Abstract
Background
The purpose of this study was to test whether long-term pair housing of male rhesus macaques ameliorated negative responses to stressful events that can occur in the course of routine husbandry or research procedures.
Methods
Twelve singly-housed individuals were videotaped during two potentially stressful events before and after social introduction into pairs. During each stressor, abnormal behavior and anxiety-related behavior were quantified from videotape.
Results
When visually exposed to the restraint and anesthesia of other monkeys, subjects showed significantly reduced frequencies of abnormal behavior when pair housed in comparison to their reactions when housed singly. Noisy and disruptive conversation between technicians standing immediately in front of the subjects’ cage did not elicit the same reduction in abnormal behavior. Neither test showed a significant difference across housing settings for anxiety-related behaviors.
Conclusions
These findings suggest that pair housing buffers adult male rhesus macaques from common stressors in the laboratory setting.
Keywords: stress, abnormal, behavior, anxiety, introduction
Introduction
It is widely accepted that procedures that are performed as part of routine husbandry have the potential to affect both physiological and behavioral parameters that are associated with stress [5]. In adult female rhesus macaques equipped with telemetry, sustained increases in heart rate were seen during cage change, and physical restraint resulted in immediate heart rate increases in juvenile female macaques [10, 25]. In addition to heart rate changes, room disturbance has been found to impact white blood cell parameters [9]. When exposed to an observer wearing leather gloves, black tufted-ear marmosets showed an increased rate of alarm calls [8]. Even small modifications to a husbandry schedule such as daily variations in feeding time have the potential to cause stress in captive nonhuman primates [41].
Social buffering typically refers to the ability of a social partner to ameliorate distress in response to stressful life events. Several prior studies have shown physiological evidence for social buffering in rhesus macaques. Mother-reared rhesus macaques showed smaller elevations in cortisol when paired than when singly housed [43]. In addition, during the stressor of a novel environment, female rhesus macaques showed a smaller change in a subset of T lymphocytes when a companion was present [19]. The same pattern was found for juvenile rhesus macaques that were exposed to the stressor of a novel peer group [20].
The benefits of social enrichment to the behavioral health of captive nonhuman primates have been well-documented [18]. Social housing has been shown to increase species-typical behavior such as allogrooming [34]. It has also been shown to decrease the frequency of abnormal and anxiety-related behaviors such as hair-pulling and nail-biting [18, 26, 31]. In addition to behavioral benefits, there are also health benefits to pair housing. Compared to singly housed controls, paired females have lower infant mortality and higher infant weight gain, and paired monkeys have been shown to receive fewer veterinary treatments [18, 35]. While efforts have been made to enrich the environment of captive nonhuman primates through interaction with humans via positive reinforcement training, there is no evidence that positive interaction with humans has the same beneficial effect as a conspecific social companion [1, 2].
Because male macaques have a reputation for isosexual aggression, there is widespread hesitance to pair them in the laboratory setting. For example, Crockett et al. found that while 100% of female cynomolgus pairs were successful, only 53% of male pairs were successful [12]. In the attempt to pair macaques, the presence of fighting and absence of grooming within the first 90 minutes are strong indicators of whether a pair of males will be compatible in the long-term [12]. At the Tulane National Primate Research Center, there is a high success rate among male social pairings, and intensive monitoring—live and/or remotely via video—during the period initially following the social introduction permits the determination of incompatibility early in the introduction before the animals become a physical danger to each other.
This study used behavioral variables to examine whether the presence of a familiar companion in the home cage environment buffers male rhesus macaques from stressors that may occur in the course of routine laboratory husbandry and clinical care. The stressors chosen for this study have been shown in prior studies to cause distress in nonhuman primates. The observation of other monkeys in the room being injected with medications such as ketamine or antibiotics has been shown to increase heart rate and decrease appetite in rhesus macaques [27, 39] In addition, the presence of an unfamiliar technician in the room caused sustained decreases in heart period and suppressed respiratory sinus arrhythmiain cynomolgus macaques [6].
In the current study, two types of behaviors were examined; both are known to increase in response to situations that might be construed as stressful in the life of a captive nonhuman primate. The first group of behaviors were species-inappropriate and consisted of repetitive abnormalbehaviors, often referred to as stereotypies. Examples of repetitive abnormal behaviors include pacing, rocking, and head-tossing. The physiological basis for these behaviors is still not fully understood and may involve positive feedback mechanisms in lower brain structures that control motor activity [13]. Several neurotransmitters such as dopamine, acetylcholine, serotonin, and opioid peptides likely interact in the development of stereotypies, and hormones such as corticotropin releasing hormone are also thought to be responsible for the physiological correlates of stereotypic behavior [24, 38]. Because they arise both in settings that are stressful and those that are understimulating, stereotypies may serve to either decrease arousal in situations of distress or increase arousal in environments that are lacking in complexity [38].
