Abstract
Membrane trafficking from the endoplasmic reticulum (ER) to the Golgi complex is mediated by pleiomorphic carrier vesicles that are driven along microtubule tracks by the action of motor proteins. Here we describe how NSP4, a rotavirus membrane glycoprotein, binds to microtubules and blocks ER-to-Golgi trafficking in vivo. NSP4 accumulates in a post-ER, microtubule-associated membrane compartment and prevents targeting of vesicular stomatitis virus glycoprotein (VSV-G) at a pre-Golgi step. NSP4 also redistributes β-COP and ERGIC53, markers of a vesicular compartment that dynamically cycles between the ER and Golgi, to structures aligned along linear tracks radiating throughout the cytoplasm. This block in membrane trafficking is released when microtubules are depolymerized with nocodazole, indicating that vesicles containing NSP4 are tethered to the microtubule cytoskeleton. Disruption of microtubule-mediated membrane transport by a viral glycoprotein may represent a novel pathogenic mechanism and provides a new experimental tool for the dissection of early steps in exocytic transport.
Keywords: glycoprotein–membrane trafficking/microtubules/virus–host interactions
Introduction
The characteristic features of virus assembly and pathological outcomes of infection are determined by specific interactions between viral and host components. Mem brane glycoproteins encoded by enveloped animal viruses represent an important group of viral components that interact with host molecules to influence factors such as cellular tropism, the site of assembly and release of viral progeny, and the immunological response of the host to infection. These interactions are mediated by a variety of amino acid sequence motifs that mimic those found in many host proteins and enable utilization of the cellular machinery for a variety of post-translational modifications. Among these motifs, membrane sorting signals have been studied intensely in recent years, and their dissection has yielded much information about the fundamental mechanisms of protein targeting in mammalian cells. For example, a diacidic Asp-X-Glu motif, present within the glycoprotein of vesicular stomatitis virus (VSV-G) and common in many cellular membrane proteins, is recognized as necessary for accumulation at COPII-coated budding sites prior to endoplasmic reticulum (ER)-to-Golgi transport (Nishimura and Balch, 1997; Nishimura et al., 1999).
Specific motifs have also been proposed to mediate retention and/or retrieval of membrane glycoproteins within endomembrane compartments. A consensus dilysine (K[X]KXX) motif at the C-terminus of the cytoplasmic domain of type I membrane proteins was recognized in several ER-resident membrane proteins following mutational analysis of the adenovirus E3/19K glycoprotein retention signal (Jackson et al., 1990). Subsequent studies have identified a role for this motif in ER retention of foamy virus glycoproteins (Goepfert et al., 1997) and redirection of the envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1) from the plasma membrane to the ER (Salzwedel et al., 1998). The prevailing model predicts that the dilysine motif acts as a signal for COPI-mediated retrieval of membrane proteins from a post-ER compartment, although recent data suggest that it may also function as a signal for permanent retention in the ER membrane (Andersson et al., 1999). However, not all ER-resident membrane proteins contain this motif. In the E2 glycoprotein encoded by hepatitis C virus, which lacks a recognizable retention motif, the transmembrane domain has been proposed to serve as a signal for static retention in the ER membrane (Cocquerel et al., 1999). Host recognition of membrane sorting signals present in viral glycoproteins may influence the site of viral assembly and/or release within the infected cell. For example, glycoproteins encoded by hepatitis B virus are retained in the ER membrane where they mediate the budding of viral nucleocapsids into the lumen prior to release of progeny via the secretory pathway (Bruss and Ganem, 1991). In contrast, alphaviruses bud directly from the cell surface following the interaction of the viral nucleocapsid with the cytoplasmic tail of virus-encoded glycoproteins present in the plasma membrane (Suomalainen et al., 1992; Strauss et al., 1995; Owen and Kuhn, 1997).
Rotavirus is a non-enveloped icosahedral virus that infects mature enterocytes lining the duodenum and small intestine of numerous animal species, causing gastroenteritis in infants (Estes, 1996). During viral morphogenesis, immature particles bud into the lumen of the ER where the outer capsid layer and haemagglutinin spike are assembled. Budding is initiated by the interaction of the particle with the cytoplasmic domain of NSP4, a non-structural viral glycoprotein. Unlike the budding of enveloped viruses, the membrane acquired by rotavirus particles during budding is removed prior to release of mature virions. Exit of viral progeny from infected cells does not proceed by secretion via the Golgi complex but has been proposed to occur via direct transport of immature particles from the ER to the plasma membrane by a non-conventional pathway (Jourdan et al., 1997). Previous studies have established that NSP4 is retained within the ER membrane, with a C-terminal domain projecting into the cytosol and two N-linked high mannose oligosaccharide residues within a short luminal region (Bergmann et al., 1989). However, a recognized ER retention motif is absent from the NSP4 polypeptide sequence, and the molecular basis for its intracellular distribution has not been elucidated.
