Abstract
Covert attention, the selective processing of visual information in the absence of eye movements, improves behavioral performance. Here, we show that attention, both exogenous (involuntary) and endogenous (voluntary), can affect performance by contrast or response gain changes, depending on the stimulus size and the relative size of the attention field. These two variables were manipulated in a cueing task while varying stimulus contrast. We observed a change in behavioral performance consonant with a change in contrast gain for small stimuli paired with spatial uncertainty, but a change in response gain for large stimuli presented at one location (no uncertainty) and surrounded by irrelevant flanking distracters. A complementary neuroimaging experiment revealed that observers’ attention field was wider with than without spatial uncertainty. Our results support key predictions of the normalization model of attention, and reconcile previous, seemingly contradictory, findings on the effects of visual attention.
Introduction
There has been a long-standing debate with regard to the neural computations underlying selective visual attention1–5. Experiments examining how covert attention modulates visual performance and neuronal activity in visual cortex have documented a variety of empirical phenomena, some of which appear to be mutually contradictory. Central to this debate are studies of the interactions between attention and stimulus contrast. The results of some experiments suggest that attention increases neuronal responses by a multiplicative response gain factor6 (Fig. 1a) and similarly improves performance via upward-scaling of the psychometric function7–12. Other neurophysiological and behavioral experiments suggest a change in contrast gain (Fig. 1b), i.e., a leftward shift of the contrast-response2,13–15 and psychometric9,10 functions. Still other results suggest an additive effect of attention across the entire contrast range or a combination of both response gain and contrast gain changes16–18. These ostensibly contradictory findings have been taken to represent alternative models of attention2,4,6,10,14,15,19.
Fig. 1.
Normalization model of attention exhibits qualitatively different forms of attentional modulation, depending on stimulus size and attention field size (adapted from Ref. 1). Each panel shows contrast-response functions for a simulated neuron, when attending to a stimulus within the neuron’s receptive field and when attending to a stimulus in the opposite hemifield. (a) Response gain (largest effects at higher contrasts; upward-shift of the contrast-response function) for large stimulus size and small attention field. (b) Contrast gain (largest effect at intermediate contrasts; appears as a leftward-shift of the contrast-response function) for small stimulus size and large attention field. Red, simulated responses as a function of contrast when stimuli in the receptive field were attended. Blue, simulated responses when attending to the opposite hemifield. Only stimulus size and attention field size were changed in simulations; all other model parameters were identical in both panels. Icons: solid black circle, simulated receptive field size; dashed red circle, simulated attention field size; vertical black grating, stimulus size.
The normalization model of attention1 was proposed to reconcile these (and other) seemingly conflicting, empirical findings of the effects of attention on sensory responses in visual cortex. This computational theory identified two critical factors that determine the effect of attention on contrast-response functions and, consequently, on behavioral psychometric functions: the size of the stimulus and the spatial spread of attention, called the “attention field”. Changing the relative sizes of these two factors allows the model to exhibit response gain changes, contrast gain changes, and various combinations of response gain and contrast gain changes. Specifically, the model predicts that attention increases response gain when the stimulus is large and the attention field small, and increases contrast gain when the stimulus is small and the attention field large (Fig. 1)1. The core idea is that the attention field reshapes the distribution of activity across the population of neurons, shifting the balance between excitation and suppression, thus yielding either a change in response gain, contrast gain, or a combination of the two (see Ref. 1 for mathematical derivation). Note that changing the size and shape of the attention field, on its own, would not predict a shift between response gain changes and contrast gain changes. Rather, it is the architecture of the model that makes this prediction; specifically, because the effect of attention is to multiply the stimulus-evoked activity before divisive normalization1. Alternatively, if attention modulated activity after normalization then it would always yield response gain changes, regardless of the size or shape of the attention field.
Other computational models of attention, although ostensibly similar to the normalization model of attention1, do not predict the shift from response- to contrast-gain changes. Some of these models presume that spatial attention always has the same effect on the excitation and suppression (i.e., the numerator and the denominator of the normalization equation), hence always yielding a contrast gain change2,20 (see also Refs. 4, 21). In another model, attention affects only the strength of the normalization, hence always yielding a response gain change19. Unique to the normalization model of attention is that the effects of attention on the numerator and denominator can differ, depending on the relative sizes of the stimulus and the attention field, hence altering the balance between excitation and suppression.
Here, we performed the first empirical test of the normalization model of attention1; we tested the key prediction that the effect of attention can systematically shift from a change in response gain to contrast gain with smaller stimuli and a broader attention field. There is evidence that the attention field is measurable, that it is flexible in size (within limits), and that it can be experimentally manipulated21–24. Previous studies, however, have not systematically manipulated the size of the attention field and its relation to stimulus size. We used spatial uncertainty to manipulate attention field size (as detailed below) and we confirmed, in a complementary experiment using functional magnetic resonance imaging (fMRI), that the attention field was larger with spatial uncertainty than without it.
We adopted established experimental protocols to investigate two types of attention, exogenous and endogenous7,25–29. Exogenous attention is involuntary, stimulus-driven, and has a transient effect, which peaks at about 100 ms and decays shortly thereafter. Endogenous attention is voluntary, conceptually driven (e.g., according to instructions), and has a sustained effect, which takes about 300 ms to be deployed and can last up to seconds. These two types of attention have been shown to have distinct effects on behavioral performance9,10,26,27,29,30. We found, for both exogenous and endogenous attention, that the effects of attention on behavioral performance shifted from one type of gain modulation to the other by manipulating only stimulus size and attention field size.
