that both high body fat and low FFM were independent predictors of all-cause mortality 7, 30, in the United States, Zhu found that FM had a negative relationship with mortality 40 which is consistent with our study results. The interplay between WC and BMI on all-cause mortality deserves further research in the elderly.
There are some limitations to our study. First, we did not measure body composition (such as FM) among our subjects, so we cannot analyze the association between FM/ FFM and mortality. However, since REE represents FFM, we reasonably assumed that subjects with high REE had higher FFM which led to lower mortality. Second, the study had limited follow-up period. The results may be confounded by previous BMI misclassification. However, we tried to exclude those deaths which occurred in the first 6 months and the results remained the same and believe that this effect of potential BMI misclassification was minimal. Third, our subjects were residents in long-term care facilities and we did not assess the proportion of indigenous Taiwanese in our population; the generalization to community-dwelling population or indigenous Taiwanese or people living in the rural area should be made with caution.
We conclude that, in the elderly living in long-term care facilities, both BMI and WC are negatively associated with mortality in Taiwan. This negative effect was enhanced among centrally obese subjects. This finding may have great public health impact as the number of instituted elderly is increasing in many developed countries. Clinicians should regularly screen these residents using both BMI and WC. Further studies regarding the association between FM and FFM in the elderly living in long-term care facilities should be done.
ACKNOWLEDGMENTS
Funding/Support: This study was financially supported by grants from the Department of Health, Executive Yuan, Taiwan (DOH92-TD-1024), National Science Council of Taiwan (NSC 93-2314-B-039-031), and China Medical University Hospital (DMR-93-021, DMR-93-078, and DMR-96-061), and the US National Institutes of Health (DK 026687).
Sponsor’s Role: None.
Footnotes
Conflict of interest: The authors declare no conflict of interest
Author Contributions: All of the authors contributed to manuscript design, data analysis, and preparation and writing of the manuscript.
REFERENCES
- 1.World Health Organization. Obesity and overweight. [Accessed at June 15, 2010];2006 Fact sheet 311: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA. 2006;295:776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 4.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 5.Wandell PE, Carlsson AC, Theobald H. The association between BMI value and long-term mortality. Int J Obes (Lond) 2009;33:577–582. doi: 10.1038/ijo.2009.36. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 7.Bigaard J, Frederiksen K, Tjonneland A, et al. Body fat and fat-free mass and all-cause mortality. Obes Res. 2004;12:1042–1049. doi: 10.1038/oby.2004.131. [DOI] [PubMed] [Google Scholar]
- 8.Bender R, Jockel KH, Trautner C, et al. Effect of age on excess mortality in obesity. JAMA. 1999;281:1498–1504. doi: 10.1001/jama.281.16.1498. [DOI] [PubMed] [Google Scholar]
- 9.Corrada MM, Kawas CH, Mozaffar F, et al. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (Silver Spring) 2007;15:1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiss A, Beloosesky Y, Boaz M, et al. Body mass index is inversely related to mortality in elderly subjects. J Gen Intern Med. 2008;23:19–24. doi: 10.1007/s11606-007-0429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazza A, Zamboni S, Tikhonoff V, et al. Body mass index and mortality in elderly men and women from general population. The experience of Cardiovascular Study in the Elderly (CASTEL) Gerontology. 2007;53:36–45. doi: 10.1159/000095783. [DOI] [PubMed] [Google Scholar]
- 14.Allison DB, Faith MS, Heo M, Kotler DP. Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol. 1997;146:339–349. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. National Nursing Home Survey -2004 Current Resident Tables. [Accessed at June 15, 2010];2008 http://www.cdc.gov/nchs/about/major/nnhsd/ResidentTables_Estimates.htm#Demographics.
- 17.Kimyagarov S, Klid R, Levenkrohn S, et al. Body mass index (BMI), body composition and mortality of nursing home elderly residents. Arch Gerontol Geriatr. 2009 doi: 10.1016/j.archger.2009.10.013. doi:10.1016/j.archger.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Grabowski DC, Campbell CM, Ellis JE. Obesity and mortality in elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2005;60:1184–1189. doi: 10.1093/gerona/60.9.1184. [DOI] [PubMed] [Google Scholar]
- 19.Department of Health, Taiwan. Definition of obesity in Taiwan. [Accessed at June 15, 2010];2002 http://www.doh.gov.tw.