NSP4 has also been implicated as an important factor in rotavirus pathogenesis. Expression of NSP4 is associated with an alteration in intracellular calcium homeostasis and a reduction in cell viability (Tian et al., 1994; Newton et al., 1997). Moreover, NSP4 has been designated a viral enterotoxin following the demonstration that a peptide derived from the cytoplasmic domain causes diarrhoea when injected intra-peritoneally or intra-ileally in 3-day-old mice (Ball et al., 1996). It is unclear how NSP4 might be either secreted or released from infected cells to effect this enterotoxin activity given its integral membrane status.
The present study was undertaken to investigate the molecular basis for the retention of NSP4 in the ER membrane and a mechanism for the cytopathic effects associated with its expression. Here we report a direct interaction between NSP4 and the microtubule cytoskeleton mediated by a domain in the cytoplasmic tail of the glycoprotein. This interaction results in accumulation of NSP4 in a post-ER membrane compartment. Expression of NSP4 also results in the arrest of ER-to-Golgi trafficking in a microtubule-dependent manner, suggesting that the NSP4–microtubule interaction causes the static retention of transport vesicles in the cytoskeleton and prevents their translocation to the Golgi complex. Direct binding to microtubules represents a potential mechanism for retention of a membrane glycoprotein in organelles of the early secretory pathway and may contribute to the pathology of rotavirus infection by restricting the transport of cellular membrane proteins to the plasma membrane.
Results
The cytoplasmic tail of NSP4 contains a tubulin-binding site
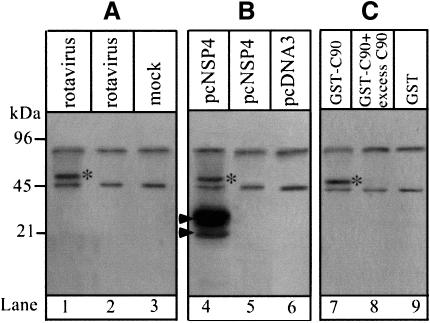
To investigate whether the intracellular location and cytopathic effects of rotavirus NSP4 are dependent on a direct association with a cellular component, 35S-labelled proteins from rotavirus-infected MA104 cells or Cos-7 cells transfected with a construct encoding NSP4 were immunoprecipitated with anti-NSP4 monoclonal antibodies. These experiments reveal an association between NSP4 and an ∼50 kDa cellular protein (Figure 1A and B). A protein of similar size was also recovered from lysates of 35S-labelled cells using a glutathione S-transferase (GST) fusion protein that contained the C-terminal 90 amino acids from the cytoplasmic tail of NSP4 (C90) (Figure 1C). The protein therefore interacts with the cytoplasmic domain of NSP4 and the interaction is saturable since excess soluble C90 blocks association with the immobilized fusion protein.
Fig. 1. NSP4 interacts with a 50 kDa cellular protein. (A) Immuno precipitation of labelled proteins from MA104 cells. Cells were 35S-labelled for 6 h and chased for 1 h prior to infection (lanes 1 and 2) or mock infection (lane 3) with SA11 rotavirus. Cells were harvested at 7 h post-infection and lysates immunoprecipitated with anti-NSP4 mAb (lanes 1 and 3) or pre-immune serum (lane 2). (B) Immunoprecipitation of labelled cellular proteins from transfected Cos-7 cells. Cells were transfected with pcNSP4 (lanes 4 and 5) or pCDNA3.1 (lane 6) and 35S-labelled for 6 h commencing 40 h after transfection. Lysates were immunoprecipitated with anti-NSP4 mAb (lanes 4 and 6) or pre-immune serum (lane 5). Arrows indicate the positions of glycosylated (upper) and unglycosylated (lower) NSP4. (C) Pull-down assay using the C-terminal 90 amino acids of NSP4 (C90) fused to GST as bait. The procedure is described in Materials and methods. The specificity of the interaction was tested by addition of excess C90 (100 µg/ml) (lane 8). Proteins were resolved by 12% SDS–PAGE and visualized by autoradiography. *, the 50 kDa cellular protein identified in each experiment.
