Abstract
Stroke is associated with long-term functional deficits. Behavioral interventions are often effective in promoting functional recovery and plastic changes. Recent studies in normal subjects have shown that sleep, and particularly slow wave activity (SWA), is tied to local brain plasticity and may be used as a sensitive marker of local cortical reorganization after stroke. In a pilot study, we assessed the local changes induced by a single exposure to a therapeutic session of IMITATE (Intensive Mouth Imitation and Talking for Aphasia Therapeutic Effects), a behavioral therapy used for recovery in patients with post-stroke aphasia. In addition, we measured brain activity changes with functional magnetic resonance imaging (fMRI) in a language observation task before, during and after the full IMITATE rehabilitative program. Speech production improved both after a single exposure and the full therapy program as measured by the Western Aphasia Battery Repetition subscale. We found that IMITATE induced reorganization in functionally connected speech-relevant areas in the left hemisphere. These preliminary results suggest that sleep hd-EEGs, and the topographical analysis of SWA parameters, is well suited to investigating brain plastic changes underpinning functional recovery in neurological disorders.
Introduction
Stroke is a neurological condition caused by the interruption in blood supply to the brain. This leads to several cellular events (eg. excitotoxicity, acidotoxicity, peri-infarct depolarization and oxidative stress) causing brain cell death (Doyle et al. 2008). Although these events might cause death or permanent impairments, many patients undergo some spontaneous recovery, which can be fostered by rehabilitative therapy (Kalra 2010). The adult brain shows, in fact, a remarkable potential for plastic changes after stroke through the same mechanisms taking place during the normal development of the nervous system (Brown et al. 2007; Carmichael et al. 2005). For instance, synapse-based mechanisms, including homeostatic plasticity (Turrigiano and Nelson 2004) and hebbian plasticity (Hebb 1949) that influence plastic events both in the developing as well as in the adult healthy central nervous system, have also been described in brain injured patients and might be responsible for the remodeling of neuronal circuits following a stroke (Russmann et al. 2009).
An increasing amount of evidence in humans and animals suggests that sleep plays an important role in regulating synaptic plasticity and reorganization (Huber et al. 2004; Liu et al. 2010; Vyazovskiy et al. 2008): in fact, while wakefulness favors synaptic potentiation, sleep might promote synaptic depression in order to obtain a general rescaling of synaptic strength, therefore serving a homeostatic function (Tononi and Cirelli 2006). A reliable measure of the homeostatic phenomena regulating sleep is the amount of non-rapid eye movement (NREM) sleep slow wave activity (SWA). An intriguing implication of the link between sleep SWA and synaptic strength is that, as synaptic efficacy is strengthened during the day in a specific brain area, subsequent sleep SWA should be higher in that area. Conversely, employing procedures leading to a reduction in local synaptic efficacy should result in reduced SWA over the same area. These predictions have been experimentally confirmed in healthy humans (Huber et al. 2006; Huber et al. 2004). Indeed, SWA changes occur in the adult intact brain during sleep, following learning, immobilization or brain stimulation protocols (Huber et al. 2006; Huber et al. 2004; Huber et al. 2008; Maatta et al. 2010). Specifically, during sleep following learning, a local increase in SWA was observed over a cluster of electrodes located over the right posterior parietal cortex (Huber et al. 2004), the same cortical area showing significant activations when performing the learning task during wakefulness (Ghilardi et al. 2000). On the other hand, arm immobilization for an entire day (Huber et al. 2006) leads to a local decrease in SWA over the contralateral sensorimotor cortex during subsequent sleep.