There is little dispute that nonhuman primates exhibit abnormal behaviors in response to situations that cause distress [21]. There is a positive correlation in rhesus macaques between the amount of stereotypic behavior exhibited and the concentration of both basal plasma cortisol and corticotropin releasing hormone found in the cerebrospinal fluid [30]. Stereotypies have been observed to increase during the stressful experience of confinement with an unfamiliar conspecific following a period of social isolation [11].
The second group of behaviors examined in this study was a constellation of self-directed species-appropriate behaviors generally associated with body care but also reflect levels of anxiety and sometimes referred to as displacement behaviors [7, 28]. Examples of these behaviors, often referred to as displacement behaviors, include scratching, autogrooming, body-shaking, and yawning [17, 40]. It is presumed that these behaviors are indicators of anxiety because they increase in response to risky social situations, such as proximity to a higher-ranking individual during feeding [14] or occurrence of vocalizations from neighboring enclosures, which can lead to intragroup aggression [4]. Pharmacologically, these behaviors increase in response to administration of known anxiogenic compounds and decrease in response to anxiolytics, such as benzodiazepines [16, 22, 36, 37]. In human medicine, self-directed behaviors such as stroking of the hands and face are seen more frequently in patients who are identified as being high-anxious than those who are identified as low-anxious [42].
This study aims to determine whether the presence of a familiar companion reduces the response to stressful events in the biomedical environment, in order to better understand the effects of social housing on psychological well-being, which is important not only from a welfare perspective but also a research standpoint. Every effort must be made to use subjects that are uniformly healthy and to avoid negative and chronic perturbations to physiological parameters.
Materials and methods
Study subjects
Twelve healthy adult male Indian-origin rhesus macaques (Macaca mulatta) were used in this study, which received prior approval from the institutional animal care and use committee (IACUC) of the Tulane National Primate Research Center (TNPRC) in Covington, LA. This study was conducted within the guidelines for ethical use of animals in United States Public Health Service policy as outlined in the Guide for the Care and Use of Laboratory Animals [29].
The study animals were born in the specific pathogen free breeding colony at the TNPRC and were antibody- and virus-negative for simian retrovirus and seronegative for simian T-lymphotrophic virus 1, simian immunodeficiency virus, and cercopithecine herpesvirus 1 prior to the start of the study. All subjects were mother-reared in social groups for at least the first two years of life. They had been singly-housed in indoor caging for 0.3 to 2.9 years prior to the beginning of the study. All subjects were sexually mature at the time of the study (age range 5 to 9 years).
Animals were maintained in Animal Biosafety Level 2 housing with a 12:12-hour light:dark cycle, relative humidity 30% to 70%, and a temperature of 17.8 to 28.9°C. Water was available ad libitum, and a standard commercially formulated nonhuman primate diet (Lab Fiber Plus Monkey DT, 5K63, PMI Nutrition International, St. Louis, MO) was provided twice daily and supplemented daily with fresh fruit and/or forage material as part of the environmental enrichment program. All subjects were housed in the same room. Each cage (Allentown, Inc., Allentown, NJ) measured 36 inches (91.4 centimeters) in height with 8.6 square feet (0.8 square meters) of floor space and contained a perch, a portable enrichment toy, a mirror, and a forage board for feeding enrichment.
Study design and procedures
The design of this study involved a phase of data collection in single housing followed by introduction to a social partner and, approximately six weeks later, a second phase of data collection in pair housing. Animals were paired with unfamiliar animals, to form six pairs, according to approved behavioral management practices employed at the TNPRC. Introductions involved at least one day of protected contact prior to full contact. During protected contact, the solid panel that separated adjacent cages was replaced with a panel that included bars that allowed for tactile contact. The monkeys were videotaped and monitored remotely in real time from an office in the same building for approximately two hours during each phase of the introduction period in order to ensure their safety and compatibility. Experimental manipulations were not performed until at least six weeks after successful social introduction.
During both single housing and pair housing, the subjects were exposed to two potentially stressful events that might normally occur through the course of routine husbandry or research procedures. Subjects were visually exposed to other monkeys in the room being restrained for administration of an injectable anesthetic. This stressor is hereafter referred to as “restraint observation” and lasted approximately five to ten minutes. The restraint and anesthesia of other monkeys in the room was not staged for the purpose of this study but occurred as scheduled for the research project in which animals were enrolled.
