Abstract
Relapse is one of the most problematic aspects in the treatment of alcoholism and is often triggered by alcohol-associated environmental cues. Evidence indicates that glutamate neurotransmission plays a critical role in cue-induced relapse-like behavior, as inhibition of glutamate neurotransmission can prevent reinstatement of alcohol-seeking behavior. However, few studies have examined specific changes in extracellular glutamate levels in discrete brain regions produced by exposure to alcohol-associated cues. The purpose of this study was to use glutamate oxidase (GluOx)-coated biosensors to monitor changes in extracellular glutamate in specific brain regions during cue-induced reinstatement of alcohol-seeking behavior. Male Wistar rats were implanted with indwelling jugular vein catheters and intracerebral guide cannula aimed at the basolateral amygdala (BLA) or nucleus accumbens (NAc) core, and then trained to self-administer alcohol intravenously. A separate group of animals was trained to self-administer food pellets. Each reinforcer was accompanied by the presentation of a light/tone stimulus. Following stabilization of responding for alcohol or food reinforcement and subsequent extinction training, animals were implanted with precalibrated biosensors and then underwent a 1 hr cue-induced reinstatement testing period. As determined by GluOx-coated biosensors, extracellular levels of glutamate were increased in the BLA and NAc core during cue-induced reinstatement of alcohol-seeking behavior. The cumulative change in extracellular glutamate in both regions was significantly greater for cue-induced reinstatement of alcohol-seeking behavior versus that of food-seeking behavior. These results indicate that increases in glutamate transmission in the BLA and NAc core may be a neurochemical substrate of cue-evoked alcohol-seeking behavior.
Introduction
Alcoholism affects approximately 17 million Americans and results in tremendous social, legal, and medical costs to society (National Epidemiologic Survey on Alcohol and Related Conditions, 2001–2002) that has been estimated at more than $184 billion per year (Keena, McGeary, & Swift, 2004). More than 700,000 people in the United States receive treatment for this disorder on any given day (NIAAA Tenth Special Report to Congress, 2000). Currently there are only three medications approved by the Food and Drug Administration specifically to treat alcoholism – disulfiram, naltrexone and acamprosate – and all of these show only a moderate degree of efficacy in promoting abstinence and preventing relapse (Heilig and Egli, 2006; Pettinati and Rabinowitz, 2006; Soyka and Roesner, 2006). Therefore, there is a need for improved understanding of the neural substrates that underlie alcoholism that would enable more effective pharmacological additions to the current cognitive, behavioral, and psychosocial treatments to be developed. It is essential to determine the neural substrates underlying relapse behavior since this is one of the most problematic obstacles to the successful treatment of alcoholism. One study has indicated that nearly 60–80% of abstinent alcoholics will relapse at least once during their lifetime (Barrick & Connors, 2002).
Glutamate is the most abundant excitatory neurotransmitter in the brain and mediates nearly 70% of synaptic transmission within the central nervous system. Receptors for glutamate are divided into either ionotropic glutamate receptors (iGluRs) that consist of ligand-gated ion channels that mediate fast excitatory transmission, or metabotropic glutamate receptors (mGluRs) that mediate slower, modulatory glutamatergic transmission. The use of various rodent models of alcohol consumption has revealed that glutamate is involved in various aspects of alcohol use. Long-term alcohol exposure has been shown to alter various aspects of glutamatergic transmission. For example, as a result of alcohol’s ability to inhibit NMDA receptor functionality, chronic alcohol exposure results in an up-regulation of NMDA receptor function and subunit expression in numerous brain regions (Tabakoff and Hoffman, 1995; Tsai and Coyle, 1998; Hoffman, 2003; Krystal et al., 2003; Gass and Olive, 2008). This up-regulation of NMDA receptor function and expression is believed to mediate the CNS hyperexcitability observed during alcohol withdrawal. In addition to alterations in NMDA receptor expression, acute alcohol withdrawal is characterized by increases in extracellular levels of glutamate in various brain regions including the nucleus accumbens and hippocampus (Dahchour and De Witte, 1999, 2003; De Witte et al., 2003).
Evidence also indicates that glutamatergic transmission is involved in relapse-like behavior. Animal studies reveal that systemically administered glutamate receptor antagonists attenuate cue-induced reinstatement of alcohol-seeking behavior. Such compounds include various non-competitive NMDA receptor antagonists (Backstrom and Hyytia, 2004; Bachteler et al., 2005), the NMDA receptor modulator acamprosate (Bachteler et al., 2005), the mGluR2/3 agonist LY379268 (Backstrom and Hyytia, 2005; Zhao et al., 2006), the mGluR5 receptor antagonist MPEP (Backstrom et al., 2004; Adams et al., 2008; Schroeder et al., 2008), and the glutamate release inhibitor lamotrigine (Vengeliene et al., 2007). A recent human study has shown that the anticonvulsant drug topiramate can reduce the percentage of heavy drinking days and improve self-reported drinking outcomes in alcohol-dependent individuals (Johnson et al., 2007). It is believed that the effects of topiramate are based partly on its ability to antagonize AMPA and kainate receptors that could moderate cue-induced craving in alcoholics. Together, these studies suggest that glutamate plays a facilitatory role in cue-induced reinstatement of alcohol-seeking behavior.
Glutamate receptors are mediators of the synaptic plasticity involved in learning and memory and may underlie several pathological disorders including drug addiction (Suvarna et al., 2005). Given the influence of alcohol-associated environmental cues on relapse, the conditioning of these cues to alcohol availability may be mediated by glutamatergic transmission in specific brain regions. Studies employing localized lesions or inactivation of specific brain regions have revealed distinct neural circuits that are necessary for the ability of drug-associated cues to elicit reinstatement of drug-seeking behavior in animals trained to self-administer cocaine or heroin (Shalev et al., 2002; See et al., 2003; See, 2005). Regions such as the mesolimbic dopamine reward circuitry, amygdala, and hippocampus appear to play a pivotal role in cue-induced reinstatement for various drugs of abuse. However, surprisingly little is known regarding the specific brain circuitry involved in cue-induced reinstatement of alcohol-seeking behavior. In a set of studies conducted by Weiss and colleagues utilizing c-fos mapping, cue-induced reinstatement of alcohol-seeking was shown to increase neural activity in the medial prefrontal cortex, nucleus accumbens (NAc) shell and core, amygdaloid complex, hippocampal formation, ventral tegmental area, and paraventricular nucleus of the hypothalamus (Zhao et al., 2006; Dayas et al., 2007). Many of these regions overlap with those that have been shown to be involved in cue-induced reinstatement of cocaine- or heroin-seeking behavior. A separate group of investigators reported similar increases in c-fos expression in the amygdala and hippocampus during contextual reinstatement of alcohol-seeking behavior (Marinelli et al., 2007). However, because c-fos is a nonspecific marker of neuronal activation, additional studies examining whether this neuronal activation is a result of increased excitatory glutamatergic transmission are needed.
The main purpose of this study was to quantify changes in extracellular glutamate within candidate brain regions during cue-induced alcohol-seeking behavior. The basolateral amygdala (BLA) was chosen to be consistent with previous clinical studies that have shown increases in metabolic activity in this structure when abstinent drug users were presented with cues that predict drug availability (for a detailed review on the role of the amygdala in drug memories see Olive, 2009). The NAc core was investigated because this structure has been implicated as a common substrate in all forms of drug-seeking behavior in animals, including drug-seeking elicited by drug-associated cues (for a detailed review on this topic please see Feltenstein & See, 2008). Human imaging studies with detoxified alcoholics have also shown activation of the NAc during exposure to alcohol-associated cues (for a review see Heinz et al., 2009). A secondary aim was to determine if similar changes could be observed during cue-elicited food seeking behavior. Changes in extracellular glutamate were monitored using a novel method of detection via glutamate oxidase (GluOx)-coated biosensors that allow for near real-time detection of changes in extracellular glutamate levels in freely moving animals. We hypothesized that cue-induced reinstatement of alcohol-seeking behavior would be correlated with significant increases in extracellular glutamate levels in the NAc core and BLA, and that these changes would be greater than those observed during cue-evoked food-seeking behavior.