In this perspective, the monitoring of sleep SWA following a session of post-stroke rehabilitative therapy might provide some hints about the principles underlying that specific rehabilitative approach. A new computer-based therapy, the Intensive Mouth Imitation and Talking for Aphasia Therapeutic Effects (IMITATE) has been proposed for post-stroke aphasia rehabilitation (Lee et al. 2010). IMITATE is an intensive six-week-long protocol that is based on action observation and imitation, which are thought to activate the brain circuits underlying a specific action in the observer, even without an explicit motor output (Buccino et al. 2001; Gallese et al. 1996). Interstingly, imitation stimulates the complex interaction between the inferior parietal lobule and the ventral premotor areas, which is thought to contribute to functional recovery (Buccino et al. 2004). Although it is based on a large body of evidence and on a strong theoretical background, IMITATE is still lacking the required objective measures showing reorganization and improvement in both physiological and behavioral outcomes (Thulborn et al. 1999; Warburton et al. 1999; Weiller 1998). For instance, while some imaging studies primarily found right hemisphere compensation, others have shown left hemisphere activity that correlated with language-related recovery (Heiss et al. 1997; Karbe et al. 1998). These findings imply a dynamic process for reorganization during aphasia recovery. As such, part of this study focused on the effects of IMITATE therapy on functional processing, specifically bilateral reorganization of a language-based core network in patients with left hemisphere stroke lesions.
The aim of this study was to begin assessing the plastic changes induced by IMITATE in post-stroke patients with non-fluent aphasia using both a quantitative analysis of sleep hd-EEG and a computational approach based on fMRI time series. Specifically, to clarify the bases of this specific rehabilitative approach we studied sleep SWA after a single IMITATE session. On the other hand, to investigate long lasting changes, we applied structural equation modeling to time series derived from functional MRI images and determined effective connectivity between core language processing regions post-stroke. Utilizing a characterized language-processing network identified in a healthy control population (Mashal et al., unpublished data), organization of this network was examined in patients prior to, during, and after IMITATE therapy assessing the changes in effective connectivity as a potential marker of recovery.
In the present work, we present preliminary data from a representative patient and discuss some implications of these results.
Methods
Subjects
Subjects were four patients who underwent ischemic stroke of the left hemisphere at least nine months before the experiment (NIH Stroke scale scores at the time of the experiment: P1=4; P2=9; P3=4; P4=3) and presented residual non-fluent aphasia (Western Aphasia Battery Aphasia Quotient: P1=95.9; P2=70.5; P3=86.9; P4=77.1) . They participated in a sleep hd-EEG study for two consecutive nights, before and after a day of a modified version of the IMITATE rehabilitation protocol. In order to maximize the amount of observable plastic changes in task-related areas, we modified the classical six week ninety minutes daily IMITATE session to a 3.5 hour intensive single day session one. The same patients underwent a fMRI study with repeated scan sessions before, during and after six weeks of the original IMITATE therapy.
IMITATE therapy
IMITATE is an intensive physiologically based therapy, which uses over 3,000 unique video clips of words and phrases spoken by six different standard American English speakers. During the therapy session, participants viewed for 20 seconds videos of six different speakers uttering a word or phrase, then a period during which to imitate the same word or phrase. The therapy uses ecologically valid stimuli (words and sentences spoken by a visible speaker using normal prosody), and has a component of graded incremental learning, such that as a patient improved in language skills, the task became more difficult. For a detailed description of IMITATE features see (Lee et al. 2010).
Sleep study procedures
Sleep procedures were carried out at the General Clinical Research Center at the University of Chicago Hospital. Sleep EEG was collected by means of a 256 sensor system (EGI, Inc). Patients were allowed to go to bed at their usual bedtime on both nights. After eight hours of time in bed, recording was stopped and the EEG sensor net was removed. Patients spent the morning after the Baseline sleep recording in their room at the Hospital, and were allowed to carry out their normal daytime activities. In the afternoon (around 2 PM), patients performed a 3 hour long IMITATE session divided in six blocks of half hour each. After dinner (around 8PM) they performed another half hour long block of IMITATE. Once rehabilitative procedures were terminated, EEG net was set up again and the patients were allowed to go to bed. Lights were turned off at the same time of the Baseline night and Post IMITATE night was recorded for eight hours. Both whole night hd-EEG recordings were staged in 20-second epochs according to standard criteria (Rechtschaffen and Kales 1968) based on standard polisomnographic derivations (C3-A2, C4-A1, Left electrooculogram, Right electrooculogram and neck electromiogram). EEG signals from the 256 channels were then filtered between 0.5 and 50 Hz and bad channels were rejected. Data were then transformed from the time to the frequency domain and average power spectra of consecutive four second artifact free epochs were calculated (Huber et al. 2004). SWA (average power spectral density between 1 and 4.5 Hz) was the range selected for topographical contrasts between the two conditions during the first NREM sleep episode.