Results
Psychophysics: Response gain and contrast gain
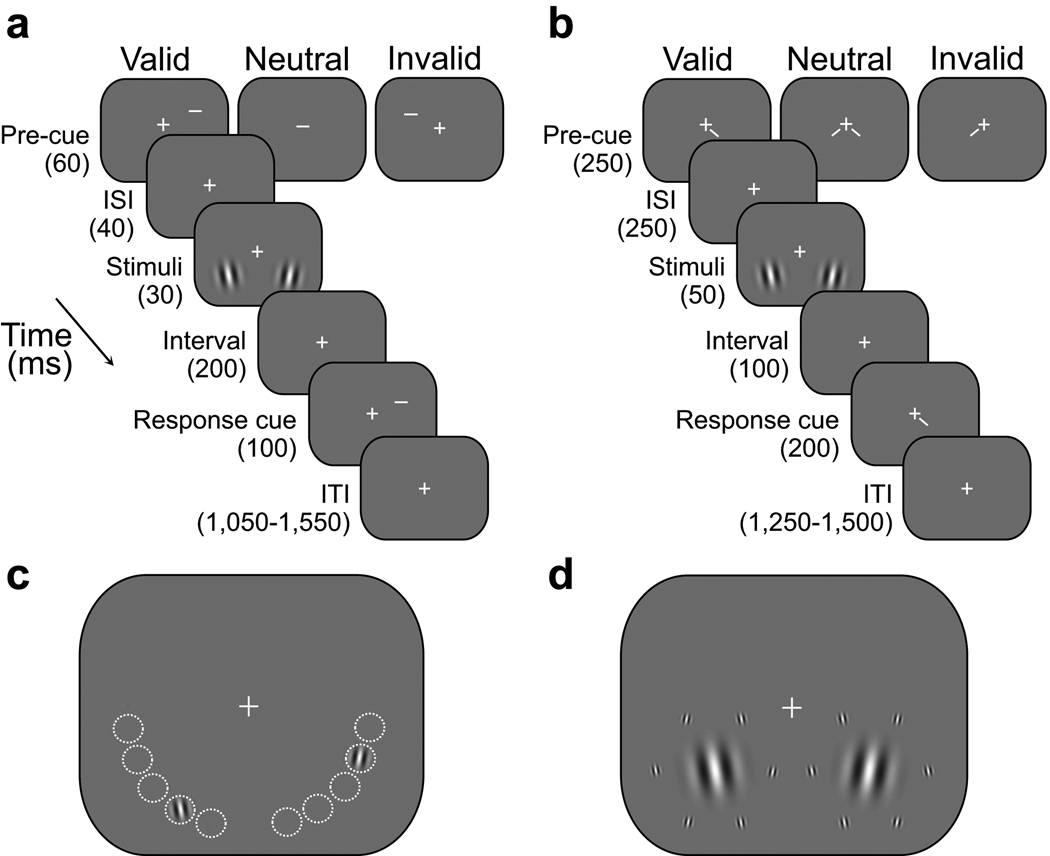
Experimental parameters were optimized (see Methods) to maximize the effects of the two types of attention: exogenous (Fig. 2a) and endogenous (Fig. 2b)7–9,27,29,31. In both experiments, observers performed an orientation discrimination task on one of two grating patches, for each of several contrasts (the contrasts of both gratings were identical on any given trial and co-varied across trials in random order). Covert attention (without eye movements) was directed by a pre-cue. A response-cue at stimulus offset indicated the target location, yielding three cue conditions: valid (pre-cue matched response-cue), invalid (mismatched), and neutral. Comparing performance accuracy (d’) for valid, neutral and invalid trials revealed differences in behavioral performance: benefits at the cued location and costs at the uncued location compared to the neutral condition. To enlarge the attention field, stimuli were presented with spatial uncertainty (1 of 5 locations randomly interleaved, Fig. 2c). To narrow the attention field, stimuli were presented at a fixed location, and for endogenous attention, they were also surrounded with irrelevant, flanking distracters. Smaller stimuli were paired with spatial uncertainty (Fig. 2c) and larger stimuli were paired with no uncertainty (Fig. 2d).
Fig. 2.
Experimental protocols. (a) Exogenous attention task. (b) Endogenous attention task. (c) Small stimuli were presented with spatial uncertainty, at one of five pre-defined isoeccentric locations. Across trials, stimulus locations varied randomly and independently on the left and right sides to encourage observers to employ a larger attention field. The dashed, white circles indicate the possible stimulus locations (not displayed during the experiments). (d) Large stimuli were presented at fixed stimulus locations with no spatial uncertainty (centered at the middle of the 5 locations of panel c). To narrow the size of the attention field for endogenous attention, the two large Gabor stimuli were each surrounded by six irrelevant distracters. See Methods for details.