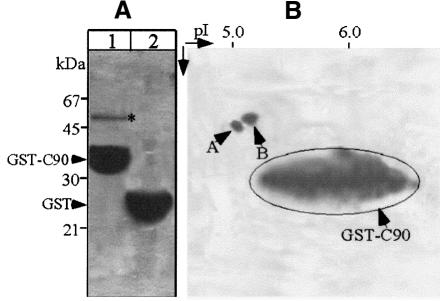
To identify the cellular protein to which NSP4 binds, proteins were purified from MA104 cells using the immobilized GST–C90 as an affinity matrix. SDS–PAGE indicated that the protein migrated as a single 50 kDa species (Figure 2A). The protein was eluted from the gel and digested with trypsin to generate a series of peptide fragments that were separated by reverse-phase HPLC. Two well-resolved peaks were each subjected to Edman degradation, yielding the sequences VGINYQPPTV and LAVNMVPFPR, which were matched to α- and β-tubulin, respectively, by reference to the SwissProt-PIR database. Migration of the protein during SDS–PAGE is consistent with the molecular weight of both α- and β-tubulin (50 kDa), which form a heterodimer (Joshi, 1998). To confirm this identification, the affinity-purified protein was resolved by two-dimensional gel electrophoresis, which yielded two polypeptides of different molecular weight and pI (Figure 2B). Each spot was excised from the gel, digested with trypsin and one HPLC-purified peptide from each sequenced, yielding the sequences FDGALNVD and EIVHIQAG. A database search matched these sequences to α- and β-tubulin, respectively.
Fig. 2. Purification and separation of the NSP4-associated cellular protein. (A) Cellular proteins were purified using immobilized GST–C90 (lane 1) or GST as a control (lane 2) as described in Materials and methods. Bound proteins were eluted with 500 mM NaCl and analysed by SDS–PAGE (12%) and Coomassie staining. (B) The eluted proteins shown in lane 1 were analysed further by two-dimensional gel electrophoresis. Note that the NSP4-associated cellular protein was resolved into two separate polypeptides (arrowheads).
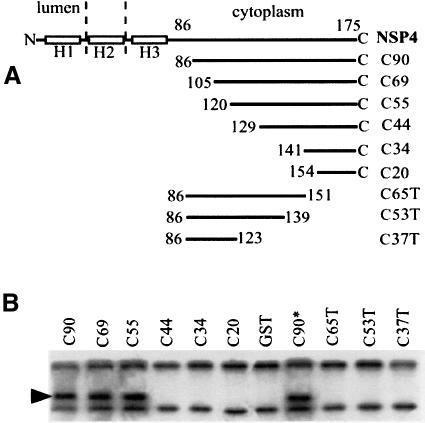
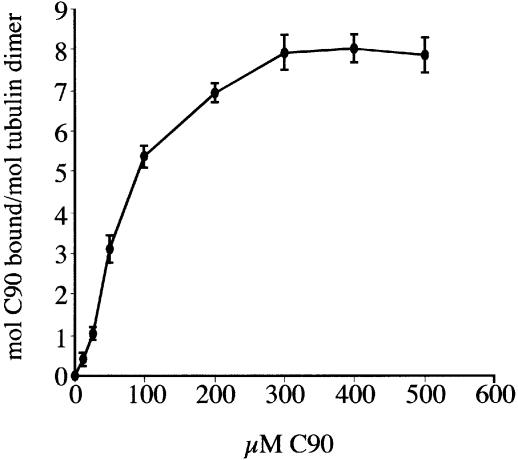
The precise location of the tubulin-binding site within the cytoplasmic tail of NSP4 was then mapped using a series of truncated NSP4–GST fusion proteins immobilized on glutathione–agarose. This approach identified a minimal region containing a functional tubulin-binding site within the C-terminal 54 residues (Figure 3). The amino acid sequence within this region did not exhibit significant primary sequence homology with any known protein, but did overlap with a region of NSP4 previously found to contain a binding site for the rotavirus inner capsid particle (Taylor et al., 1996). Evidence that the cytoplasmic domain of NSP4 binds directly to microtubules, and not via another microtubule-associated protein, was obtained following incubation of C90 with purified bovine brain microtubules, polymerized in vitro and stabilized with taxol. 32P-labelled C90 was added to taxol-stabilized microtubules and the amount of protein bound determined by scintillation counting of the pellet following sedimentation of the microtubule–C90 complexes (Figure 4).
Fig. 3. The microtubule-binding domain of NSP4 is located within the C-terminal 54 amino acids. (A) Schematic representation of the structure of truncated variants of NSP4 used; H1–H3 denotes the positions of hydrophobic domains. (B) Cleared lysates from 35S-labelled MA104 cells were incubated with the indicated fusion protein immobilized on glutathione–agarose. Beads were recovered, washed, and bound proteins were detected by SDS–PAGE and autoradiography. C90* denotes a mutant form of C90 with a C-terminal methionine to isoleucine substitution previously shown to abolish binding of the rotavirus inner capsid particle (Taylor et al., 1993).
Fig. 4. Binding of 32P-labelled C90 to taxol-polymerized microtubules. Experiments were carried out as described in Materials and methods. Data points represent the mean and SEM from four independent experiments.