MATERIALS AND METHODS
Subjects
All experimental procedures conformed to the 1996 National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, the 2003 Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research, and with the approval of an Institutional Animal Care and Use Committee. Male Wistar rats (250–275 g upon arrival, Harlan, Indianapolis, IN) were housed in an animal colony room with temperature and humidity within NIH guidelines, and were allowed to recover from transport to the facility for at least 3 days prior to initiation of experiments. Animals were maintained on a reversed 12 h light-dark cycle (lights off at 0700 h) with ad libitum access to food unless otherwise stated. All experimentation was conducted during the dark phase of the light-dark cycle, with the exception of overnight food training sessions that commenced at approximately 1600 h and terminated at 0800 h the following morning.
Self-Administration Apparatus
Intravenous alcohol self-administration was conducted in operant self-administration chambers (ENV-008, Med Associates, St. Albans, VT, USA; Gass and Olive 2007). Each chamber was housed in a melamine sound-attenuating cubicle equipped with a house light and exhaust fan designed to mask external noise and odors, and was interfaced to a PC computer. Chambers were each equipped with two stainless steel response levers located on one wall that flanked a 4.2 × 5 cm food pellet receptacle. Each response lever was located approximately 7 cm above a stainless steel rod floor, and positioned above each lever was a 2.5 cm diameter white stimulus light. Located near the top of the self-administration chambers was a Sonalert speaker that provided an auditory stimulus (2900 Hz, ~65 dB) during alcohol delivery. Outside each chamber was a syringe pump that was interfaced to the computer and delivered the alcohol solution via a single-channel liquid swivel mounted atop the chamber via polyethylene tubing.
Self-Administration Procedures
Prior to self-administration training, rats that were designated to undergo intravenous alcohol self-administration and reinstatement procedures were prepared with intravenous catheters into the jugular vein (Gass and Olive 2007). Briefly, rats were anesthetized with 2% isoflurane vaporized in medical grade breathing air at a flow rate of 0.4 L/min, and the right jugular vein was isolated and a sterile silastic catheter filled with 100 U/ml heparin was inserted 2.5 cm into the vein. The catheter was secured to the surrounding tissue with sutures, and the opposite end of the catheter was tunneled subcutaneously to the dorsum where it exited the skin between the scapulae. This end of the catheter was secured to the surrounding tissue via sutures and a mesh collar attached to a threaded vascular access port (Plastics One, Roanoke, VA, USA). The access port was sealed with a piece of Tygon tubing closed at one end and a protective cap. During the same surgical procedure, rats were also unilaterally implanted with plastic guide cannula (MD-2250, Bioanalytical Systems, Inc., West Lafayette, IN) aimed at the NAc core (AP +1.8, ML ±1.5, DV −5.0 mm from bregma and skull surface) or BLA (AP −2.5, ML ±5.0, DV −6.4 mm from bregma and skull surface) according to the atlas of Paxinos and Watson (2005). Guide cannula were fitted with obturators and a protective cap to prevent contamination and obstruction, and secured to the skull with stainless steel screws and dental cement. The wound was then treated with a topical antibiotic ointment and 2% w/v xylocaine (Henry Schein Veterinary Supply, Melville, NY). Following surgical procedures, rats were allowed at least 5 days of recovery and received daily intravenous infusions of 70 U/ml heparin (0.2 ml volume) to maintain catheter patency and 100 mg/ml cefazolin (0.1 ml volume) to protect against infection. Animals were also administered carprofen (2.5 mg/kg s.c.) once daly for 5 days following surgical procedures to minimize post-surgical discomfort. Catheter patency was tested periodically throughout the experiment by infusion of 10 mg/ml sodium methohexital (0.2 ml volume) and observation of brief loss of postural muscle tone. Rats designated to undergo food self-administration and reinstatement procedures were not implanted with intravenous catheters to avoid unnecessary post-surgical discomfort, but received identical surgical implantation of intracranial guide cannula and post-operative care.
Following recovery from surgical procedures, all animals were limited to access to 20 g of food per day for the remainder of the experiments to facilitate operant self-administration performance. In our experience this mild food restriction results body weights equivalent to 90% of those under free-feeding conditions while still allowing weight gain, and results in more robust patterns of self-administration than those under free-feeding conditions (Oei and Singer, 1979). To initiate operant responding, rats were placed in the self-administration chambers for 16 h overnight training sessions whereby each press on the designated active lever delivered a 45 mg food pellet (Bio-Serv, Frenchtown, NJ) into the food receptacle on a fixed-ratio 1 (FR1) schedule of reinforcement. Each food pellet delivery was followed by a 4-sec timeout period, during which additional active lever presses were recorded but produced no programmed consequences. Presses on the designated inactive lever were recorded but produced no consequences at any time during the experiment. Approximately 24 hr following the initial overnight training session, rats were then placed into the chambers and 60 min alcohol or food self-administration sessions were conducted twice daily, with approximately 4 h elapsed between the first and second sessions. For rats designated to undergo intravenous alcohol self-administration and reinstatement procedures, each press on the active lever resulted in delivery of alcohol (1% v/v, delivered in a volume of 0.03 ml over a 1 sec period, 1 mg/kg/infusion) on a FR1 schedule of reinforcement. Each alcohol infusion was followed by a 4-sec timeout period, during which additional active lever presses were recorded but produced no programmed consequences. Alcohol was delivered to the vascular access port by polyethylene tubing housed in a stainless steel spring tether that was attached to the liquid swivel. Each alcohol infusion was accompanied by concurrent illumination of the stimulus light and presentation of an auditory stimulus for 1 sec. Self-administration sessions were conducted 5 consecutive days per week, and each was preceded by intravenous infusion of 0.1 ml of 70 U/ml heparin and followed by infusion of 0.1 ml of 100 mg/ml cefazolin. For rats designated to undergo food self-administration and reinstatement procedures, rats underwent identical overnight training procedures, and during daily self-administration sessions each active lever press resulted in delivery of a 45 mg food pellet rather than an infusion of alcohol. Visual and auditory stimuli that were presented during reinforcer delivery, as well as the duration of the time out period, were identical between alcohol and food self-administration groups.
Extinction & Reinstatement Procedures
After reaching criteria for stability of responding (<20% variation in the number of active lever presses across three consecutive days), once daily 1 hr extinction trials were initiated, whereby presses on the active lever no longer produced any programmed consequences. Prior to each extinction session, animals were briefly observed for physical signs of alcohol withdrawal (agitation, tail stiffness, abnormal body posture, and excessive vocalizations upon handling). Extinction training was conducted once daily (instead of twice daily, as during the maintenance phase of self-administration) since reinstatement test sessions were planned to be conducted only once daily to avoid possible confounds due to multiple reinstatement tests on a single day. After reaching extinction criteria (<25% of the average number of active lever presses made during the last 2 days of alcohol or food self-administration, minimum 10 days), animals underwent reinstatement testing. During reinstatement test sessions, animals were placed in the operant self-administration chamber, lever presses were recorded and each press on the active lever resulted in a 1 sec presentation of the light-tone stimulus that was previously paired with alcohol or food pellet delivery, followed by a 4 sec timeout, but no alcohol or food pellet was delivered. Reinstatement test sessions were 1 hr in length and were conducted in the same operant self-administration chamber as was used during the maintenance and extinction phases.