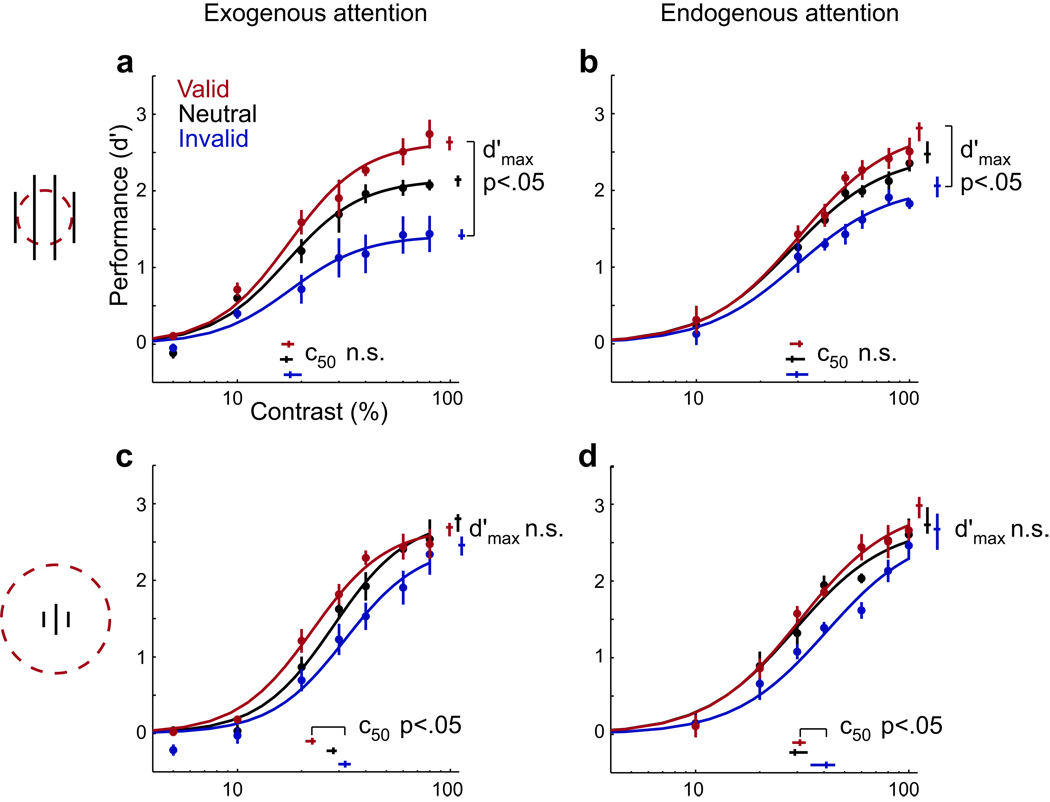
Both exogenous and endogenous attention exerted different gain changes on psychometric functions (either contrast gain or response gain changes), depending on stimulus size and uncertainty. The measured psychometric functions were fit (nonlinear least-squares, see Methods) with a parametric function. The two parameters d’max (asymptotic performance at high contrast levels) and c50 (the contrast yielding half maximum performance) determined response gain and contrast gain, respectively. They were estimated separately for each attention condition, while the exponent n (slope) was constrained to have the same value for all conditions.
When the targets were large and the spatial uncertainty minimal (encouraging deployment of a relatively small attention field), attention increased asymptotic performance at high contrasts, as predicted by a response gain change (Fig. 3a,b, Suppl. Tables 1–2; robust differences in d’max but no evidence for a change in c50). Conversely, when the targets were small and the attention field relatively large due to spatial uncertainty, attention altered performance primarily for intermediate contrasts, as predicted by a contrast gain change (Fig. 3c,d, Suppl. Tables 1–2; reliable differences in c50). There was a trend for a change in d’max that was not statistically significant; this trend was not inconsistent with the predictions of the normalization model of attention because the model predicts a pure contrast gain change only in the limit as the attention field size increases. Best-fitting values for the exponents of the psychometric functions were similar for the exogenous (n=2.48; 68%-CI=[2.37; 2.66]) and endogenous (n=2.00, 68%-CI=[1.85; 2.22]) attention experiments (Suppl. Fig. 1), and were in the range of values reported for contrast-response functions in early visual cortex32. We refit the data, allowing the exponent to vary independently across conditions, and observed the same pattern of results: a response gain change for large stimuli with no uncertainty and a contrast gain change for small stimuli with spatial uncertainty.
Fig. 3.
Effects of exogenous and endogenous attention on performance (d’) as a function of contrast. (a,b) Large stimulus with small attention field. (c,d) Small stimulus with large attention field. (a,c) Exogenous attention. (b,d) Endogenous attention. Each panel plots psychometric functions for each attentional condition (valid, neutral and invalid pre-cues) and parameter estimates (c50 : contrast yielding half-maximum performance, d’max: asymptotic performance at high contrast). Exponent n (slope) was constrained to have the same value for all pre-cue conditions. Each data point represents the mean across observers. Error bars on data points are ±1 SEM (n=4 observers in each experiment, 9,408 to 14,910 trials per observer, except one observer who completed 21,000 in the exogenous experiment). Error bars on parameter estimates are 68%-confidence intervals, obtained by bootstrapping.
The same results were evident in the psychometric functions from individual subjects (Fig. 4). Large stimuli with small attention fields yielded similar c50 values for valid and invalid pre-cues (Fig. 4a, red symbols; one-tailed Wilcoxon: p=.500), whereas small stimuli with large attention fields resulted consistently in smaller c50 values for valid pre-cues (Fig. 4a, blue symbols; one-tailed Wilcoxon: p=.007). Large stimuli with small attention fields resulted reliably in larger d’max values for valid compared to invalid pre-cues (Fig. 4b, red symbols; one-tailed Wilcoxon: p<.001), whereas small stimuli with large attention fields resulted in similar d’max values for the two pre-cue conditions (Fig. 4b, blue symbols; one-tailed Wilcoxon: p=.221).
Fig. 4.
Effects of stimulus and attention field size on parameter estimates of individual observers. (a) Contrast yielding half-maximum performance (c50), estimated for valid pre-cues versus invalid pre-cues. (b) Asymptotic performance at high contrast (d’max). Red symbols, large stimulus with small attention field. Blue symbols, small stimulus with large attention field. Open symbols, individual observers. Filled symbols, mean across observers. Squares, exogenous attention. Circles, endogenous attention. Observer 1 participated in both the exogenous and endogenous attention experiments and is marked with diagonal lines through the symbols.