NSP4 co-localizes with microtubules in Cos-7 cells
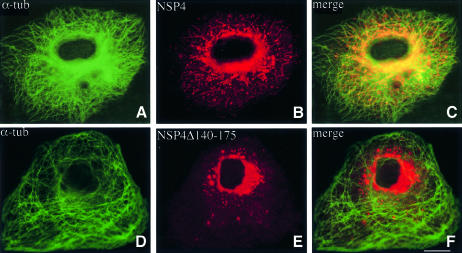
Cos-7 cells were transfected with a plasmid encoding the complete cDNA sequence of NSP4 (pcNSP4) or a C-terminally truncated variant that lacked a functional tubulin-binding site (pcNSP4Δ140–175). Both proteins were observed to be glycosylated and endoglycosidase H (endo H) sensitive (data not shown), consistent with previous data indicating that oligosaccharide residues present on NSP4 are not modified by transit through the Golgi complex (Both et al., 1983; Bergmann et al., 1989). Localization of each protein by confocal laser scanning microscopy revealed a marked difference in their respective distributions within the cell (Figure 5). Both forms of NSP4 are abundant in the perinuclear area, but a significant fraction of wild-type protein exhibits a punctate distribution throughout the cytoplasm. In contrast, NSP4Δ140–175 is concentrated in a dense juxtanuclear body that may reflect misfolding and aggregation of the truncated glycoprotein (Figure 5E and F).
Fig. 5. Localization of NSP4 and NSP4Δ140–175 in vivo. Cos-7 cells were transiently transfected with a construct encoding either NSP4 or NSP4Δ140–175. After 48 h, the cells were fixed, permeabilized, and double-labelled with α-tubulin mAb (A and D) and a polyclonal antibody against NSP4 (B and E) prior to analysis by confocal laser scanning microscopy. Note the co-localization of α-tubulin with NSP4 (C), but not with the truncated mutant (F). The scale bar represents 10 µm.
Further analysis of the staining pattern exhibited by NSP4 revealed a tendency for spots to follow distinct linear tracks that radiate outward toward the periphery of the cell. When the distribution of NSP4 is compared with that of the microtubule network in the same cell, co-localization is observed between the discrete NSP4 spots and individual microtubules (Figure 5C). No such correlation was apparent for the truncated NSP4 variant that lacks a functional microtubule-binding domain. We conclude that membrane-anchored NSP4 associates with microtubules and that this association gives rise to the alignment of membraneous structures containing NSP4 with microtubules.
Expression of NSP4 blocks ER-to-Golgi trafficking of VSV-G
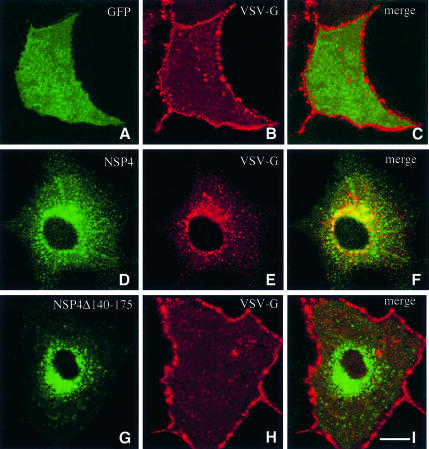
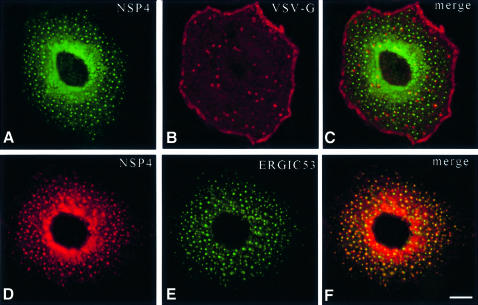
Previous immunocytochemical and biochemical studies have concluded that NSP4 is located in the ER membrane (Bergmann et al., 1989). The integral membrane status of NSP4 coupled with its microtubule-binding activity is intriguing given the role played by microtubules as tracks along which membrane vesicles are driven by motor complexes (Lippincott-Schwartz, 1998). To evaluate the effect of NSP4 expression on membrane traffic, we investigated transport using VSV-G as a marker. A dual transfection system enabled the sequential delivery of plasmid DNA encoding NSP4 and in vitro-synthesized mRNA encoding VSV-G. VSV-G is targeted to the plasma membrane via the classical exocytotic pathway (Gabel and Bergmann, 1985; Bergmann, 1989). Expression of the cytoplasmic green fluorescent protein (GFP) did not alter targeting of VSV-G to the plasma membrane (Figure 6A). In contrast, expression of NSP4 markedly alters the distribution of VSV-G, which accumulates at discrete sites throughout the cytoplasm (Figure 6E). Dual-labelling experiments show that NSP4 and VSV-G co-localize when expressed in the same cell (Figure 6F). Co-expression of VSV-G with NSP4Δ140–175 failed to alter the cellular distribution of either protein (Figure 6G–I).