fMRI study procedures
Imaging sessions were conducted 6 times in total. The first five sessions were 3 weeks apart, with the third being just before the start of IMITATE therapy and the fifth being at the end of the 6 week therapy window. The sixth imaging session was a follow-up 9 months post-therapy. During fMRI imaging, patients performed an “observation” task, in which they passively watched and listened to a video of a woman articulating four syllables: /pa/, /fa/, /ta/, and /tha/. These syllables were chosen as their varying lip and tongue movements create different articulatory profiles. The imaging run contained 120 event-related randomized stimuli (30 of each syllable, ISI=0 to 12 seconds), and was 6 min 30 seconds in duration (with 260 time points collected).
Structural Equation Modeling (SEM) was performed using the AMOS software package (SPSS, Inc., Chicago, IL). Patient models of language processing during the “observation” task were compared to previously identified normative left and right hemisphere models for this same task (Mashal et al., unpublished data). The normative models, derived from a healthy control population, were constructed using time series extracted from the peak voxel of each of 6 regions of interest (ROIs): primary sensorimotor cortices (M1), dorsolateral pre-motor cortex (LPMCd), ventral premotor cortex (LPMCv) including pars opercularis of the inferior frontal gyrus, inferior parietal lobule (IP) including the intraparietal sulcus, posterior superior temporal gyrus and sulcus (postST), and anterior superior temporal gyrus and sulcus (antST). ROIs were identified by anatomical parcellation of the cerebral cortex for each patient using the Freesurfer software package (http://surfer.nmr.mgh.harvard.edu).
Left and right hemisphere models of this core language network were constructed individually for each patient using extracted time series from said regions of interest, and comparing the patient model to the normative model at each time point during the study. Using the normative model (Figure 1) as the predicted model, structural equations relating weights and directional influence of network nodes were derived and iterative maximum likelihood solutions were obtained. An acceptance (p≥0.05) or rejection (p<0.05) of the null hypothesis (no difference between predicted and observed models) was determined from the Chi-squared solution distribution.
Figure 1.
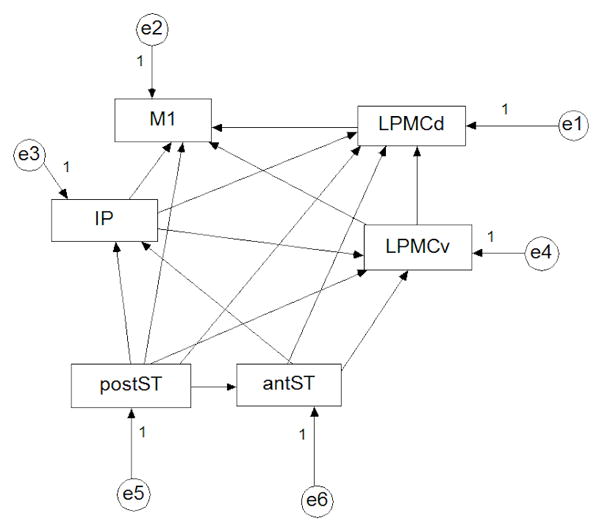
The normative unweighted model for the “observation” task (same for left and right hemispheres), with directional influences between endogenous variables (ROIs: M1: primary sensorimotor cortices; LPMCd: dorsolateral pre-motor cortex; LPMCv: lateral ventral premotor cortex; IP: inferior parietal lobule; postST: posterior superior temporal gyrus and sulcus; antST: anterior superior temporal gyrus and sulcus) shown with arrows. Error terms represented by “e” variables.