Saline Substitution Procedure
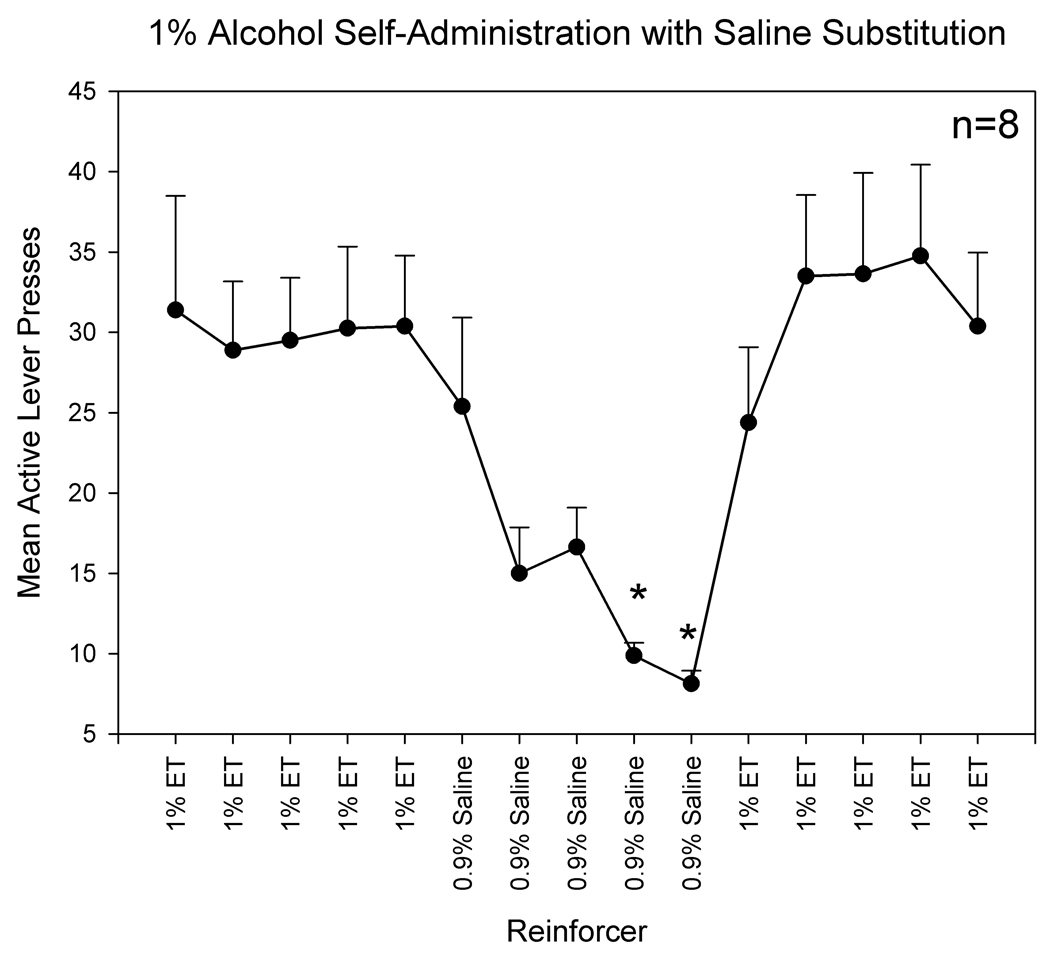
In order to determine the reinforcing efficacy of i.v. alcohol self-administration a saline substitution experiment was employed. The training procedures used in this component of the experiment were the same as those utilized in the training for i.v. alcohol self-administration. Animals were implanted with indwelling jugular vein catheters and trained to self-administer the same 1% alcohol solution used in the original studies, but after stabilization of responding, the reinforcer was switched to a 0.9% saline solution. After five sessions of saline self-administration, the reinforcer was reverted back to the original 1% alcohol solution. Active and inactive lever presses were monitored during all phases of the experiment. All parameters, such as the light-tone complex stimulus, were identical to those used in the operant procedures involving the glutamate biosensor.
Biosensor Implantation and Recording Procedures
Glutamate biosensors were purchased from Pinnacle Technologies (Lawrence, KS) (Hu et al., 1994). Each biosensor was equipped with a 1 mm sensor cavity (176 µm O.D.) that was constructed of a platinum-iridium electrode coated with Nafion (a sulfonated Teflon-like polymer that repels anionic interferants) as well as an enzyme layer containing immobilized glutamate oxidase (GluOx) and ascorbate oxidase (AscOx). GluOx catalyzes the breakdown of glutamate to α-ketoglutarate and hydrogen peroxide, the latter of which is electrochemically active at the electrode surface and gives a near real-time indirect measurement of changes in extracellular glutamate levels. AscOx catalyzes the breakdown of ascorbate (a highly abundant electroactive constituent of brain extracellular fluid) to dehydroascorbate and water, thus dramatically reducing potential interference arising from oxidation of ascorbate at the electrode surface. Biosensors were interfaced to a PC via a potentiostat (Biostat, ESA Inc., Chelmsford, MA). An amperometric voltage of +600 mV was applied to the biosensor during calibration as well as during in vivo measurements.
On the day prior to reinstatement testing, each biosensor was calibrated in vitro in phosphate buffered saline (PBS, pH=7.4) with four 10 µM increments of L-glutamate. Biosensors were also verified to be nonresponsive to 50 µM DOPAC, 12.5 nM dopamine, and 200 µM ascorbate. The concentrations of DOPAC, dopamine, and ascorbate were selected based on previous studies that have estimated basal extracellular concentrations of these analytes in the brain to be in the range of 1–20 µM for DOPAC (Glick, Dong, Keller, & Carlson, 1994), 1–5 nM for dopamine (Glick et al., 1994), and 40–60 µM for ascorbate (Tsai, Wu, Lin, Kuo, & Yang, 1996). Therefore, a lack of response of the biosensor to the concentrations of analytes used during calibrations, which are in excess of the estimated values in the brain, suggests that these molecules do not interfere with the signal obtained from the biosensor in vivo.
Following calibration, obturators were removed from the guide cannula and biosensors were lowered into the BLA or NAc core. Animals were then allowed to recover overnight prior to reinstatement testing. To obtain stable baseline measurements of glutamate oxidation currents, prior to the initiation of the reinstatement session each animal was placed in the operant chamber for approximately 45 minutes. During this time levers were retracted, the house light was not illuminated, and exhaust fan was turned off. After a stable baseline currents were observed, 2 minutes of baseline recording from the biosensors occurred prior to the beginning of each reinstatement test.
Drugs
Alcohol (95% v/v), physiological saline (0.9% w/v sodium chloride), methohexital, cefazolin and heparinized saline were obtained from the MUSC Pharmacy Distribution Center. For intravenous self-administration, alcohol (95% v/v) was diluted to 1% v/v in sterile saline. All other chemical reagents were obtained from Fisher Scientific (Suwanee, GA) or Sigma-Aldrich (St. Louis, MO).
Histological verification of biosensor probes
Following experimental procedures, animals were anesthesized with isoflurane and euthanized by decapitation. Brains were then removed, immersed in 10% v/v formalin for at least one week at 4°C, and then immersed in a 30% (w/v) sucrose solution for at least 72 hours at 4°C followed by immersion in 15% (w/v) sucrose for at least 72 hours at 4°C. Brains were then cut into 40 µm coronal sections on a cryostat (Leica CM1900, Leica Microsystems, Bannockburn, IL), mounted onto microscope slides, and stained with cresyl violet for histological verification of cannulae placement under light microscopy.