As a complementary way of testing the predictions of the model, we used nested hypothesis tests (nested F-tests) to compare the full model fit with four restricted models: (1) normalization model prediction, constraining c50 to be constant across cue conditions (valid, neutral, invalid) for large stimuli without uncertainty and constraining d’max for small stimuli with uncertainty; (2) opposite to the normalization model prediction, constraining d’max for large stimuli without uncertainty and constraining c50 for small stimuli with uncertainty; (3) always response gain, constraining c50 parameter for both experimental conditions; (4) always contrast gain, constraining d’max for both experimental conditions. As predicted by the normalization model of attention, the full model fit the mean data (across subjects) similarly well as model 1 (exogenous p>.1 and endogenous p>.1), and fit better than any of the other restricted models (model 2: exogenous p<.001 and endogenous p=.002; model 3: exogenous p=.035 and endogenous p>.1; model 4: exogenous p<.001 and endogenous p=.006). Only one of these 8 nested hypothesis tests failed to reject an alternative to the normalization model: for endogenous attention, the full model fit better than model 3, as predicted by the normalization model, but it was not statistically significant. A non-parametric cross-validation analysis of the model fits yielded results consistent with the nested F-tests. In short, for exogenous attention we can reject contrast-gain-only and response-gain-only in favor of the normalization model; for endogenous attention we can reject contrast-gain-only but did not have enough statistical power to reject response-gain-only. As stated above, this is not inconsistent with the predictions of the normalization model of attention because the model predicts a pure contrast gain change only in the limit as the attention field size increases.
Fixation was stable during stimulus presentation in the exogenous and endogenous attention experiments (Suppl. Fig. 2 depicts gaze positions of a representative observer). The recorded gaze positions of all observers had a standard deviation of 0.185° horizontally and 0.300° vertically in the exogenous experiment, and 0.257° horizontally and 0.426° vertically in the endogenous attention experiment. Gaze position distributions were statistically indistinguishable for all conditions (leftward, rightward and neutral pre-cues; with or without spatial uncertainty) in both experiments. Saccades were detected in 0.113% and 0.749% of the trials in the exogenous and endogenous attention experiments, respectively.
fMRI: attention field size
The size of the attention field was larger when stimuli were presented with spatial uncertainty compared to when the stimuli were presented at the same location without spatial uncertainty (Fig. 5). We used fMRI to measure attention field size while observers performed the endogenous attention task in the MRI scanner (see Methods). We analyzed activity in primary visual cortex (V1), as opposed to other visual areas (cortical or subcortical), for the following reasons: (1) V1 is known to process contrast and orientation, (2) attention robustly modulates fMRI responses in V128,33–37 and concurrently improves behavior28,34, and (3) V1 is relatively large compared to other visual areas, with a precise retinotopic map, enabling us more easily to quantify the spatial extent of activation. To evaluate whether spatial uncertainty encouraged observers to enlarge their attention field, we compared the spread of cortical activity brought about by attention in two conditions: spatial uncertainty and no spatial uncertainty. In one condition, a pair of stimuli (10% contrast) appeared in the left and right hemifields, randomly and independently at one of five predefined locations in each hemifield. In the second condition, stimuli of the same size and contrast were always shown at a fixed pair of locations (the middle of the 5 locations in the first condition) in the left and right hemifields, with placeholders continuously indicating these two locations.
Fig. 5.
Attention field size depends on spatial uncertainty. (a) fMRI responses at a series of locations in left primary visual cortex (V1), for a typical observer, when a small stimulus was presented at the center of the 5 possible locations, during the experiment with spatial uncertainty. Upper abscissa, distance along a 6° isoeccentric arc in the visual field that included the stimulus location. Zero corresponds to the location where the arc crosses the lower vertical meridian. Lower abscissa, distance in deg of polar angle. Black symbols, fMRI response differences (cued minus uncued), plotted at locations corresponding to the centroids of each of several V1 ROIs, defined based on retinotopic mapping. Open symbol, location corresponding to the center of the stimulus. Gray symbol, estimated center of the attention field. Gray line, estimated spread of the attention field. (b) fMRI measurements of the spread of cortical activity in V1, in units of arc deg of visual angle in the visual field, with spatial uncertainty versus without spatial uncertainty. Each symbol shape corresponds to one observer. Gray, left hemisphere. Black, right hemisphere.
fMRI response amplitudes were measured at each of several locations in V1 corresponding retinotopically to an arc in the visual field that included the 5 stimulus locations (see Methods). Data were analyzed from the first (spatial uncertainty) condition only for trials on which the stimuli were presented at the middle of the 5 locations. Thus, responses were measured for stimuli of identical size at the identical stimulus location, but with and without spatial uncertainty. We then subtracted the response amplitudes for uncued stimulus presentations from the response amplitudes for cued stimuli presented at the same stimulus location, largely removing any stimulus-evoked response. This resulted in a spatial distribution of response differences, i.e., the attention field, for each condition (with and without spatial uncertainty), separately for left and right hemisphere V1 from each observer. Finally, attention field size was quantified as the spread of this spatial distribution of response differences (Fig. 5a), and found to be larger when stimuli were presented with spatial uncertainty than without it (one-tailed Wilcoxon-test: p=.0057; Fig. 5b).
Eye position was measured during the fMRI experiment and, as in the behavioral experiments; fixation was stable (Suppl. Fig. 3 depicts gaze positions of a representative observer for both conditions). We analyzed eye movements for the spatial uncertainty condition only for trials in which stimuli were presented at the middle of the 5 locations. Recorded gaze positions of all observers during stimulus presentation had a standard deviation of 0.326° horizontally and 0.443° vertically with spatial uncertainty, and 0.438° horizontally and 0.532° vertically without spatial uncertainty. Gaze position distributions were statistically indistinguishable for all conditions (leftward and rightward pre-cues; with or without spatial uncertainty), and no saccades were detected.