The other stressor was referred to as “staged rudeness”, which involved a two-minute visit by two technicians with whom the monkeys were not familiar. These individuals talked loudly, moved abruptly, and made eye contact with the monkeys. These are actions that TNPRC technicians are trained to avoid but still occasionally occur. Experimental stressors were not presented in the same order in all animals, and at least three weeks elapsed between the staged rudeness stressors on different subjects.
Behavioral data acquisition and coding
Wall-mounted cameras with remote monitoring were employed to obtain video recordings of the monkeys during each phase of the study. Video recordings were obtained of each monkey’s response to the potentially stressful events in single housing and then again in pair housing. Behavioral coding began ten minutes after the cessation of each stressor. Interruptions by animal care staff for routine husbandry procedures that had the potential to alter the animals’ behavior occurred at varying lengths of time following the stressors. Because a standard duration of data collection was desired for each stressor, the longest, uninterrupted duration of data that was available for all of the monkeys per stressor was used. While the duration of coding was the same for all of the monkeys within a stressor, it was different between the stressors due to husbandry practices that were beyond the control of the authors. Following restraint observation, four minutes of videotaped data were obtained for eight subjects. Data for four subjects could not be collected. However, following the staged rudeness test, ten minutes of videotaped data were obtained for all twelve subjects. The two experimental stressors were never presented on the same day.
In addition to post-stressor observation, three to six hours of video were collected for each individual in both single and pair housing, for a total of 108 hours. This observation condition consisted of routine, quiet circumstances during which no husbandry or research activities were being carried out in the room. The schedule of data collection was balanced for time of day, with two start times in the morning and two in the afternoon. These data were collected prior to the onset of stress tests, and in the case of pairs, no sooner than two months after social introduction.
Videos were coded and quantified utilizing Observer XT® software (Noldus Information Technology Inc., Leesburg, VA) and a standard, exhaustive, and mutually-exclusive ethogram of rhesus macaque behavior. For observations during routine conditions and during post-stressor observations, all instances of each abnormal and anxiety-related behavior was recorded and calculated as frequencies per hour for each individual. If bouts of behaviors occurred, a new entry for a particular behavior was made if three or more seconds passed without the behavior occurring. In addition to all-events sampling for calculating frequencies, instantaneous scan sampling with 30-second inter-sample intervals were used to quantify social interactions within each pair.
Two behavioral categories were used to measure potential stress. The first category included repetitive stereotypies (pacing, rocking, and head-tossing), which were the only category of abnormal behavior observed in the study subjects. Each of these behaviors was coded if the onset of the bout involved three repetitions in succession. The second category was anxiety-related behaviors, including scratching, autogrooming, body-shaking, and yawning. Affiliative social behaviors included grooming and other affiliative contact, social play, mounting, and lipsmacking. Agonistic behavior included submissive behaviors (e.g. fear grimacing and cringing), facial threats, lunges, and aggressive contact. For each pair, dominance rank was determined based upon ad libitum observation of displacements. This aspect of social relationships was explored in order to examine separately the correspondence between rank and levels of abnormal and anxiety-related behavior.
Statistical analysis
Not all subjects performed all stereotypic behaviors in each condition, so these behaviors were analyzed as a single category for analysis. For the same reason, analysis of anxiety-related behaviors involved summed frequencies of individual displacement behaviors. Statistical analysis was performed using Statistica® software (Statsoft, Tulsa, OK) to compare individual frequencies per hour of abnormal and anxiety-related behaviors. Data were analyzed using two-tailed Wilcoxon matched pairs test to compare each individual’s behavior during stressful events in single housing with its behavior during pair housing (since comparisons were paired and the analysis restricted to nonsocial behavior, data are assumed to be independent in the paired condition). These comparisons were also made using values calculated by subtracting frequencies during routine conditions from frequencies during the stress tests. Spearman rank order correlations coefficients were used to test for a correspondence between prior tenure in social housing and changes in levels of behavior. Mann-Whitney U test was used compare levels of behavior in the dominant versus subordinate member of a pair. For all statistical tests, alpha was set at 0.05.
Results
Descriptively, responses to the two stressors varied considerably in that while other monkeys were being restrained and anesthetized, study subjects spent the bulk of the test quiet and still. In contrast, during the staged rudeness test the monkeys attempted to flee, hid behind each other, or threatened and lunged at the person.