Fig. 6. Expression of NSP4 blocks targeting of VSV-G to the plasma membrane. Cos-7 cells were transiently transfected with a construct encoding NSP4 (D–F) or NSP4Δ140–175 (G–I) and grown for 36 h. Cells were then re-transfected with in vitro synthesized mRNA encoding VSV-G and grown for a further 10 h. Cells were fixed, permeabilized, and double-labelled with a polyclonal antibody against VSV-G (B, E and H) and anti-NSP4 mAb (D and G). As a control, Cos-7 cells were transfected first with a construct encoding GFP (A–C) prior to transfection of VSV-G mRNA. The scale bar represents 10 µm.
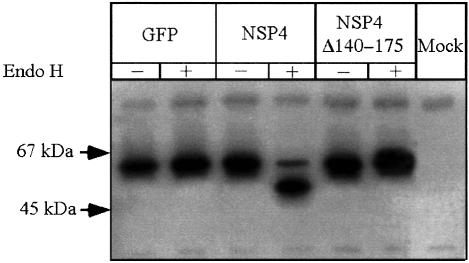
VSV-G is glycosylated at two asparagine residues as it is translocated across the ER membrane, and these high mannose oligosaccharides are subsequently trimmed and modified during transit through the medial Golgi complex (Gabel and Bergmann, 1985). Analysis of VSV-G glycosylation in the presence of NSP4 revealed that N-linked carbohydrate remained sensitive to endo H digestion, indicating that it was not modified by Golgi enzymes (Figure 7). Neither GFP nor NSP4Δ140–175 affected the endo H sensitivity of VSV-G, indicating that trafficking of VSV-G through the Golgi had occurred in the presence of each polypeptide. Thus, we conclude that NSP4 blocks entry of VSV-G to the Golgi and that the inhibition of VSV-G trafficking is mediated selectively by the microtubule-binding properties of NSP4.
Fig. 7. Endo H sensitivity of VSV-G synthesized in the presence of NSP4 or NSP4Δ140–175. Cells were transfected as described in the legend to Figure 5. The cells were 35S-labelled for 2 h commencing 45 h post-DNA transfection and chased for a further 1 h prior to harvest. Lysates were immunoprecipitated with anti-VSV-G antiserum and half of the sample incubated with endo H. Proteins were visualized by SDS–PAGE and autoradiography.
Redistribution of ERGIC53 and COPI complexes by NSP4
The previous experiments indicate that NSP4 blocks the transport of VSV-G at a pre-Golgi step. This result could arise from a block in export from the ER or could reflect the inhibition of a post-ER step in vesicular transport. To address this issue, we examined the effect of NSP4 expression on the distribution of markers of COPII and COPI vesicle coat proteins. Recruitment of the COPII coat complex (composed of Sec23/24, Sec13/31 complexes and Sar1p) mediates budding and export of cargo from specialized ER sites (Barlowe, 1998). Budded COPII-coated vesicles then coalesce to form morphologically distinct vesicular tubular clusters (VTCs), also referred to as the ER–Golgi intermediate compartment (ERGIC). Subsequent replacement of COPII proteins with COPI is essential for translocation of VTCs to the cis-Golgi (Pepperkok et al., 1993; Shima et al., 1999).
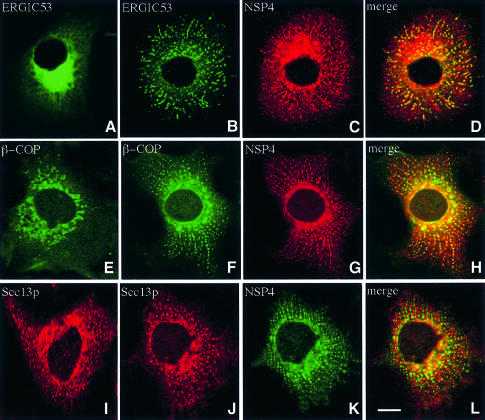
ERGIC53 is a well characterized integral membrane marker of VTCs that dynamically cycles between the ER and Golgi (Itin et al., 1995). Steady-state localization of ERGIC53 reveals a perinuclear distribution with diffuse background fluorescence evident throughout the cytoplasm (Figure 8A). Expression of NSP4 induced the redistribution of ERGIC53 into a spotty pattern that suggested the presence of numerous discrete structures spread throughout the cytoplasm. Under these conditions, structures containing ERGIC53 are aligned along distinct linear tracks that appear to radiate from the centre of the cell toward the periphery. Merged fluorescent images of ERGIC53 and NSP4 from the same cell indicate a high degree of co-localization, suggesting that ERGIC53 and NSP4 might be present in the same membrane structure. β-COP, a marker of the COPI coat complex, undergoes a similar redistribution in the presence of NSP4 (Figure 8E–H), with co-localization of the two proteins again evident. In contrast, the cellular distribution of Sec13p, a COPII coat complex marker, was not significantly altered when co-expressed with NSP4 (compare Figure 8I and J). These findings argue against a block in the export of membrane proteins from the ER, and suggest that a significant fraction of NSP4 is located in a post-ER membrane compartment that co-localizes with both ERGIC53 and COPI and therefore is likely to represent the VTC.