Discussion
The interpretation of the behavioral data is based on a model of the noise or variability in neuronal activity that limits behavioral performance along with a model of the decision-making process10,38. Performance accuracy, d’, is proportional to the signal-to-noise ratio (SNR) of the underlying neuronal responses. Hence, with additive, independent, and identically-distributed (IID) noise, and a maximum-likelihood decision rule, any change in the neuronal contrast-response functions would be reflected as a parallel change in performance accuracy. A change in response gain of the underlying neuronal responses (Fig. 1a) would yield a scaling of the psychometric function (Figs. 3a,b); a change in contrast gain of the underlying neuronal responses (Fig. 1b) would yield a horizontal shift (on the log contrast axis) of the psychometric function (Figs. 3c,d). This interpretation of our behavioral results depends on the assumption that behavioral performance is limited by additive IID noise, and although there are psychophysical data suggesting that this may be the case39, we must also consider the possibility that performance is instead limited by the Poisson-like noise evident in single-cell firing rates40,41. But this alternative model of the noise, coupled with a maximum-likelihood decision rule38 leads to exactly the same interpretation of the psychometric functions10.
The exogenous attention task may have involved some degree of endogenous attention as well. The advantage of using an exogenous cue is that it results in a highly controlled manipulation of attention, not dependent on the observer’s strategy7,8,27,29. However, observers knew before each trial that the discrimination task was to be performed on one of two stimuli and hence could have directed endogenous attention to both, and it is not known exactly how exogenous attention (driven by the pre-cue) and endogenous attention (driven by the observer’s knowledge of the task) combine. One possibility, which is theoretically tractable and consistent with our results, is that they combine multiplicatively. An alternative possibility is that exogenous attention completely overrides endogenous attention; our results suggest that this is not the case, because adding spatial uncertainty shifted performance from a response gain change to a contrast gain change.
Spatial uncertainty was used to manipulate attention field size, but we ensured that this manipulation did not affect task difficulty and performance accuracy. Specifically, we adjusted the degree of stimulus tilt separately for each observer and for each of the two stimulus and attention field sizes, so that performance was approximately 85% correct at full contrast with a neutral cue. Moreover, we did not compare performance between conditions with different amounts of uncertainty. Rather, within each condition (small stimuli with large uncertainty, large stimuli with no uncertainty), we compared performance for valid, invalid, and neutral cues; hence, there was no difference in spatial uncertainty within each of these comparisons.
We used fMRI to measure attention field size by taking advantage of the fact that neuromodulatory inputs contribute to the fMRI responses. The attention field, according to the normalization model of attention, is hypothesized to be a neuromodulatory input, largely invisible in extracellular electrophysiological measurements of spiking activity. Although it is known that the fMRI signal is triggered by metabolic demands of neural activity, the details of this process are only partially understood, and it is likely that a mixture of synaptic and spiking activity may contribute42,43. The extent of decoupling between synaptic and spiking activity depends on the nature of cortical processing, i.e., whether the cortical activity is dominated by local recurrent circuitry or by synaptic inputs to a cortical area (either feedforward or feedback) from other brain areas. Consequently, fMRI measurements may be highly correlated with the output spiking activity of a visual area under some circumstances, but with sub-threshold neuromodulatory input under other circumstances. Indeed, strong neuromodulatory input due to attention might explain the apparent discrepancy in the literature between attentional effects measured with fMRI and electrophysiologically. The implication is that fMRI is not merely a low-resolution, noninvasive measure of spiking activity, but rather that it constitutes an independent, complementary measure44.
We measured attention field size using low contrast stimuli with high attentional demands to put the cortical circuits into a regime in which the cortical activity is dominated by feedback synaptic inputs. The spread of cortical activity was significantly larger with spatial uncertainty than with no uncertainty. These results provide converging evidence to the idea that manipulating spatial uncertainty can affect attention field size21–24,45,46. In future studies, behavioral and neuroimaging data, acquired simultaneously, will be used to further test predictions of the normalization model of attention1, e.g., by simultaneously fitting measurements of behavioral performance and attention field size, or by binning trials according to attention field size and testing for systematic differences in behavioral performance.
Previous studies have reported ostensibly conflicting results with regard to the gain changes induced by selective attention. A review of the literature1 suggests that discrepancies in the neurophysiological findings may have resulted from differences in the experimental protocols, specifically the relative sizes of the stimuli and attention fields, which have not been systematically controlled and manipulated. Based on the present results, we suggest that such differences in the experimental protocols may also explain previous discrepancies among psychophysical studies7–12,18. For instance, with constant stimulus size some studies demonstrated that exogenous attention altered performance via a response gain change, whereas endogenous attention did so via a contrast gain change7–10. A response gain change could have been elicited by brief peripheral cues nearby the stimulus, whereas a contrast gain change could have resulted from endogenous cues at fixation, not adjacent to the stimulus, which may have encouraged a narrower or larger attention field, respectively. Here we show that with both types of attention we can manipulate the stimulus size and the size of the attention field to yield either contrast gain or response gain changes.
We conclude that attention modulates activity in visual cortex in a manner that can resemble either a change in response gain or contrast gain, depending on stimulus size and attention field size. Our results provide the first experimental evidence supporting key and unique predictions of the normalization model of attention, thereby furthering our understanding of the processing in visual cortex and the neural computations underlying visual attention.
Methods
Experiments were conducted with the written consent of each observer, and procedures were approved by the University Committee on Activities Involving Human Subjects at New York University.