Behavioral testing
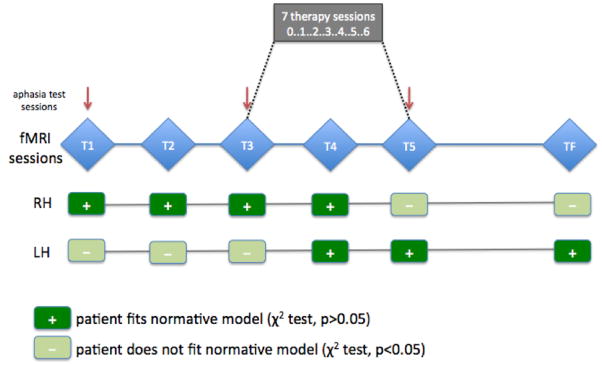
The Western Aphasia Battery (WAB) (Kertesz, 1982) was used in order to assess patients' aphasia level before and after the therapy. In the sleep study, WAB was administered before the Baseline and after the Post IMITATE night of sleep, so as to evaluate the effect of the intensive rehabilitation on speech performance. On the other hand, for the fMRI study, WAB was administered at the beginning of the study (T1), at the beginning of the therapy (T3) and at the end of the therapy (T5) (see Figure 3) so as to evaluate the long lasting effect of the full IMITATE therapy protocol.
Figure 3.
Time table of imaging sessions, IMITATE therapy sessions, and aphasia testing sessions, and results of comparison to normative model for one representative patient. Five time points (3 week intervals) of fMRI sessions are shown as T1-T5 plus follow-up session at 9 months post-therapy (TF). Behavioral aphasia testing sessions are indicated by red arrows, and the 7 therapy sessions are shown between T3 and T5. Results for comparison between the normative model and single patient at each time point in the left (LH) and right (RH) hemispheres are shown in the lower half of the figure. + indicates that the null hypothesis was accepted (p>0.05; no difference between predicted and observed models) at that time point.
Results
The four patients showed an average 6% ±0.6 increase in the Repetition WAB subscale after the single exposure to IMITATE, thus suggesting a potential positive functional outcome deriving from the intensive rehabilitation session. Although data acquisition for the fMRI study is still ongoing for three out of the four patients, preliminary observations presented below for a representative patient are suggestive of a positive sustained behavioral outcome also after the six week IMITATE treatment.
Sleep EEG SWA
Results for a representative patient (P3) who completed both studies are shown in Figure 2. This patient showed a slight increase in the Repetition WAB subscale score (Pre Baseline: 86, Post IMITATE: 88) after a single exposure to the 3.5 hour rehabilitation session. We found a local increase in SWA during the initial 30 minutes of the first NREM sleep cycle calculated as percentage difference from the Baseline night. This part of the night is the one showing the highest SWA and has been reported elsewhere to present SWA changes after learning (Huber et al. 2004). These changes were mainly involving the hemisphere ipsilateral to the lesion (Figure 2). Specifically, the electrodes showing SWA increase were located in a scalp area overlying the left pre motor and inferior parietal cortices. There was also a marked increase in SWA over the frontal areas that involved both hemispheres with a peak at the right frontal derivations.
Figure 2.