Fig. 8. Distribution of ERGIC53, β-COP and Sec13p in cells expressing NSP4. Cos-7 cells were transiently transfected with pcNSP4 (B–D, F–H and J–L) or mock transfected (A, E and I) and grown for 48 h. Cells were fixed, permeabilized and double-labelled with a polyclonal antibody against NSP4 and a monoclonal antibody against either ERGIC53 (A–D), β-COP (E–H) or Sec13p (I–L) prior to analysis by confocal laser scanning microscopy. The scale bar represents 10 µm.
The NSP4-mediated arrest of membrane trafficking is released by nocodazole
To confirm that the arrest of ER-to-Golgi trafficking by NSP4 was due to its interaction with microtubules, we examined the distribution of VSV-G and ERGIC53 in nocodazole-treated cells that lacked a functional microtubule network. The experimental rationale was based on the observation that VSV-G can undergo transport to the plasma membrane even in the absence of microtubules, albeit with reduced efficiency (Rogalski et al., 1984). Therefore, static retention of vesicles containing VSV-G and NSP4 on the microtubule surface should be released by nocodazole treatment, which would enable transport of VSV-G to resume. Depolymerization of microtubules following nocodazole treatment was confirmed by fluorescent microscopy, revealing a diffuse cytoplasmic staining pattern for α-tubulin (data not shown). Nocodazole treatment resulted in release of the NSP4-mediated block in VSV-G trafficking and the accumulation of the latter protein in the plasma membrane (Figure 9). Nocodazole treatment also altered the distribution of ERGIC53 in NSP4-transfected cells, yielding a pattern of randomly scattered spots throughout the cytoplasm, consistent with the distribution of ERGIC53 previously observed in several cell types following depolymerization of microtubules (Tang et al., 1995; Yang and Storrie, 1998). A significant fraction of NSP4 was also found in structures that co-localize with ERGIC53 after nocodazole treatment. These experiments show that NSP4 can immobilize elements of the early secretory pathway and prevent the targeting of membrane proteins to the Golgi in a microtubule-dependent manner.
Fig. 9. Distribution of NSP4, VSV-G and ERGIC53 in nocodazole-treated cells. Cells were transfected with either pcNSP4 and VSV-G mRNA as in Figure 5 (A–C) or pcNSP4 alone (D–F). At 48 h post-transfection, the cells were treated with 5 µM nocodazole for 3 h and then double-labelled for VSV-G and NSP4 (A–C) or ERGIC53 and NSP4 (D–F). The scale bar represents 10 µm.
Discussion
NSP4 is a membrane-anchored viral MAP
Microtubule-associated proteins (MAPs) constitute a heterogeneous group of proteins involved in the function and dynamics of microtubules (Drewes et al., 1998). Several organelle-specific MAPs have been identified recently, including p63, an integral membrane protein located in the ER (Klopfenstein et al., 1998), and GMAP-210, a peripheral protein associated with the cis-Golgi (Infante et al., 1999). Microtubule binding by these proteins in vivo might play a role in the maintenance of organelle morphology and position. NSP4 is the first membrane-anchored MAP shown to be expressed by an animal virus. The microtubule-binding domain of NSP4 is located within a 54 amino acid sequence in the cytoplasmic tail of the protein. This sequence does not share significant homology with any other known MAP. Moreover, the microtubule-binding domain of NSP4 has a net negative charge (11 acidic, eight basic residues). The abundance of acidic residues is in contrast to the basic nature of microtubule-binding domains found in other MAPs.
The microtubule-binding domain of NSP4 lies adjacent to a region of the protein predicted to form an α-helical coiled-coil structure (Taylor et al., 1996), and similar structures are recognized or predicted to exist within many different MAPs. When potential sequence similarities with this region of NSP4 were examined by searching the SwissProt database using BLAST, limited homology was detected within the coiled-coil domains of other microtubule-binding proteins, including kinesin.
NSP4 arrests ER-to-Golgi membrane transport
Transport of membrane and secretory proteins from the ER to the Golgi complex is mediated by pleiomorphic membrane vesicles referred to as VTCs or the ERGIC (Bannykh et al., 1998). These structures are defined by several transmembrane marker proteins such as ERGIC53 and by the presence of the COPI coat complex. Transport of VTCs to the Golgi is dependent on both microtubule integrity and a functional dynactin complex (Burkhardt et al., 1997; Burkhardt, 1998). The most compelling evidence for microtubule-directed translocation of VTCs to the Golgi has come from studies in living cells that express a temperature-sensitive mutant of VSV-G tagged with GFP (Presley et al., 1997; Scales et al., 1997). These studies reveal the rapid translocation of VTCs in a series of saltatory movements to a central site presumed to represent the cis-Golgi.