Statistical Analysis
Self-administration & Reinstatement data
Behavioral data from the self-administration phase of this study were analyzed based on the stage of the experiment. During the extinction phase, lever presses on the last two days of active self-administration (i.e., maintenance) were averaged and compared to the final day of extinction training (for both alcohol- and food-reinforcement groups) using a repeated-measures ANOVA with experimental phase (maintenance vs. extinction) as the primary factor. Results from this analysis provided a statistical basis for determination of extinguished responding. The number of lever presses recorded during the reinstatement session was compared with those from the last day of the extinction phase using a repeated measures ANOVA with experimental phase (extinction vs. reinstatement) as the primary factor. Separate ANOVAs were performed on the number of both active and inactive lever presses. Results from this analysis provided indication of successful reinstatement of alcohol and food-seeking behavior. For the saline substitution experiment, both active and inactive lever presses were analyzed using a repeated-measures ANOVA. Significant changes in active lever presses during the different reinforcer contingencies (alcohol or saline reinforcement) were used to determine the reinforcing efficacy of the i.v. 1% alcohol solution.
Glutamate biosensor data
After stabilization all changes in biosensor current output during reinstatement testing (i.e., relative to the average pre-session baseline, in nA) were grouped into 1 min time bins, since this was the temporal resolution of recording of lever presses in the self-administration chambers. This change in current in 1 min bins was then converted to a change in glutamate concentration (in µM), based on the individual standard curve generated for each biosensor prior to implantation. This allowed for a correlation between active lever presses and associated changes in glutamate concentration in 1 min time bins. For statistical comparisons between experimental and control groups, changes in extracellular glutamate levels between alcohol-and food-reinforced groups during the reinstatement phase were analyzed using a 2 × 2 ANOVA with Group (alcohol or food reinforcement) and Region (BLA or NAc core) serving as the independent variables. Cumulative glutamate served as the dependent variable. This analysis was used to determine any significant changes in extracellular glutamate levels during the reinstatement session between the two groups and brain regions. Post-hoc tests were used to determine specific differences among the groups. P-values less than 0.05 were considered significant for all tests. All statistical tests were performed using SPSS version 18.0 (SPSS, inc., Chicago, IL).
RESULTS
Exposure to alcohol- or food-associated cues significantly increased responding on the active lever
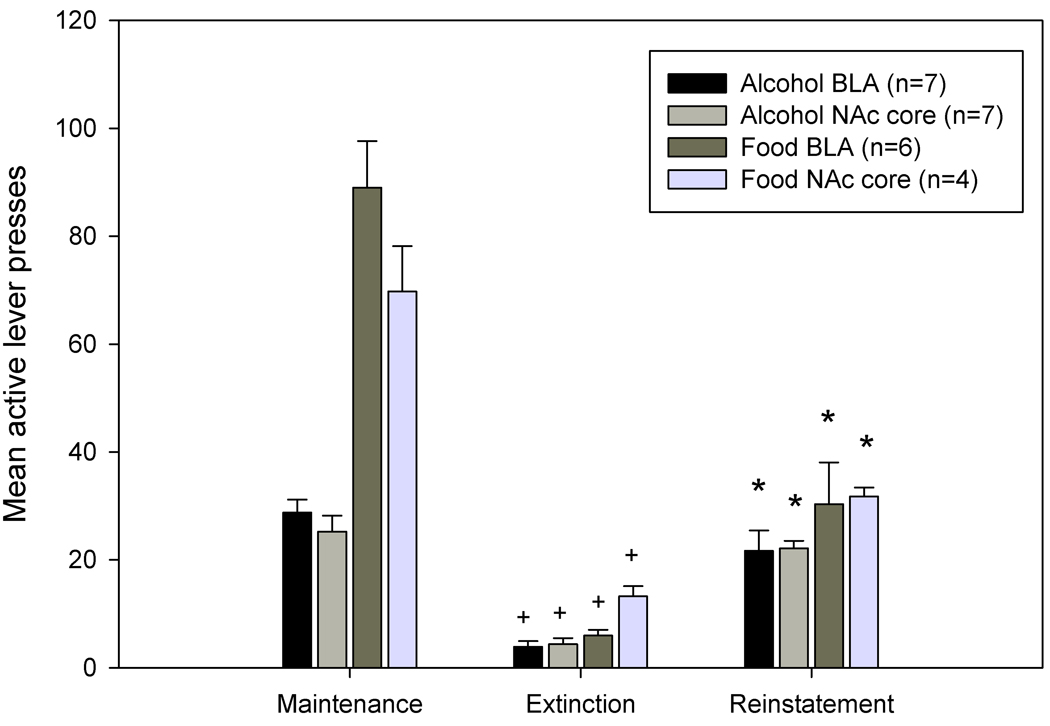
Analysis of lever pressing behavior revealed there were significant differences between mean active lever presses during the different phases of the experiment (i.e., maintenance, extinction (EXT), and reinstatement phases) in both alcohol- and food-reinforced groups. A repeated-measures ANOVA revealed a significant experimental phase X group interaction (F6,46=10.029, p<0.001). Pair-wise comparisons revealed that animals from both alcohol and food reinforced groups showed a significant decrease in responding on the active (previously reinforced) lever during extinction when compared to self-administration training (p values <0.001). Additionally, both alcohol- and food-reinforced groups showed a significant increase in responding on the active lever during reinstatement testing when compared to the extinction phase (p values <0.01). These data are displayed in Figure 1. There were no significant differences in the number of inactive pressing during any of the phases for both alcohol- and food-reinforced animals. There were also no significant differences in lever pressing when the data were collapsed across region of biosensor implantation (BLA or NAc core). Together, these data indicate that alcohol and food cues served as reinforcers and that reinstatement of drug-and food-seeking behavior was observed.
Figure 1.
Maintenance of self-administration, extinction, and reinstatement of alcohol- and food-seeking behavior. Active lever presses that previously resulted in alcohol or food delivery were significantly decreased during extinction training as compared to when alcohol or food was available (+, p <0.001). During reinstatement tests, response-contingent presentation of the light-tone stimulus that was previously paired with either alcohol or food significantly increased active but not inactive lever presses as compared to those observed during extinction (*, p<.01). Data are presented as mean ± SEM.
Cue-induced reinstatement of alcohol-seeking behavior is associated with greater changes in extracellular glutamate levels in the NAc core and BLA as compared with cue-induced reinstatement of food-seeking behavior
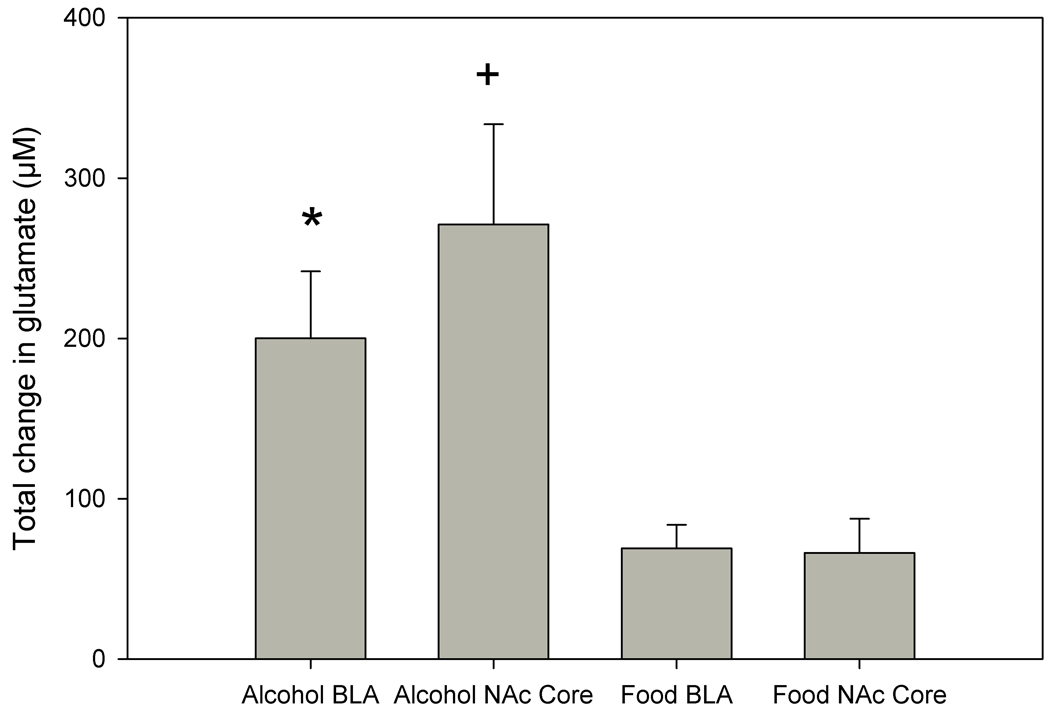
Analysis of glutamate biosensor data revealed significant differences among the groups in changes in glutamate transmission during the cue-induced reinstatement phase. A two-way ANOVA showed a significant increase in extracellular glutamate elicited by alcohol-associated cues (F1,20=12.883, p<0.01). Post-hoc comparisons revealed a significantly greater increase in extracellular glutamate levels in the BLA during cue-induced alcohol-seeking behavior when compared to food-seeking behavior (p<0.05). There was also a significantly greater increase in extracellular glutamate in the NAc core during alcohol-seeking behavior when compared to food-seeking behavior in the NAc core (p<0.01). These data are displayed in Figure 2. There were no significant differences in cumulative changes in extracellular glutamate levels between the two brain regions (NAc core and BLA) in alcohol-reinforced animals, nor were there any differences observed across brain regions in food-reinforced animals.