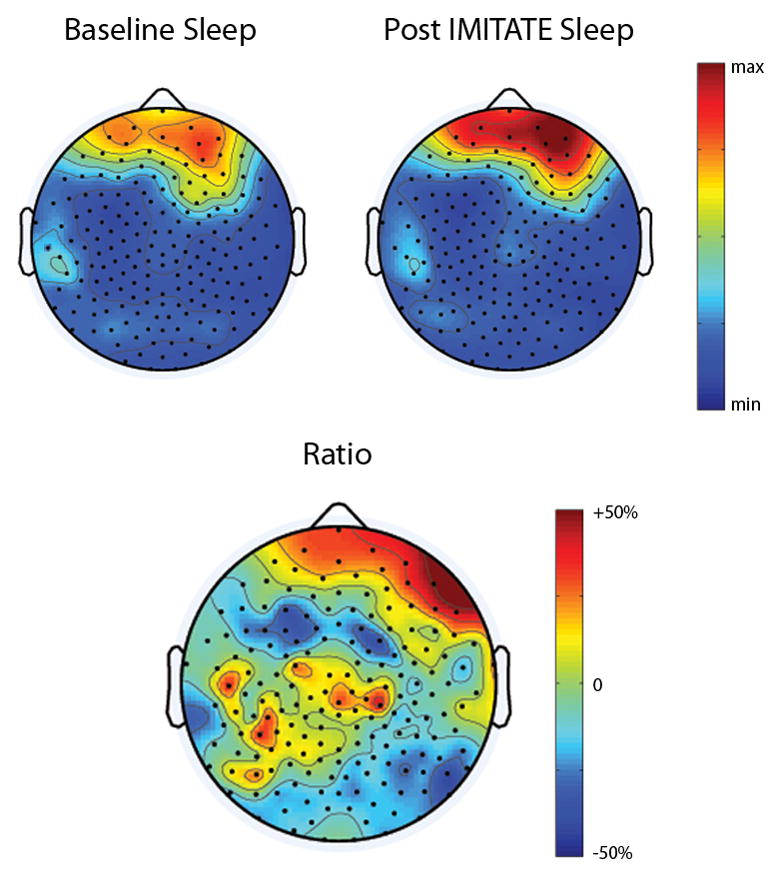
(top) Topographic distribution of power in the SWA range during the first 30 minutes of the first NREM sleep episode. Data collected at baseline (top-left) and after IMITATE speech therapy (top-right) on a single representative patient. Topographic features of SWA are highly reproducible across nights showing a right asymmetry due to the left hemispheric brain lesion. (bottom) Local effects of IMITATE on sleep SWA. Changes in sleep SWA after IMITATE measured as percentage difference from the baseline.
SEM
Findings from the same representative patient are shown in Figure 3, with an indication of whether or not the patient model was a good “fit” (i.e., Χ2 distribution of solution indicates no significant difference) to the normative model at each fMRI session time point (T1-T5 and TF). While the right hemisphere language processing core network trended away from the normative model over time, the left hemisphere network appeared to reorganize more like the normative model. For the right hemisphere, the patient resembled healthy controls before (T1 and T2) and at the start of therapy (T3 and T4), but by the end of the therapy protocol no longer resembled the normative group (T5). Additionally, at the follow-up session 9 months post-therapy (TF), the patient still did not resemble the normative model. The left hemisphere reorganization, in contrast, appeared to trend towards the control model during therapy. Before (T1 and T2) and at the start of therapy (T3) the patient network model did not resemble the control model, but did during (T4) and after therapy (T5) as well as at the 9 month follow-up (TF). Additionally, as in the sleep study, the patient showed a modest behavioral improvement during therapy on the Repetition subscore (T1: 91, T3: 87, T5: 94). Intriguingly, after the six week therapy, also Aphasia Quotient WAB scores sensibly improved (T1: 80.9, T3: 78.6, T5: 84).