Observers and psychophysical sessions
Seven observers (5 female; 25–40 years old) with normal or corrected vision participated in the experiments. Half were assigned to the exogenous and the other half to the endogenous attention experiment (one participated in both). For each experiment, observers completed both conditions (small stimuli paired with large attention field and vice versa) in a counter-balanced order. For each condition, observers participated in practice sessions to determine individual orientation discrimination thresholds, followed by 4 to 6 1-hr experimental sessions.
Exogenous attention task
On each trial, a pair of 5 cycles/deg Gabor stimuli were presented in the lower quadrants of the left and right hemifields at 5° eccentricity, one of which was the target. Contrast varied from trial to trial in randomly shuffled order, and stimuli were presented briefly, to avoid any possible dependence of attentional state on stimulus contrast. Observers performed an orientation discrimination task to indicate whether the 30 ms target was tilted left or right of vertical, and received auditory feedback if their response was incorrect. On two-thirds of the trials, exogenous attention was directed by flashing the 60 ms pre-cue (0.5° white line) above one of the stimulus locations, but not at the stimulus location to avoid masking. On the remaining trials, a neutral pre-cue was presented at fixation, providing a baseline against which to evaluate the effects of attention. A 40 ms inter-stimulus interval (ISI) between cue offset and stimulus onset, yielding a cue-stimulus onset asynchrony of 100 ms, maximized the effect of exogenous attention27,29,31. Target location was indicated by a peripheral 100 ms response-cue (0.5° green line). A valid cue was defined as a match between the pre-cue location and the response-cue location (one-third of the trials); a mismatch yielded an invalid cue (one-third of the trials). Observers were explicitly told that pre-cues were randomized and uninformative about the target location.
Endogenous attention task
Similar to the exogenous attention task, except for the following modifications. Durations of the central pre-cues (250 ms) and ISI (250 ms) were increased to ensure observers had enough time to deploy endogenous attention27,29,47. Stimuli were presented at 6° eccentricity. A 200 ms response-cue appeared right or left of the fixation cross. One-fourth of the trials had neutral cues. Of the remaining trials, two-thirds were valid and one-third invalid to ensure that observers followed the cue25,29. Observers were explicitly told that the cue was informative regarding the target location and that there was a benefit in using the cue to perform the task. Using a partially valid cue allowed us to assess both the benefit and cost of attention at the attended and unattended locations, respectively, even though observers are known to treat the ~70% valid cue much as they would have treated a cue with 100% validity48.
Psychophysical manipulations of the stimulus and attention field size
Stimulus size and attention field size co-varied systematically in separate sessions. Small stimuli (σ=0.4°, standard deviation of Gabor window) were presented at one of five pre-defined isoeccentric locations in each of the lower right and left quadrants. Across trials, stimulus locations varied randomly and independently on the left and right sides. Peripheral pre-cue locations co-varied with the stimulus locations in the exogenous attention experiment. Large stimuli (σ=1°) were presented at fixed locations (same eccentricity as small stimuli), with no spatial uncertainty. In the endogenous attention experiment, the two large Gabor stimuli were each surrounded by six irrelevant distracters (Gabor flankers, σ=0.2°). Flanker contrast was identical to that of the Gabor stimuli on each trial. Flankers were tilted either 30° clockwise or counter-clockwise (randomized on each trial) from vertical to perturb responses in the neural subpopulations most sensitive for discriminating the near-vertical target orientation so that performance would benefit by narrowing the attention field. The center-to-center distance between the stimuli and flankers was 3.5°. From the point of view of the model, adding the flankers was the same as increasing stimulus size (beyond the excitatory part of the classical receptive field) but without increasing attention field size (because attending the flankers would have impaired performance). We verified through model simulations that the magnitude of the response gain change at high contrasts was predicted by the model to be larger with than without the flankers (see also Eq. 8 of Ref. 1) but not as large as when both the flankers were present and the attention size was narrowed.
Analysis of psychophysical data
For each observer, performance, d' = z (hit rate) – z (false alarm rate), was assessed across experimental sessions, for each contrast and each trial condition (valid, invalid and neutral). Counter-clockwise response to counter-clockwise stimulus tilt was (arbitrarily) considered a hit, and counter-clockwise response to clockwise stimulus was considered a false alarm. The psychometric data were fit (nonlinear least-squares) to the mean performance (across observers):
where d'(c) is performance as a function of contrast, d’max determines the asymptotic performance at high contrasts, c50 is the contrast corresponding to half the asymptotic performance, and n is an exponent that controls the slope of the psychometric function. The two parameters, d’max and c50, determined response gain and contrast gain, respectively. We estimated these two parameters for each attention condition while the exponent was treated as one free parameter, constrained to have the same value across conditions (Suppl. Tables 1–2). Best-fit parameters were obtained separately for the exogenous and endogenous attention experiments.
A bootstrap procedure was used to determine confidence intervals for the fitted response gain (d’max) and contrast gain (c50) parameters (Fig. 3, error bars along the edges). The same bootstrap procedure was used to determine if changes in response and/or contrast gain were statistically significant. Specifically, we randomly resampled individual psychophysical trials with replacement to generate a resampled data set, which was refit, and this procedure of resampling and refitting was repeated 10,000 times to generate bootstrap distributions of the psychometric data and of the fitted parameters. Confidence intervals and p-values were extracted for each parameter estimate from these bootstrap distributions. We compiled the bootstrap distribution of the differences between the conditions (e.g., valid versus invalid trials) and determined the percentage of the values in the tail of the distribution of the differences greater than zero for response gain changes (d'max), or less than zero for contrast gain changes (c50). The use of these one-tailed statistical tests was justified based on previous studies, reporting a benefit for valid and a cost for invalid, compared to neutral cues7–9,29.