Figure 2.
Cumulative change in extracellular glutamate levels during cue-induced reinstatement of alcohol- or food-seeking behavior in the BLA or NAc core. Significantly greater cumulative increases in extracellular glutamate in the BLA during cue-induced reinstatement of alcohol-seeking behavior were observed as compared to those observed in these regions during cue-induced reinstatement of food-seeking behavior (indicated by asterisk). A similar observation was noted for cumulative changes in extracellular glutamate levels in the NAc core (indicated by plus sign). Data are presented as mean ± SEM.
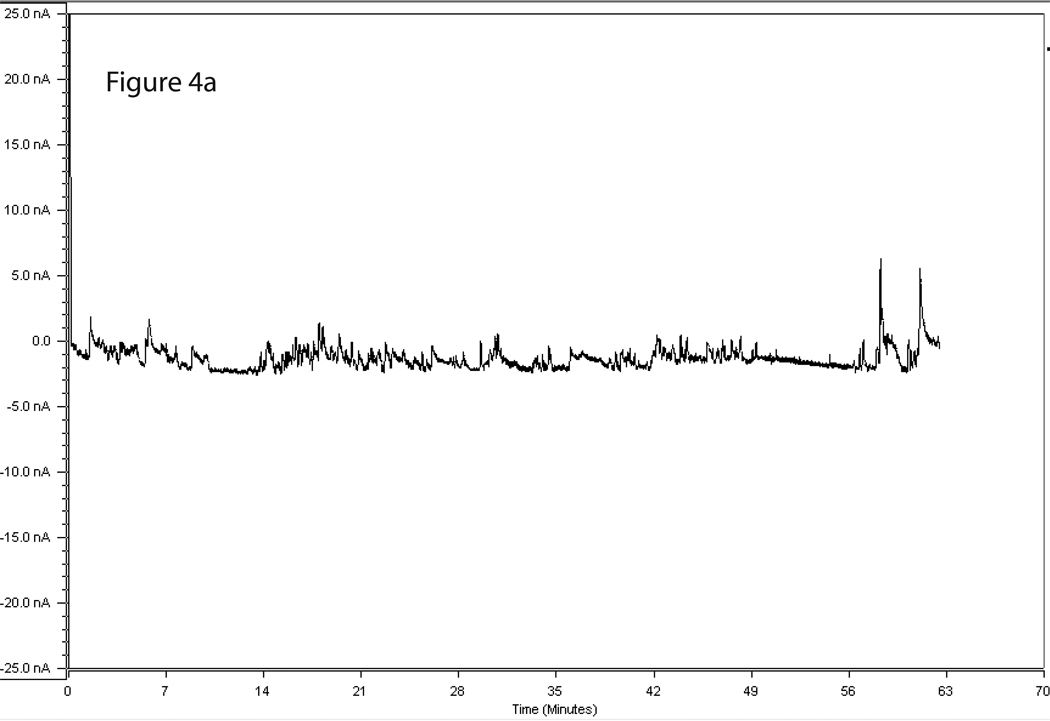
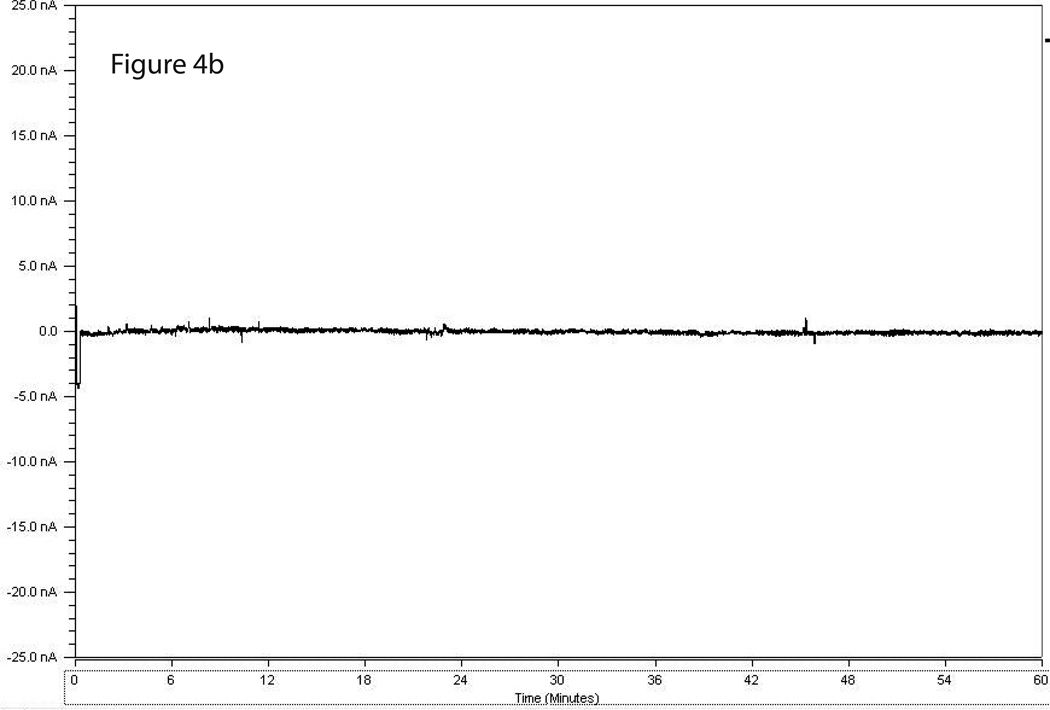
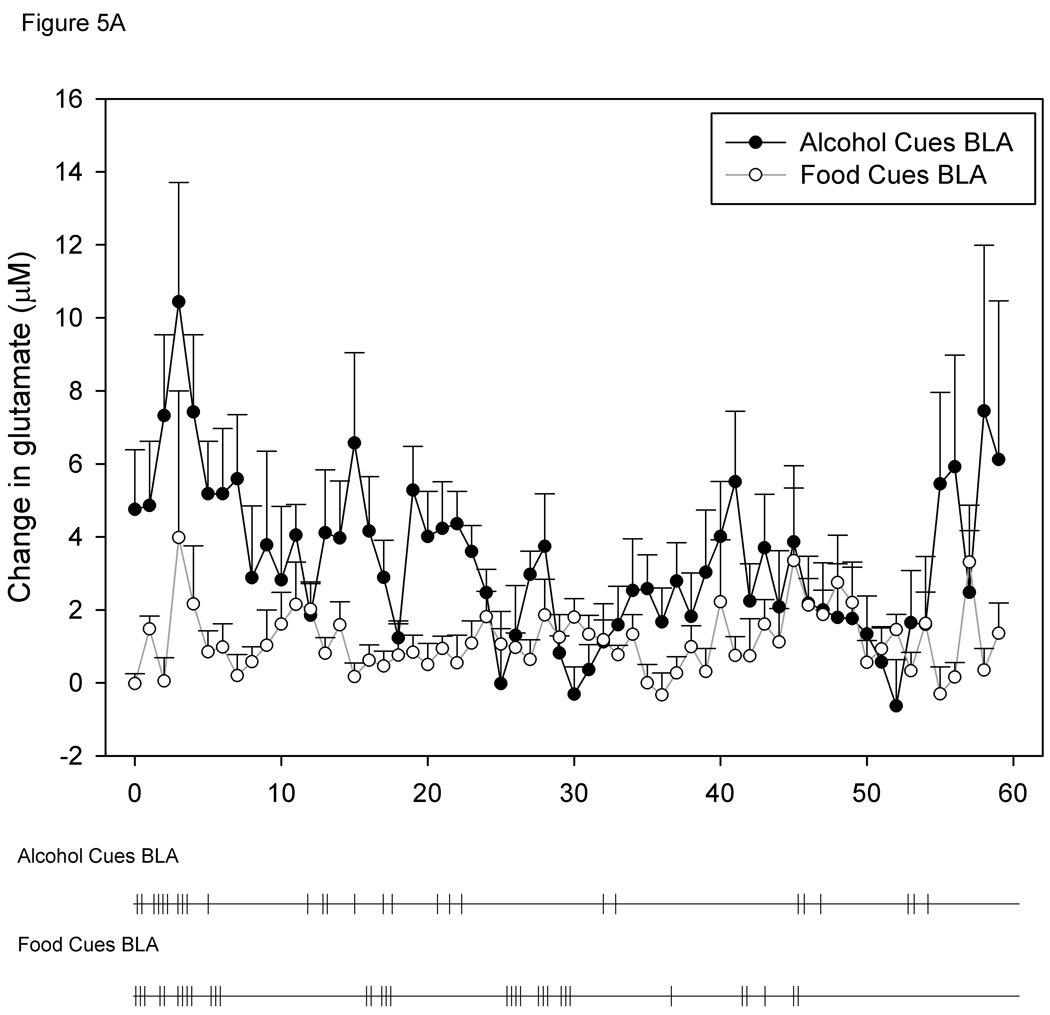
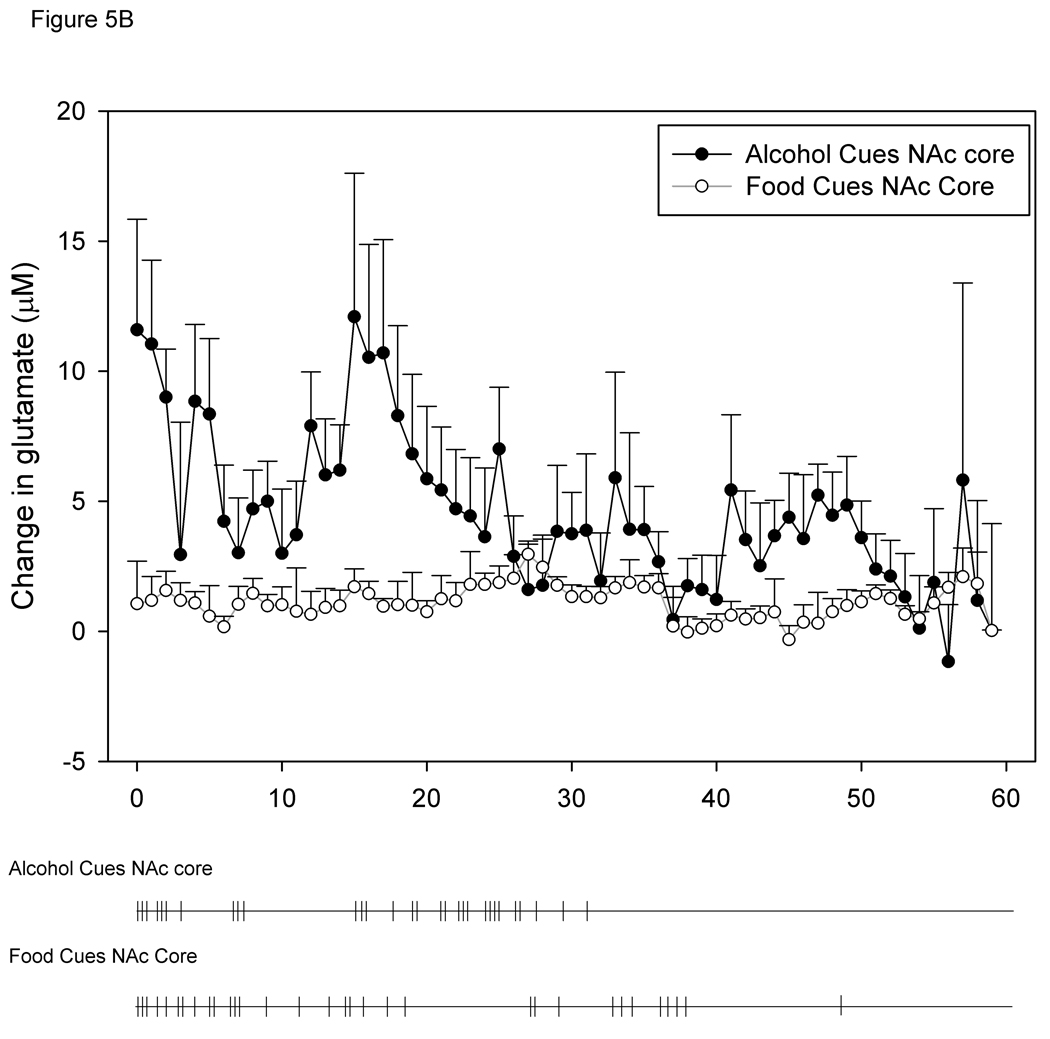
Figure 3 shows a representative calibration trace of a biosensor prior to implantation, and Figure 4 shows a representative trace of biosensor recording during cue-induced reinstatement of alcohol-seeking behavior. Figures 5A and 5B show the time course of operant responding and changes in extracellular glutamate levels in the BLA and NAc core, respectively, during the reinstatement test session. Active lever presses generally tended to occur in bouts, and were generally more frequent during the first half of the session as compared to the latter half. Similarly, the larger increases in extracellular glutamate-mediated biosensor currents tended to occur in the first half of the session as compared to the latter half. Figure 6 shows the approximate placement of the 1 mm sensing cavity of the biosensor in the BLA or NAc core.