Discussion
In this study we employed sleep hd-EEG for the first time as a tool to assess functional physiological measures of rehabilitation in a neurological population. Preliminary results show that IMITATE induced acute local changes in SWA during the night following an intensive session of the rehabilitative protocol. SWA changes were particularly evident over the left hemisphere in the areas predicted by the therapeutic rationale. IMITATE also produced long term reorganization of a left hemispheric functional network whose effective connectivity trended towards a normative model after the administration of the full six week therapy on the same patient. The right hemisphere network also showed a trend after therapy, away from the normative model, which potentially suggests a different but existent effect of IMITATE on reorganization contralateral to the lesion. Behaviorally, IMITATE exposure resulted in improved language skills as measured by WAB Repetition Scale both after a single exposure and after the full six week approach. Particularly intriguing is the dissociation between the right and the left hemisphere relative to IMITATE exposure. In fact, while right hemisphere involvement seem to be more associated with early therapeutic effect, left hemisphere involvement in areas sensitive to IMITATE protocol seem to be more stable both in an acute phase and on the long term and might be predictive of positive long term behavioral outcome. Right hemisphere involvement during and post-IMITATE is unclear, though may not be as predictive of long term behavioral outcome as the left hemisphere. These data are in line with previous human and animal models of stroke recovery revealing functional and structural neural plasticity occurring both in perilesional areas and in brain regions distant from the lesion site (Heiss et al. 1997; Karbe et al. 1997; Nudo 1997; Zhang et al. 2004), and further imply a dynamic process for aphasia recovery in language processing regions. Altogether, these preliminary findings are suggestive of plastic changes occurring in areas activated during the execution of IMITATE, and may reflect the effectiveness of such intervention. Moreover, these results reinforce the notion that sleep hd-EEGs, and the topographical analysis of SWA parameters, is well suited to investigating local brain plastic changes underpinning functional recovery in neurological populations, allowing for a non-invasive and repeatable assessment of such changes.
Acknowledgments
Study funded by the National Institutes of Health under grant number R01DC007488, the National Institute of Neurological Disorders and Stroke under grant number R01NS055185 and by the James S. McDonnell Foundation grant to the Brain Network Recovery Group (BrainNRG). Their support is gratefully acknowledged.
References
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: An event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119 ( Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Heiss WD, Karbe H, Weber-Luxenburger G, Herholz K, Kessler J, Pietrzyk U, Pawlik G. Speech-induced cerebral metabolic activation reflects recovery from aphasia. J Neurol Sci. 1997;145:213–217. doi: 10.1016/s0022-510x(96)00252-3. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Maatta S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra L. Stroke rehabilitation 2009: old chestnuts and new insights. Stroke. 2010;41:e88–90. doi: 10.1161/STROKEAHA.109.572297. [DOI] [PubMed] [Google Scholar]
- Karbe H, Herholz K, Kessler J, Wienhard K, Pietrzyk UE, Heiss WD. Recovery of language after brain damage. Adv Neurol. 1997;73:347–358. [PubMed] [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64:215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- Lee J, Fowler R, Rodney D, Cherney L, Small SL. IMITATE: An intensive computer-based treatment for aphasia based on action observation and imitation. Aphasiology. 2010;24:449–465. doi: 10.1080/02687030802714157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta S, Landsness E, Sarasso S, Ferrarelli F, Ferreri F, Ghilardi MF, Tononi G. The effects of morning training on night sleep: a behavioral and EEG study. Brain Res Bull. 2010;82:118–123. doi: 10.1016/j.brainresbull.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. Remodeling of cortical motor representations after stroke: implications for recovery from brain damage. Mol Psychiatry. 1997;2:188–191. doi: 10.1038/sj.mp.4000188. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, Md: Neurological Information Network; 1968. [Google Scholar]
- Russmann H, Lamy JC, Shamim EA, Meunier S, Hallett M. Associative plasticity in intracortical inhibitory circuits in human motor cortex. Clin Neurophysiol. 2009;120:1204–1212. doi: 10.1016/j.clinph.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature Reviews Neuroscience. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C. Imaging recovery from stroke. Exp Brain Res. 1998;123:13–17. doi: 10.1007/s002210050539. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]