Analysis of both VSV-G glycosylation and its cellular distribution indicates that trafficking of this protein to the plasma membrane is arrested by NSP4 at a pre-Golgi step. This effect is attributable directly to the NSP4–micro tubule interaction since expression of neither NSP4Δ140– 175 nor GFP adversely affected targeting of VSV-G to the plasma membrane. NSP4 also altered the steady-state distribution of ERGIC53 and β-COP to a series of discrete structures that co-localized with NSP4, and this redistribution was again dependent on the presence of a microtubule-binding site within NSP4 (Figure 8). ERGIC53 has been observed to undergo redistribution to discrete cytoplasmic structures following the depolymerization of microtubules, incubation of cells at 15°C, or during the inhibition of cytoplasmic dynein/dynactin (Tang et al., 1995; Burkhardt et al., 1997; Yang and Storrie, 1998). Under these conditions, VTCs are unable to move along the microtubule toward their destination and persist in discrete structures randomly distributed throughout the cytoplasm. The redistribution of ERGIC53 induced by NSP4 differs from that observed in these studies in that both proteins exhibit a non-random distribution within structures that are clearly aligned along linear tracks radiating throughout the cytoplasm. The lack of a reticular pattern exhibited by ERGIC53 in these cells suggests that the NSP4-mediated block in trafficking occurs in a post-ER membrane compartment that probably corresponds to the VTC or to a similar post-ER transport vesicle. This conclusion is supported by the observation that NSP4 and ERGIC53 also co-localized following treatment of cells with nocodazole, but in structures that were randomly distributed throughout the cytoplasm (Figure 9). This pattern is distinct from the redistribution of the ER marker calreticulin in similarly treated cells (data not shown), which was restricted to a continous perinuclear zone, probably reflecting retraction of the ER, as observed previously in cells lacking a functional microtubule network (Lee et al., 1989).
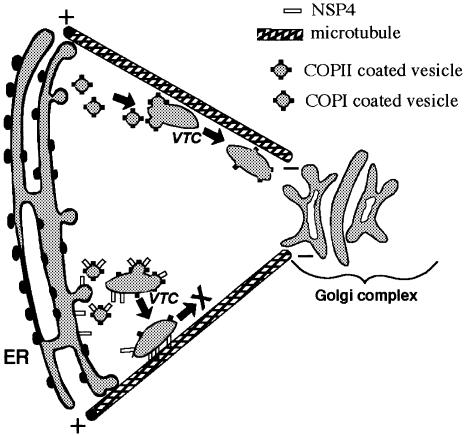
We conclude that translocation of vesicles involved in ER-to-Golgi trafficking is prevented by a direct interaction between NSP4 and microtubules. This block in VSV-G trafficking could be overcome by nocodazole treatment, suggesting that vesicles might be anchored directly to the microtubule surface by NSP4. Depolymerization of micro tubules would therefore release the anchored vesicles and permit VSV-G to reach the plasma membrane via a microtubule-independent trafficking pathway (Rogalski et al., 1984). The ability of VSV-G to reach the plasma membrane in the presence of nocodazole indicates that the block in trafficking imposed by NSP4 is not due to inhibition of export from the ER and supports a post-ER, microtubule-associated site for vesicle arrest (Figure 10).
Fig. 10. Schematic representation of NSP4-mediated arrest of ER-to-Golgi transport. Normal ER-to-Golgi translocation of VTCs is depicted along the upper microtubule. The presence of NSP4 causes a direct attachment of VTCs to the microtubule surface and prevents subsequent translocation.
Previous studies have shown that overexpression of certain MAPs inhibits organelle motility in vivo (Bulinski et al., 1997; Ebneth et al., 1998). However, to our knowledge, this is the first report of a membrane protein that blocks membrane transport through the static retention of vesicles on the microtubule track. Additionally, microtubule binding by the cytoplasmic domain of a membrane glycoprotein represents a plausible mechanism for retention in the ER or intermediate compartment by preventing subsequent transport along the secretory pathway.
Implications for the assembly and pathogenesis of rotavirus
The ability of NSP4 to block ER-to-Golgi traffic may have implications for viral pathogenesis and the unusual maturation pathway of rotavirus. Unlike other viruses that mature in the ER lumen, rotavirus is not released via the classical exocytotic pathway. Instead, recent data suggest that this virus may undergo direct vesicular transport from the ER to the apical plasma membrane of polarized epithelial cells without transit through the Golgi complex (Jourdan et al., 1997).