- 20.World Health Organization. International Association for the study of Obesity, International Obesity Task Force. [Accessed at June 15, 2010];Sydney: Health Communications; The Asia-Pacific perspective: redefining obesity and its treatment. 2000 http://www.iotf.org/asiapacific/.
- 21.Liu HY, Lu YF, Chen WJ. Predictive equations for basal metabolic rate in Chinese adults: A cross-validation study. J Am Diet Assoc. 1995;95:1403–1408. doi: 10.1016/S0002-8223(95)00369-X. [DOI] [PubMed] [Google Scholar]
- 22.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of chemotherapeutic agents. Columbia Univ Press; 1949. p. 196. [Google Scholar]
- 23.Lee ET, Go OT. Survival analysis in public health research. Annu Rev Public Health. 1997;18:105–134. doi: 10.1146/annurev.publhealth.18.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Ghali WA, Quan H, Brant R, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 25.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: A review of four unresolved questions. Int J Obes (Lond) 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 26.Kalmijn S, Curb JD, Rodriguez BL, et al. The association of body weight and anthropometry with mortality in elderly men: The Honolulu Heart Program. Int J Obes Relat Metab Disord. 1999;23:395–402. doi: 10.1038/sj.ijo.0800832. [DOI] [PubMed] [Google Scholar]
- 27.Woo J, Ho SC, Sham A. Longitudinal changes in body mass index and body composition over 3 years and relationship to health outcomes in Hong Kong Chinese age 70 and older. J Am Geriatr Soc. 2001;49:737–746. doi: 10.1046/j.1532-5415.2001.49150.x. [DOI] [PubMed] [Google Scholar]
- 28.Ho SC. Health and social predictors of mortality in an elderly Chinese cohort. Am J Epidemiol. 1991;133:907–921. doi: 10.1093/oxfordjournals.aje.a115970. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher D, Visser M, Sepulveda D, et al. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 30.Heitmann BL, Erikson H, Ellsinger BM, et al. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old swedish men-a 22-year follow-up. The study of men born in 1913. Int J Obes Relat Metab Disord. 2000;24:33–37. doi: 10.1038/sj.ijo.0801082. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 32.Price GM, Uauy R, Breeze E, et al. Weight, shape, and mortality risk in older persons: Elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 33.Chang CJ, Wu CH, Chang CS, et al. Low body mass index but high percent body fat in Taiwanese subjects: Implications of obesity cutoffs. Int J Obes Relat Metab Disord. 2003;27:253–259. doi: 10.1038/sj.ijo.802197. [DOI] [PubMed] [Google Scholar]
- 34.Wu CH, Heshka S, Wang J, et al. Truncal fat in relation to total body fat: Influences of age, sex, ethnicity and fatness. Int J Obes (Lond) 2007;31:1384–1391. doi: 10.1038/sj.ijo.0803624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 36.Sahyoun NR, Jacques PF, Dallal G, et al. Use of albumin as a predictor of mortality in community dwelling and institutionalized elderly populations. J Clin Epidemiol. 1996;49:981–988. doi: 10.1016/0895-4356(96)00135-7. [DOI] [PubMed] [Google Scholar]
- 37.Inui A, Meguid MM. Cachexia and obesity: Two sides of one coin? Curr Opin Clin Nutr Metab Care. 2003;6:395–399. doi: 10.1097/01.mco.0000078989.18774.74. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 39.Stevens J, Juhaeri CaiJ. Changes in body mass index prior to baseline among participants who are ill or who die during the early years of follow-up. Am J Epidemiol. 2001;153:946–953. doi: 10.1093/aje/153.10.946. [DOI] [PubMed] [Google Scholar]
- 40.Zhu S, Heo M, Plankey M, et al. Associations of body mass index and anthropometric indicators of fat mass and fat free mass with all-cause mortality among women in the first and second National Health and Nutrition Examination Surveys follow-up studies. Ann Epidemiol. 2003;13:286–293. doi: 10.1016/s1047-2797(02)00417-9. [DOI] [PubMed] [Google Scholar]