For the individual subject analysis (Fig. 4), we computed the d’ performance across experimental sessions, separately for each condition and contrast, to obtain the psychometric functions. We then followed the same fitting procedures as outlined above to estimate d’max and c50, separately for each observer.
Observers and fMRI scanning sessions
Functional Magnetic Resonance Imaging (fMRI) data were acquired from five healthy observers with normal or corrected vision (3 female; 25–31 years old), three of whom participated in the psychophysics experiments. Each observer participated in several scanning sessions: one to obtain a high-resolution anatomical volume for cortical surface extraction and coregistration across sessions, one to define retinotopically organized visual areas, and two to three sessions to measure fMRI responses in the main experiment (spatial uncertainty vs. no uncertainty, described below).
MRI data acquisition
MRI data were acquired at 3T (Allegra, Siemens Medical Systems) equipped with a transmit head coil (NM-011) and an eight-channel phased array receive surface coil (NMSC-071; both Nova Medical). We used a standard echoplanar pulse sequence with the following parameters: repetition time TR = 1.2 s; echo time TE = 30 ms; flip angle = 72°; voxel size = 3×3×3 mm; 22 slices oriented approximately parallel to the calcarine sulcus.
Stimulus, task and fMRI procedure
The experimental protocol was similar to the endogenous attention psychophysics experiment (see above). After an initial fixation (250 ms), a pre-cue (250 ms) indicated the upcoming target location. All trials were validly cued (one hemifield was cued, the other uncued) to maximize the number of trials to be included in the analysis. A blank period of 1250 ms allowed observers to covertly deploy endogenous attention. A pair of Gabor stimuli (5 cycles/deg, σ=0.4° standard deviation of the Gaussian window) were presented (50 ms) in the lower quadrants of the left and right hemifields (6° eccentricity), one of which was the target. The stimuli were tilted slightly clockwise or counter-clockwise of vertical. Stimulus contrast was 10%, the lowest contrast at which observers could perform the task above chance, to minimize the contribution of stimulus-evoked activity to the fMRI measurements. While fixating, observers reported the orientation of the cued stimulus (clockwise or counter-clockwise of vertical) by pressing one of two buttons. Inter-trial intervals were variable in duration (5.4 to 9 s, randomly interleaved).
Two conditions (spatial uncertainty, no spatial uncertainty) were conducted in separate sessions on different days. In one condition, a pair of stimuli (10% contrast) appeared in the left and right hemifields, randomly and independently at one of five predefined locations in each hemifield (see Psychophysics methods and Fig. 2c). In the other condition, the stimuli were always shown at the same pair of locations (the middle of the 5 locations in the first condition) in the left and right hemifields. In this condition, to further minimize spatial uncertainty, placeholders (white line edges of 0.25° length) were displayed continuously throughout each run at the corners of the fixed stimulus locations in each hemifield. Stimulus tilt was adjusted for each observer, such that performance was ~70% correct in both conditions.
fMRI data analysis
Standard retinotopic mapping procedures were used to identify primary visual cortex (V1), for each observer49. Within V1, we defined fourteen ROIs (7 in each hemisphere) corresponding to a series of locations along an arc in the visual field, including the 5 stimulus locations in the lower left and right visual quadrants along with two additional locations in the upper visual field quadrants.
fMRI responses from the attention experiments were preprocessed with standard methods, and a response amplitude was measured for each of the two conditions (spatial uncertainty, no spatial uncertainty), and for each of the ROIs (for details, see Ref. 50). In the spatial uncertainty condition, we restricted the analysis to trials in which stimuli were presented at the middle of the 5 locations. Thus, the responses were measured for stimuli of identical size at the identical location, with and without spatial uncertainty. We then subtracted the response amplitudes for uncued stimulus presentations from the response amplitudes for cued stimuli presented at the same location. This resulted in a spatial distribution of response differences (i.e., the attention field) with one response difference for each of the ROIs, corresponding to a series of locations along an arc in each visual hemifield. Left and right hemisphere responses were analyzed separately and were treated as statistically independent.
To quantify the attention field size, we computed the spread of the spatial distribution of response differences (cued minus uncued) across the seven ROIs. Specifically, the response differences were treated as a probability distribution and we computed its mean and standard deviation. The mean was computed as:
where i indexes over the seven ROIs, xi is a location along the arc in the visual field corresponding to the ROI center, and p(xi) is the cued-uncued response difference for that ROI. Negative values were truncated, and response differences were normalized such that they added to 1. E(x) therefore represented the center of the attention field and had units of arc deg of visual angle. The standard deviation was computed as:
The size of the attention field was then defined as twice the standard deviation in units of arc deg of visual angle in the visual field. This analysis was repeated for both conditions (spatial uncertainty vs. no uncertainty), for both hemispheres of each observer (Fig. 5).
Supplementary Material
Acknowledgments
Supported by NIH grants R01-EY019693 (DJH and MC), R01-MH06980 (DJH), and R01-EY016200 (MC). We thank Mike Landy and members of the Carrasco and Heeger labs for their helpful comments. The psychophysical experiments were presented at the Annual Meeting of the Vision Science Society (VSS, 2009) and the European Conference on Visual Perception (ECVP, 2009).
Footnotes
Contributions.
KH programmed, conducted and analyzed the experiments, and co-wrote the manuscript; LMK conducted and analyzed the psychophysics experiments and assisted in conducting the fMRI experiment; MC and DH conceived and supervised the project and co-wrote the manuscript.