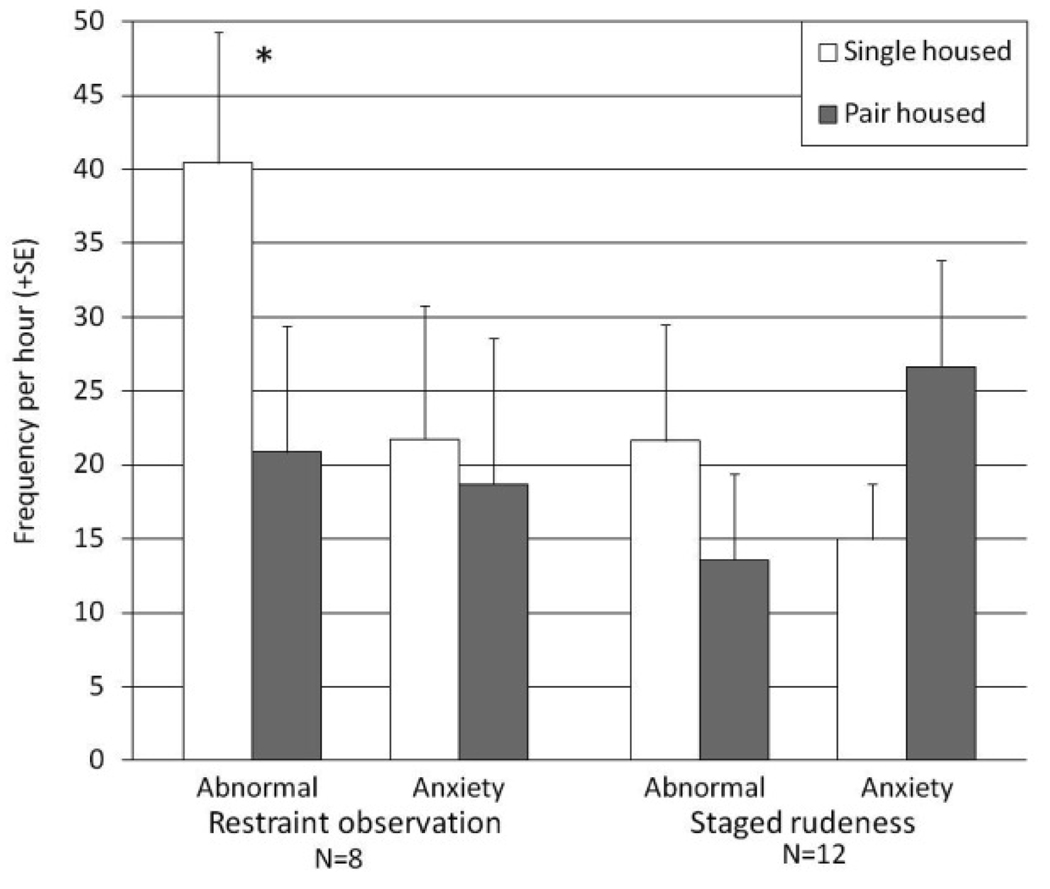
During routine, quiet conditions, there was no difference between single and pair housing with respect to frequency of either abnormal (N = 12, Z = 1.51, P = 0.13) or anxiety-related behavior (Z = 1.33, P = 0.18). During the restraint observation test, the frequency of abnormal behavior was significantly lower in the pair housing setting than in single housing (N = 8, Z = 2.20, P = 0.03; see Figure 1). A comparable change in anxiety-related behavior during this test was not observed (Z = 0.42, P = 0.67). The staged rudeness test revealed no effect of housing condition on abnormal (N = 12, Z = 1.07, P = 0.28) or anxiety-related behavior (Z = 1.07, P = 0.29). Analyzing data from the first four minutes of the staged rudeness test (the same time period used in the restraint observation test) also showed no significant contrasts (abnormal: N = 12, Z = 1.02, P = 0.31; anxiety-related: Z = 0.76, P = 0.45).
Figure 1.
Change in frequencies of anxiety-related and abnormal behavior (* = P < 0.05)
Adjusting for levels of these behaviors during routine conditions in single and pair housing did not influence the direction or significance of results (restraint observation: abnormal [Z = 1.96, P = 0.049], anxiety [Z = 0.42, P = 0.67]; staged rudeness: abnormal [Z = 0.71, P = 0.48], anxiety [Z = 1.07, P = 0.29]). In addition, duration of prior single housing and changes in levels of behaviors did not correspond; Spearman (F restraint observation: anxiety R = 0.21; Spearman rank order correlations coefficients ranged from −0.38 to 0.45 and did not reach significance for any behavioral category in either housing condition or stressors.