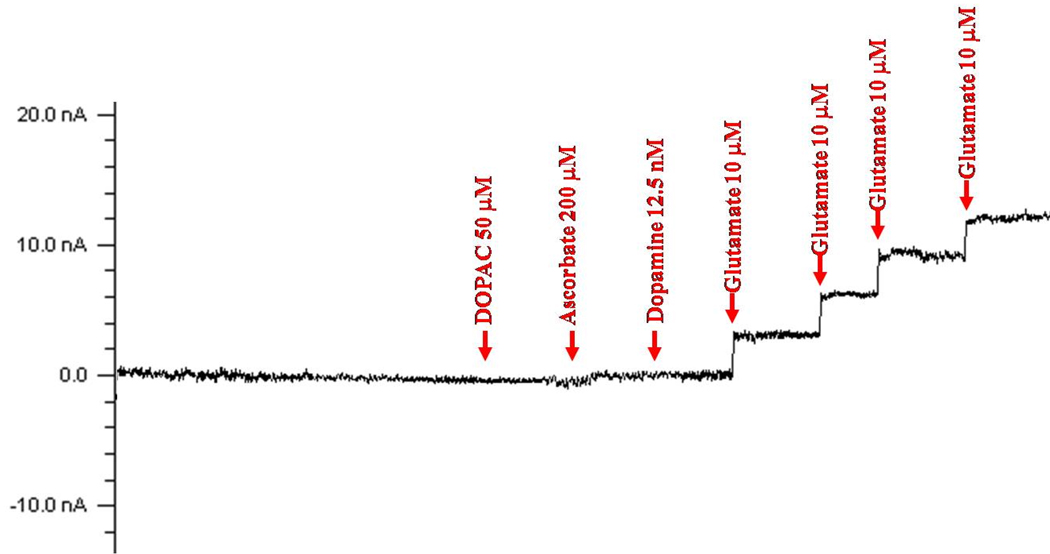
Figure 3.
Representative calibration of a glutamate biosensor to determine specificity for glutamate. Prior to implantation, biosensors were calibrated in vitro in PBS with four 10 µM increments of glutamate and verified to be non-responsive to 200 µM ascorbate, 50 µM DOPAC, and 12.5 nM dopamine.
Figure 4.
4A & 4B. Representative trace of a biosensor recording during cue-induced reinstatement of alcohol-seeking (a) and food-seeking (b) behavior. The X axis displays time points during the reinstatement session and the Y axis displays detected GluOx-derived currents (in nA).
Figure 5.
5A and 5B. Time course of changes in BLA (A) and NAc core (B) extracellular glutamate levels during alcohol- and food-seeking behavior. Below each graph is a time course of active lever pressing behavior from a representative animal from each of the groups. Each tick mark represents one bar press during the reinstatement session.
Figure 6.
Coronal diagrams of the rat brain showing the location of the tips of the biosensor in the BLA (a) and NAc core (b). Biosensor placement was determined from postmortem histological analysis of cresyl violet stained tissue sections. The distance of each section from bregma was −2.92 mm for the BLA and +1.80 mm for the NAc core. Diagrams were taken from the atlas of Paxinos and Watson (2005).
Analysis of the saline substitution data revealed significant changes in active lever presses based on the type of reinforcer (1% alcohol or 0.9% saline) [F(14,98)=7.184, p<.001]. Specifically, there was a significant decrease in active lever responding when saline was substituted as the reinforcer and a significant increase in active lever presses when alcohol was reintroduced as the reinforcer. These data are displayed in Figure 7.
Figure 7.
The effects of saline substitution for alcohol on active lever presses. These data indicate that responding on the lever that previously delivered a 1% alcohol solution is significantly decreased when saline is substituted as the reinforcer (indicated by asterisk).
Discussion
To our knowledge, this study is the first to use GluOx-coated biosensors implanted in discrete brains regions to assess changes in extracellular levels of glutamate during reinstatement of alcohol- and food-seeking behavior. We found increases in extracellular glutamate levels in the NAc core and the BLA during cue-induced reinstatement of alcohol-seeking behavior that were significantly greater than those compared to changes observed during cue-induced reinstatement of food-seeking behavior. These changes did not appear to result from differences in lever pressing between the alcohol and food self-administering groups, as both groups of animals displayed similar levels of lever pressing during the reinstatement testing. There were also no significant differences in inactive lever presses during the reinstatement phase, which indicates that all animals displayed similar rates of general activity.
These results are consistent with other studies in the field that have pharmacologically examined the role of glutamate transmission in alcohol-seeking behavior. For instance, pharmacological blockade or genetic inactivation of ionotropic glutamate receptors (i.e., NMDA or AMPA receptors) attenuates cue-induced reinstatement of alcohol-seeking (Backstrom and Hyttia, 2004 Sanchis-Segura et al., 2006). In addition, dampening of glutamate transmission via stimulation of presynaptic mGluR2/3 autoreceptors or antagonism of postsynaptic mGluR5 receptors attenuates cue-induced alcohol-seeking behavior (Backstrom and Hyytia, 2005; Zhao et al., 2006; Schroeder et al. 2008; Adams et al., 2008; Adams et al., 2010). Suppression of glutamate release by anticonvulsant drugs such as lamotrigine also attenuates cue-induced alcohol-seeking (Vengeliene et al., 2007). Combined with our observations that cue-evoked alcohol-seeking is associated with elevated extracellular levels of glutamate in the NAc core and BLA, two regions that are known to be involved in regulated goal-directed drug-seeking behavior and stimulus-reward associations (Grant et al., 1996; Meil & See, 1997; Childress et al., 1999; Cornish & Kalivas, 2000; Kilts et al., 2001; Kruzich & See, 2001; Bonson et al., 2002; Fuchs & See, 2002; McLaughlin & See, 2003; Di Ciano & Everitt, 2004; Fuchs et al., 2004; Dayas et al., 2007; Di Ciano et al., 2008; Rogers et al., 2008), respectively, these various lines of study indicate that increased glutamate transmission mediates cue-induced alcohol-seeking behavior. Future studies employing intracranial microinjection procedures in these regions are currently underway in our laboratory to determine if local inhibition of glutamatergic transmission in these regions attenuates cue-induced reinstatement of alcohol-seeking behavior.
Our results also suggest that the BLA and NAc core could be critical brain regions involved in cue-evoked alcohol-seeking behavior. Although very few studies have examined the neural regions that mediate this behavior, the present data corroborate previous c-fos mapping studies that show neuronal activation within the areas of the nucleus accumbens and amygdala during cue-induced alcohol-seeking behavior (Zhao et al., 2006; Dayas et al., 2007). The NAc core is considered to be a final common pathway for all forms of reinstatement of drug-seeking behavior, including cue-induced drug seeking (Feltenstein & See, 2008). Consistent with our findings that alcohol-associated cues increase extracellular glutamate levels in the NAc core, it has been shown that inhibition of glutamatergic transmission by local infusion of mGluR5 antaqonists into this region attenuate cue-induced cocaine-seeking behavior (Backstrom and Hyytia, 2006). Antagonism of these receptors in the NAc shell also attenuates cue-induced reinstatement of cocaine-seeking behavior (Kumaresan et al., 2009). Further investigation is needed to determine other brain structures where glutamatergic transmission is increased during alcohol-associated cue-induced relapse-like behaviors, and which of these structures play a key role in mediating relapse.
It has become apparent that the brain circuits and neural substrates that underlie drug addiction overlap with those that mediate normal learning and memory processes. As a result, drug addiction has been theorized to be a disorder of learning and memory (Pulvirenti & Diana, 2001; Kelly, 2004; O’Brien, et al., 1992; Robbins & Everitt, 2002; White, 1996; Di Chiara, 1998; Di Chiara, 1999; Volkow et al., 2002; Wise, 2004; Hyman, 2005). Repeated drug use could lead to an “overlearning” of the associations between a drug’s effects and the environmental stimuli that predict their availability. In this regard, the associations between drugs and specific cues and contexts could become overly salient and, therefore, lead to increased craving and relapse in abstinent individuals. With the prominent role that glutamate plays in mnemonic processes, these theories suggest that modulation of glutamate could serve as a possible target to reduce the influence of drug-associated cues on addictive behaviors. The ability of medications currently being tested and/or used for the treatment of addiction to alcohol and other drugs of abuse, such as acamprosate, topiramate, and N-acetylcysteine, may result from their ability to modulate glutamatergic transmission. Clearly, future studies will need to elucidate the exact mechanisms by which glutamate contributes to the formation of drug-associated memories that elicit drug-seeking behavior.