References
- 1.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco M. Covert attention increases contrast sensitivity: Psychophysical, neurophysiological and neuroimaging studies. Prog Brain Res. 2006;154:33–70. doi: 10.1016/S0079-6123(06)54003-8. [DOI] [PubMed] [Google Scholar]
- 4.Boynton GM. A framework for describing the effects of attention on visual responses. Vision Res. 2009;49:1129–1143. doi: 10.1016/j.visres.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 6.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Res. 2005;45:1867–1875. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Pestilli F, Viera G, Carrasco M. How do attention and adaptation affect contrast sensitivity? J Vis. 2007;7(9):1–12. doi: 10.1167/7.7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling S, Carrasco M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res. 2006;46:1210–1220. doi: 10.1016/j.visres.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pestilli F, Ling S, Carrasco M. A population-coding model of attention's influence on contrast response: Estimating neural effects from psychophysical data. Vision Res. 2009;49:1144–1153. doi: 10.1016/j.visres.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrone MC, Denti V, Spinelli D. Color and luminance contrasts attract independent attention. Curr Biol. 2002;12:1134–1137. doi: 10.1016/s0960-9822(02)00921-1. [DOI] [PubMed] [Google Scholar]
- 12.Morrone MC, Denti V, Spinelli D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Res. 2004;44:1389–1401. doi: 10.1016/j.visres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Lu ZL, Tjan BS, Dosher BA, Chu W. Blood oxygenation level-dependent contrast response functions identify mechanisms of covert attention in early visual areas. Proc Natl Acad Sci U S A. 2008;105:6202–6207. doi: 10.1073/pnas.0801390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 16.Buracas GT, Boynton GM. The effect of spatial attention on contrast response functions in human visual cortex. J Neurosci. 2007;27:93–97. doi: 10.1523/JNEUROSCI.3162-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williford T, Maunsell JH. Effects of spatial attention on contrast response functions in macaque area V4. J Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain. Vision Res. 2005;45:1201–1212. doi: 10.1016/j.visres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Maunsell JH. A normalization model of attentional modulation of single unit responses. PLoS One. 2009;4:e4651. doi: 10.1371/journal.pone.0004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta R, DeYoe EA. I know where you are secretly attending! The topography of human visual attention revealed with fMRI. Vision Res. 2009;49:1037–1044. doi: 10.1016/j.visres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksen CW, St James JD. Visual attention within and around the field of focal attention: a zoom lens model. Percept Psychophys. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- 23.Muller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the "Zoom Lens" of visual attention. J Neurosci. 2003;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castiello U, Umilta C. Size of the attentional focus and efficiency of processing. Acta Psychol (Amst) 1990;73:195–209. doi: 10.1016/0001-6918(90)90022-8. [DOI] [PubMed] [Google Scholar]
- 25.Ling S, Carrasco M. When sustained attention impairs perception. Nat Neurosci. 2006;9:1243–1245. doi: 10.1038/nn1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu ZL, Dosher BA. Spatial attention: different mechanisms for central and peripheral temporal precues? J Exp Psychol Hum Percept Perform. 2000;26:1534–1548. doi: 10.1037//0096-1523.26.5.1534. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron. 2005;45:469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano AM, McElree B, Carrasco M. On the automaticity and flexibility of covert attention: a speed-accuracy trade-off analysis. J Vis. 2009;9(30):31–10. doi: 10.1167/9.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeshurun Y, Montagna B, Carrasco M. On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Res. 2008;48:80–95. doi: 10.1016/j.visres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonides J, Irwin DE. Capturing attention. Cognition. 1981;10:145–150. doi: 10.1016/0010-0277(81)90038-x. [DOI] [PubMed] [Google Scholar]
- 32.Sclar G, Maunsell JH, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Res. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- 33.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 34.Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tootell RB, et al. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 36.Brefczynski JA, DeYoe EA. A physiological correlate of the 'spotlight' of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 37.Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jazayeri M, Movshon JA. Optimal representation of sensory information by neural populations. Nat Neurosci. 2006;9:690–696. doi: 10.1038/nn1691. [DOI] [PubMed] [Google Scholar]
- 39.Katkov M, Tsodyks M, Sagi D. Inverse modeling of human contrast response. Vision Res. 2007;47:2855–2867. doi: 10.1016/j.visres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Dean AF. The variability of discharge of simple cells in the cat striate cortex. Exp Brain Res. 1981;44:437–440. doi: 10.1007/BF00238837. [DOI] [PubMed] [Google Scholar]
- 41.Carandini M. Amplification of trial-to-trial response variability by neurons in visual cortex. PLoS Biol. 2004;2:E264. doi: 10.1371/journal.pbio.0020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–151. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- 43.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 44.Bartels A, Logothetis NK, Moutoussis K. fMRI and its interpretations: an illustration on directional selectivity in area V5/MT. Trends Neurosci. 2008;31:444–453. doi: 10.1016/j.tins.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez M, Costa A, Humphreys GW. The size of an attentional window affects working memory guidance. Atten Percept Psychophys. 72:963–972. doi: 10.3758/APP.72.4.963. [DOI] [PubMed] [Google Scholar]
- 46.Belopolsky AV, Zwaan L, Theeuwes J, Kramer AF. The size of an attentional window modulates attentional capture by color singletons. Psychon Bull Rev. 2007;14:934–938. doi: 10.3758/bf03194124. [DOI] [PubMed] [Google Scholar]
- 47.Liu T, Stevens ST, Carrasco M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Res. 2007;47:108–113. doi: 10.1016/j.visres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Sperling GDBA, editor. Handbook of Perception and Human Performance. John Wiley & Sons; 1986. pp. 1–65. [Google Scholar]
- 49.Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Donner TH, Sagi D, Bonneh YS, Heeger DJ. Opposite neural signatures of motion-induced blindness in human dorsal and ventral visual cortex. J Neurosci. 2008;28:10298–10310. doi: 10.1523/JNEUROSCI.2371-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.