For pairs, under routine conditions, social rank did not influence levels of either abnormal or anxiety-related behavior (abnormal: U = 16, P = 0.75; anxiety-related: U = 13, P = 0.42). During the restraint observation test, neither the frequency of abnormal nor anxiety-related behavior varied with rank (abnormal: U = 7, P = 0.77; anxiety-related: U = 1, P = 0.13). Similarly, during the staged rudeness test, neither abnormal nor anxiety-related behavior varied with rank (abnormal: U = 12, P = 0.34; anxiety-related: U = 12, P = 0.30).
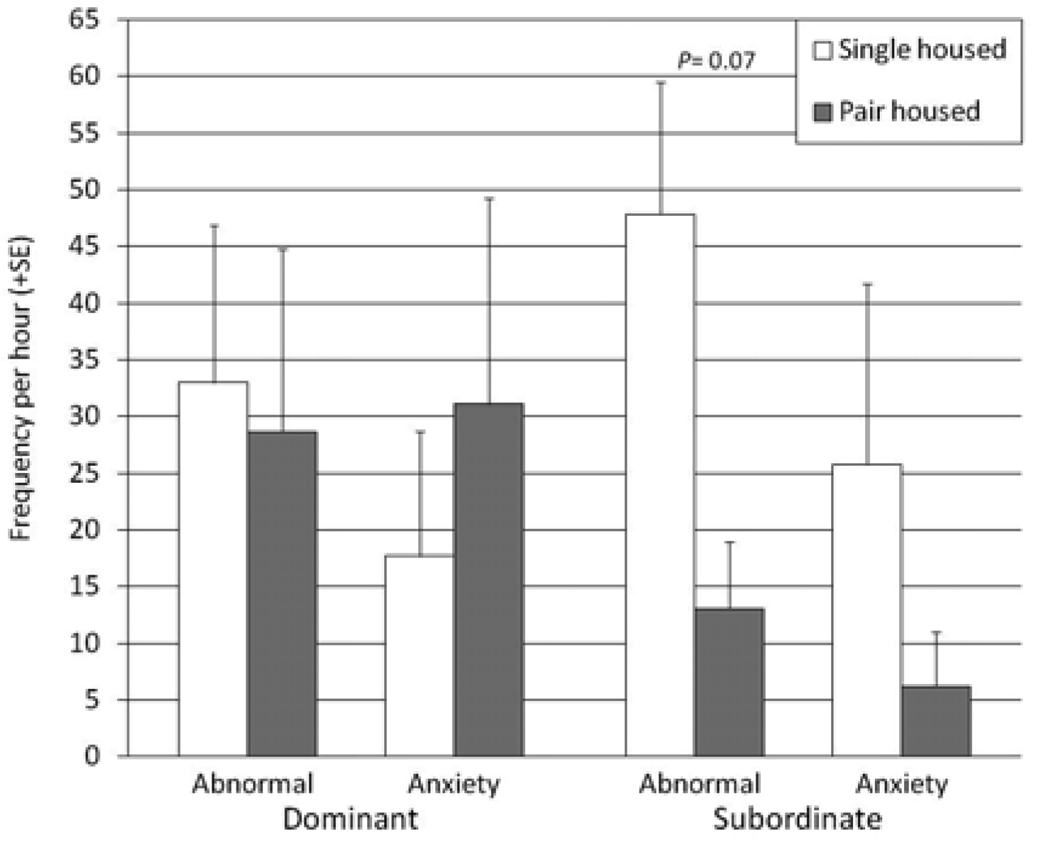
The direction of change in abnormal behavior between single housing and pair housing during the restraint observation test was consistent in both dominant and subordinate individuals (see Figure 2). However, in subordinate animals the reduction in abnormal behavior associated with pair housing was notable and approached statistical significance (Z = 1.82, P = 0.07), whereas it did not in dominant animals (Z = 1.07, P = 0.29). As with the full set of subjects, anxiety-related behavior showed no significant changes in either the dominant (Z = 0.36, P = 0.72) or subordinate subset of subjects (Z = 1.10, P = 0.27).
Figure 2.