One component of this experiment that needs further investigation is the source of the detected changes in extracellular glutamate. The present study did not investigate whether the source of detected glutamate was of neuronal or glial origin. In addition to synaptic glutamate release, glutamate can be released into the extracellular space via nonexocytotic mechanisms such as the cystine-glutamate exchanger (xc) located on glial cells (Melendez et al., 2005; McBean, 2002; Baker et al., 2002; Moran et al., 2005). Evidence for the influence of the xc on glutamate levels, particularly within the nucleus accumbens, comes from studies showing agents that regulate this exchanger, such as N-acetylcysteine, can greatly influence glutamate concentrations in the extracellular environment (Baker et al., 2002). In addition, extracellular glutamate levels are tightly controlled by excitatory amino acid transporters (EAATs). Future studies incorporating local infusions of tetrodotoxin, calcium channel blockers, and activators or inhibitors on EAATs and xc in close proximity to the biosensor are needed to determine the origin of the glutamate signals measured by glutamate biosensor methodology.
One interesting finding from the current study was the inability to food cues to elicit similar increases in glutamate concentrations in the BLA and NAc core as alcohol cues. Studies that have examined the role of neuronal glutamate in drug- and other reinforcer-seeking behaviors have generally shown that food-seeking behavior is unchanged with glutamatergic blockade. For instance, an array of experiments have shown that blockade of metabotropic glutamate receptors attenuate drug-seeking behaviors for nicotine (Bespalov et al., 2005; Dravolina et al., 2007), cocaine (Kumaresan et al., 2009; Xie et al., 2010), and methamphetamine (Gass et al., 2009) without affecting food-seeking behavior (but see Martin-Fardon et al., 2009). However, suppression of glutamate release via activation of group II metabotropic receptors has been shown to attenuate both drug- and food-seeking behaviors at high doses (Peters & Kalivas, 2006) and attenuate food-induced reward seeking behavior but not food self-administration (Bossert et al., 2006). The discrepancy among these findings may result from type of metabotropic glutamate receptor that is modulated. Group I mGluRs, located predominately on post-synaptic dendrites, appear to regulate drug-seeking over food-seeking behavior and Group II mGluRs, which are located mainly on the presynaptic terminal have been shown to regulate both drug- and food-seeking behavior. Findings from the current study suggest that food cues may be less salient than alcohol cues and thus are less likely to be formed through glutamate-mediated mnemonic associations.
While this study revealed increases in extracellular glutamate in the BLA and NAc core that correlated with the presentation of alcohol cues, other studies have shown that extracellular glutamate are modified by chronic alcohol exposure or drug-associated cues. For example, extracellular glutamate concentrations in the NAc are increased in rats in the presence of cocaine cues or contexts (Bell et al., 2000; Hotsenpiller et al., 2001). In addition, extracellular levels of glutamate show a sensitized response to an acute alcohol challenge in the NAc following chronic alcohol administration (Szumlinski et al., 2007). However, the extent to which glutamatergic transmission specifically in the NAc core or BLA mediates conditioned reinforcement from drug/cue associations warrants further investigation.
One potential limitation of the current study is the use of intravenous alcohol self-administration. This method is not the natural route of alcohol self-administration used by humans, but it allows for direct delivery of ethanol to the bloodstream and thus a more direct measure of the reinforcing properties of alcohol than the standard oral route of self-administration. In addition, the precise amount of alcohol delivered into the bloodstream can be calculated from the number of alcohol infusions obtain by the animals. For example, in the current study, alcohol-reinforced animals received approximately 25–30 infusions of 1 mg/kg alcohol during alcohol self-administration training, which corresponds to approximately 25–30 mg/kg per session. This is, however, approximately 1/20 of the amount of alcohol consumed in standard oral self-administration studies (which typically range from 600 mg/kg to 1000 mg/kg per session). Nonetheless, statistical analysis revealed that alcohol-reinforced animals responded significantly more on the active lever than the inactive lever, and that responding was significantly higher when alcohol was delivered (i.e., during the acquisition and maintenance phases) as compared to when alcohol was not delivered (i.e., extinction sessions). To further address this issue a separate group of animals were trained to self-administer a 1% alcohol solution that was subsequently replaced with a 0.9% saline solution. The results indicated that responding on the active lever significantly decreased when saline was substituted for alcohol and returned to pre-substitution levels when alcohol was reintroduced as the reinforcer. Together these data indicate that intravenous alcohol administration served as a reinforcer during these experiments. Further research is needed to determine the molecular targets and brain circuitries activated by these low levels of self-administered alcohol. Studies in our laboratory are underway to determine if similar increases in extracellular glutamate levels in the NAc core and BLA are observed in rats with a history of oral alcohol self-administration.
An additional limitation of this study is the inability of the glutamate biosensor to reveal true extracellular basal concentrations of glutamate in the targeted brain regions. This is an inherent constraint of the biosensor technology. One possibility is that the current results could reflect increased extracellular glutamate concentrations prior to the reinstatement session in the alcohol-seeking groups. This limitation of the biosensor does not allow for the assessment of extracellular glutamate concentrations prior to behavioral testing. Future studies could employ the zero-net-flux microdialysis procedure to accurately determine basal extracellular glutamate concentrations. Nonetheless, the ability of the biosensor technology to detect changes in glutamate concentrations revealed significant increases during alcohol-seeking behavior when compared to food-seeking behavior.
In conclusion, we have demonstrated that cue-induced reinstatement of alcohol-seeking behavior is accompanied by significant increases in extracellular glutamate concentrations in the BLA and NAc core, and that these increases are greater in magnitude as compared to those observed during cue-induced reinstatement of food-seeking behavior. These results suggest that increased glutamate transmission may be a neural correlate of cue-evoked relapse to alcohol-seeking behavior and provide a mechanistic basis by which glutamate-based therapeutics may exert their beneficial effects on preventing relapse in human alcoholics.
Acknowledgements
This work was supported by Public Health Service grants AA017820-01A1, AA013852, and AA007474 and from the National Institute on Alcohol Abuse and Alcoholism.
References
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- Adams CL, Short JL, Lawrence AJ. Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49 Suppl 1:167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol Clin Exp Res. 2007;31:1441–1445. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Dong N, Keller RW, Jr, Carlson JN. Estimating extracellular concentrations of dopamine and 3,4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: relationships between in vitro and in vivo recoveries. J Neurochem. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Mitchell KM, Albahadily FN, Michaelis EK, Wilson GS. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994;659:117–125. doi: 10.1016/0006-8993(94)90870-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. A learning model of addiction. Res Publ Assoc Res Nerv Ment Dis. 1992;70:157–177. [PubMed] [Google Scholar]
- Oei TP, Singer G. Effects of a fixed time schedule and body weight on ethanol self-administration. Pharmacol Biochem Behav. 1979;10:767–770. doi: 10.1016/0091-3057(79)90330-7. [DOI] [PubMed] [Google Scholar]
- Olive MF. Role of the amygdala in drug associated memories. Cell Science. 2009;6 [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti L, Diana M. Drug dependence as a disorder of neural plasticity: focus on dopamine and glutamate. Rev Neurosci. 2001;12:141–158. doi: 10.1515/revneuro.2001.12.2.141. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol Biochem Behav. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Tsai PJ, Wu JP, Lin NN, Kuo JS, Yang CS. In vivo, continuous and automatic monitoring of extracellular ascorbic acid by microdialysis and on-line liquid chromatography. J Chromatogr B Biomed Appl. 1996;686:151–156. doi: 10.1016/s0378-4347(96)00224-1. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Heidbreder CA, Spanagel R. The effects of lamotrigine on alcohol seeking and relapse. Neuropharmacology. 2007;53:951–957. doi: 10.1016/j.neuropharm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. discussion 951–965. [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]