Frequencies of abnormal and anxiety-related behavior during the restraint observation test in dominant and subordinate individuals
During routine circumstances when no husbandry or research activities were being carried out in the room, pairs spent 23.02% ± 4.41 of their time engaged in affiliative behavior and 1.90% ± 1.15 of their time engaged in agonistic behavior. These levels of interaction are consistent with larger samples of compatible pairs and demonstrate that pairs had established affiliative bonds [3, 15]. Following the restraint observation tests, affiliation was virtually absent in all pairs except one, which spent 55% of the observation time engaged in affiliative behavior. Surprisingly, levels of affiliation were virtually unchanged following the staged rudeness test (24.77 ± 9.66%). No agonistic behavior was observed during either observation period.
Discussion
The results of this study support the hypothesis that pair housing buffers caged rhesus macaques from potential stressors that commonly occur through the course of routine husbandry and clinical care. When visually exposed to the restraint and anesthesia of another monkey in the room, monkeys showed significantly less abnormal behavior when paired than when housed singly. Although the levels did not achieve statistical significance, the results for the “staged rudeness” stressor showed the same pattern. The behavioral effects differed between the two stressors used in this study, which indicates that some human activities are more distressing to captive nonhuman primates than others. Based on the results of this study and others that measured the response to the observation of other monkeys being restrained and injected, it appears that this is a distressful experience for laboratory nonhuman primates [27, 39]. For monkeys that reside in rooms where others are routinely injected and anesthetized, the buffering effects of social housing may be especially beneficial. It is also of practical significance that animals’ reaction during a stressor may not predict the response after the stressor, and that the absence of overt fear did not signal that the animal had not been distressed by the experience.
The results of this study contribute to the growing body of evidence that a social companion can serve as a buffer from stress in nonhuman primates [23]. In addition, these findings are consistent with previous research indicating that social isolation intensifies the stress experienced during alarming situations [33]. Because the measurement of behavioral parameters is the least invasive method for assessing stress, it is important to measure relevant behaviors, i.e. those behaviors that would be expected to change in frequency during a stressful situation. While repetitive abnormal behaviors after stressful events followed the expected decrease when the subject was housed with a social companion, anxiety-related behaviors did not show the same pattern. One possible explanation for this finding might be that the stressors utilized in this study were sufficient to elicit what is really a ritualized, space-constrained escape attempt rather than displacement behavior, which often appears in the context of possible risk rather than imminent danger. For example, animals observing the sedation of other animals are often about to be sedated themselves, whereas human ‘rudeness’, once it is over, is not a harbinger of a subsequent frightening experience. This suggestion could be tested by evaluating a larger array of potential stressors that vary across this axis. Another possible direction for future research is to assess the behavioral response to a stressor while the stressor is occurring. This would also allow for observation of the subjects’ behavioral interactions with the humans involved. Because this study involved the opportunistic use of animals that were enrolled in an infectious disease study, there was little control over rearing or prior social history. The response of individuals with rearing backgrounds other than mother-reared in a social setting should be explored since rearing background is a critical factor in the development of aberrant behavior in nonhuman primates and may influence the effect of social housing or response to stressors themselves. Last, the correspondence between presence or degree of social buffering and relationship quality could be explored.
Prior research has found that pairing is generally a positive experience for both dominant and subordinate monkeys (as evidenced by greater activity levels, less self-grooming, and less time spent in abnormal behavior for both members of the pair) [18, 31]. The results of the current study hint that under particularly stressful conditions, subordinate animals may benefit more than dominant animals, perhaps because a higher-ranking partner may be a more effective ally. A larger subject pool would be needed to thoroughly assess the interaction between buffering and rank. Further research could also involve counterbalancing the order of housing conditions or employing an A-B-A design to control for possible order effects. This phenomenon is unlikely to have confounded the results of the current study, since all subjects had been thoroughly habituated to both stressors over the preceding years, but this design could test this assumption. Another possible direction for this research topic would be to examine the relationship between behavioral and physiological responses to stress in the context of social buffering by using surgically implanted telemetry devices to measure heart rate and temperature.
Last, because of their reputation for aggressive behavior, there is widespread hesitance to pair house adult male rhesus macaques in the laboratory environment for fear of injury. However, this study supports the findings of previous research in that even adult male rhesus macaques can reap the benefits of pair housing. While the current study did not show housing-related reductions in abnormal or anxiety-related behavior under routine conditions, other studies have found that pair housing is associated with reductions in anxiety-related behavior, as well as reduced inactivity and fecal cortisol, and increased locomotion [3, 15]. This study suggests that benefits accrue under stressful conditions as well [15, 32].
Acknowledgments
We thank the animal care and environmental enrichment staff for their assistance with this study and excellent care of the monkeys. We also thank Brooke Oettinger and Lauren Cox for scheduling and carrying out the monkeys’ exposure to stressors.
Funding
NIH grant P01-MH076388 to The Joseph Stokes, Jr. Research Institute at the Children’s Hospital of Philadelphia and NIH grants RR000167, RR00164, RR019628, RR012112, and RR05169, and RR024231 to the Tulane National Primate Research Center
References
- 1.Baker KC, Bloomsmith M, Neu K, Griffis C, Maloney M. Positive reinforcement training as enrichment for singly-housed rhesus macaques. Anim Welfare. in press. [PMC free article] [PubMed] [Google Scholar]
- 2.Baker KC, Bloomsmith M, Neu K, Griffis C, Maloney M, Oettinger B, Schoof V, Martinez M. Positive reinforcement training moderates only high levels of abnormal behavior in singly housed rhesus macaques. J Appl Anim Welf Sci. 2009;12:236–252. doi: 10.1080/10888700902956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker KC, Bloomsmith M, Neu K, Griffis C, Oettinger B, Schoof V, Clay A, Maloney M. Benefits of isosexual pairing of rhesus macaques (Macaca mulatta) vary with sex and are limited by protected contact but not by frequent separation of pairs. Am J Primatol. 2008;70:44. [Google Scholar]
- 4.Baker KC, Aureli F. Behavioural indicators of anxiety: an empirical test in chimpanzees. Behaviour. 1997;134:1031–1050. [Google Scholar]
- 5.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim. 2004;43:42–51. [PubMed] [Google Scholar]
- 6.Bowers CL, Crockett CM, Bowden DM. Differences in stress reactivity of laboratory macaques measured by heart period and respiratory sinus arrhythmia. Am J Primatol. 1998;45:245–261. doi: 10.1002/(SICI)1098-2345(1998)45:3<245::AID-AJP2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw RH. Displacement activities as potential covert signals in primates. Folia Primatol. 1993;61:174–176. doi: 10.1159/000156746. [DOI] [PubMed] [Google Scholar]
- 8.Cagni P, Gonçalves I, Jr, Ziller F, Emile N, Barros M. Humans and natural predators induce different fear/anxiety reactions and response pattern to diazepam in marmoset monkeys. Pharmacol Biochem Behav. 2009;93:134–140. doi: 10.1016/j.pbb.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Capitanio JP, Mendoza SP, McChesney M. Influences of blood sampling procedures on basal hypothalamic-pituitary-adrenal hormone levels and leukocyte values in rhesus macaques (Macaca mulatta) J Med Primatol. 1996;25:26–33. doi: 10.1111/j.1600-0684.1996.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Clarke AS, Mason WA, Mendoza SP. Heart rate patterns under stress in three species of macaques. Am J Primatol. 1994;33:133–148. doi: 10.1002/ajp.1350330207. [DOI] [PubMed] [Google Scholar]
- 11.Coelho AM, Carey KD, Shade RE. Assessing the effects of social environment on blood pressure and heart rate of baboons. Am J Primatol. 1991;23:257–267. doi: 10.1002/ajp.1350230406. [DOI] [PubMed] [Google Scholar]
- 12.Crockett CM, Bowers CL, Bowden DM, Sackett GP. Sex differences in compatibility of pair-housed adult longtailed macaques. Am J Primatol. 1994;32:73–94. doi: 10.1002/ajp.1350320202. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R. Behavioral, physiological and functional aspects of stereotyped behavior: a review and a re-interpretation. J Anim Sci. 1986;62:1776–1786. doi: 10.2527/jas1986.6261776x. [DOI] [PubMed] [Google Scholar]
- 14.Diezinger F, Anderson JR. Starting from scratch: a first look at a “displacement activity” in group-living rhesus monkeys. Am J Primatol. 1986;11:117–124. doi: 10.1002/ajp.1350110204. [DOI] [PubMed] [Google Scholar]
- 15.Doyle LA, Baker KC, Cox LD. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am J Primatol. 2008;70:542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn AJ, Guild AL, Kramarcy NR, Ware MD. Benzodiazepines decrease grooming in response to novelty but not ACTH or β-endorphin. Pharmacol Biochem Behav. 1981;15:605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- 17.Easley SP, Coelho AM, Taylor LL. Scratching, dominance, tension, and displacement in male baboons. Am J Primatol. 1987;13:397–411. doi: 10.1002/ajp.1350130405. [DOI] [PubMed] [Google Scholar]
- 18.Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) Am J Primatol. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- 19.Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiol Behav. 1994;55:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 20.Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of companions in modulating stress associated with new group formation in juvenile rhesus macaques. Physiol Behav. 1996;59:941–945. doi: 10.1016/0031-9384(95)02164-7. [DOI] [PubMed] [Google Scholar]
- 21.Honess PE, Marin CM. Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci Biobehav Rev. 2006;30:390–412. doi: 10.1016/j.neubiorev.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Insel TR, Ninan PT, Aloi J, Jimerson DC, Skolnick P, Paul SM. A benzodiazepine receptor-mediated model of anxiety. Arch Gen Psychiatry. 1984;41:741–750. doi: 10.1001/archpsyc.1984.01790190015002. [DOI] [PubMed] [Google Scholar]
- 23.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Phil Trans R Soc B. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladewig J, de Passillé AM, Rushen J, Schouten W, Terlouw EMC, von Borell E. Stress and the physiological correlates of stereotypic behavior. In: Lawrence, Rushen, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. Wallingford: CAB International; 1993. pp. 97–118. [Google Scholar]
- 25.Line SW, Markowitz H, Morgan KN, Strong S. Effects of cage size and environmental enrichment on behavioral and physiological responses of rhesus macaques to the stress of daily events. In: Novak, Petto, editors. Through the Looking Glass. Washington, DC: American Psychological Association; 1991. pp. 160–179. [Google Scholar]
- 26.Line SW, Morgan KN, Markowitz H, Roberts JA, Riddell M. Behavioral responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Lab Prim Newsl. 1990;29:1–5. [Google Scholar]
- 27.Line SW, Morgan KN, Markowitz H, Strong S. Heart rate and activity of rhesus monkeys in response to routine events. Lab Prim Newsl. 1989;28:9–12. [Google Scholar]
- 28.Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Anim Behav. 1992;44:967–979. [Google Scholar]
- 29.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 30.Novak MA, Meyer JS, Lutz C, Tiefenbacher S. Stress and the performance of primate stereotypies. In: Mason G, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications for welfare. Wallingford: CAB International; 2007. p. 248. [Google Scholar]
- 31.Reinhardt V, Houser D, Eisele S, Cowley D, Vertein R. Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. Am J Primatol. 1988;14:135–140. doi: 10.1002/ajp.1350140204. [DOI] [PubMed] [Google Scholar]
- 32.Roberts SJ, Platt ML. Effects of isosexual pair-housing on biomedical implants and study participation in male macaques. Contemp Top Lab Anim Sci. 2005;44:13–18. [PubMed] [Google Scholar]
- 33.Rowell TE, Hinde RA. Responses of rhesus monkeys to mildly stressful situations. Anim Behav. 1963;11:235–243. [Google Scholar]
- 34.Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl Anim Behav Sci. 1996;48:159–171. [Google Scholar]
- 35.Schapiro SJ, Bushong D. Effects of enrichment on veterinary treatment of laboratory rhesus macaques (Macaca mulatta) Anim Welfare. 1994;3:25–36. [Google Scholar]
- 36.Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2:186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Schino G, Troisi A, Perretta G, Monaco V. Measuring anxiety in nonhuman primates: effect of lorazepam on macaque scratching. Pharmacol Biochem Behav. 1991;38:889–891. doi: 10.1016/0091-3057(91)90258-4. [DOI] [PubMed] [Google Scholar]
- 38.Shulman LM, Sanchez-Ramos JR, Weiner WJ. Defining features, clinical conditions, and theoretical constructs of stereotyped movements. In: Sprague RL, Newell KM, editors. Stereotyped movements: brain and behavior relationships. Washington, DC: American Psychological Association; 1996. pp. 17–34. [Google Scholar]
- 39.Springer DA, Baker KC. Effect of ketamine anesthesia on daily food intake in Macaca mulatta and Cercopithecus aethiops. Am J Primatol. 2007;69:1080–1092. doi: 10.1002/ajp.20421. [DOI] [PubMed] [Google Scholar]
- 40.Troisi A, Schino G. Environmental and social influences on autogrooming behavior in a captive group of Java monkeys. Behaviour. 1987;100:292–302. [Google Scholar]
- 41.Waitt C, Buchanan-Smith HM. What time is feeding? How delays and anticipation of feeding schedules affect stump-tailed macaque behavior. Appl Anim Behav Sci. 2001;75:75–85. [Google Scholar]
- 42.Waxer v. Nonverbal cues for anxiety: an examination of emotional leakage. J Abnorm Psychol. 1977;86:306–314. doi: 10.1037//0021-843x.86.3.306. [DOI] [PubMed] [Google Scholar]
- 43.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]