Rotavirus-infected individuals exhibit decreased activity of intestinal brush border disaccharidases [sucrase– isomaltase (SI), lactase and maltase–glucoamylase], which may contribute to the development of diarrhoea (Davidson et al., 1977; Collins et al., 1988). Jourdan et al. (1998) have demonstrated that the reduced SI activity induced by rotavirus infection of Caco-2 cells is not due to decreased biosynthesis of the enzyme, but rather is the result of disruption of the delivery of the enzyme to the apical plasma membrane in rotavirus-infected cells. The NSP4-mediated arrest of ER-to-Golgi membrane traffic thus offers a plausible mechanism by which an animal virus could limit the activity of enzymes in the plasma membrane and thus contribute to the onset and/or persistence of diarrhoea.
Materials and methods
Antibodies and reagents
Monoclonal antibodies (mAbs) against α-tubulin and β-COP were purchased from Sigma. Anti-NSP4 mAb was kindly provided by Harry Greenberg (Stanford University, CA), rabbit polyclonal serum against a β-galactosidase–NSP4 fusion was obtained from Paul Atkinson (MAF, Wallaceville, New Zealand), mAb against ERGIC53 (Schweizer et al., 1988) was a generous gift from Hans-Peter Hauri (Basel, Switzerland), rabbit polyclonal antibody against VSV-G (Machamer and Rose, 1988) was provided by Carolyn Machamer (University of California San Diego, CA) and mAbs against human Sec13p (Tang et al., 1997) were a generous gift from Wanjin Hong (University of Singapore). Fluoro link™ Cy™ 2-labelled donkey anti-mouse IgG (H+L) was purchased from Amersham Life Science, rhodamine Red™-x-conjugated AffiniPure goat anti-rabbit IgG (H+L) from Jackson Immunoresearch, USA, and protein A– Sepharose CL-4B beads and glutathione–Sepharose CL-4B beads from Amersham Pharmacia.
Cell culture and virus infection
Rhesus monkey kidney cell lines MA104 or Cos-7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum at 37°C. MA104 cells were infected by the SA11 strain of rotavirus as previously described (Xu et al., 1998).
Plasmid construction and expression of recombinant proteins
Expression of truncated NSP4 domains in Escherichia coli as GST fusions has been described previously (Taylor et al., 1996). Additional C-terminally truncated variants were constructed using PCR to generate the appropriate cDNA fragments encoding C65T, C53T and C37T, which were cloned as BamHI fragments into the vector pGEX.2T. Transient expression of NSP4 in Cos-7 cells was achieved by insertion of the coding sequence into pCDNA3.1 (Invitrogen), under transcriptional control of the cytomegalovirus (CMV) promoter to yield pcNSP4. pcNSP4Δ140– 175 was generated using PCR mutagenesis to insert a stop and BamHI site and replacement of the wild-type sequence in pcNSP4. Expression of VSV-G in Cos-7 cells was achieved by transfection of RNA. Briefly, cDNA was excised from pB5/G (provided by Dr Carolyn Machamer) using BamHI and cloned into pCDNA3.1. This construct was then used as a template for in vitro mRNA synthesis using T7 RNA polymerase (Promega). Transfection of DNA and RNA into Cos-7 cells was performed using Lipofectamine Plus (Life Technologies) following the manufacturer’s instructions.
Metabolic labelling, immunoprecipitation and endoglycosidase H digestion
MA104 or Cos-7 cells were labelled with 35S Trans-label (ICN) as described previously (Xu et al., 1998). The cells were solubilized in lysis buffer [25 mM HEPES pH 7.5, 5 mM EDTA, 5 mM EGTA, 50 mM NaCl, 50 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml aprotinin and 2 µg/ml pepstatin], and centrifuged to remove debris. The clarified lysate was pre-incubated with protein A–Sepharose beads (1 h) and subsequently incubated with the appropriate antibody for 3 h at 4°C. Immune complexes were recovered by addition of protein A–Sepharose beads for 1 h, washed three times in lysis buffer and the proteins analysed by SDS–PAGE and autoradiography or phosphoimaging.
For experiments in which endo H digestion was performed prior to SDS–PAGE, the precipitated immune complexes were released from protein A–Sepharose beads by incubating with 0.1 M NaOAc pH 5.5, 0.25% SDS at 95°C for 5 min and centrifuged at 13 000 r.p.m. for 2 min. The recovered supernatants were divided into two equal aliquots. Endo H was added into one aliquot from each sample. These samples were incubated at 37°C for 2 h and analysed by SDS–PAGE and autoradiography.
GST fusion ‘pull-down’ assay and analysis of bound proteins
GST–C90 or GST was expressed in E.coli and purified on glutathione– Sepharose (Taylor et al., 1996). For analytical binding experiments, 20 µl of a 50% slurry of glutathione–agarose to which ∼2 µg of GST–C90 or GST was bound, were incubated for 3 h at 4°C with a clarified lysate prepared from 2 × 106 35S-labelled MA104 cells. The beads were then washed with lysis buffer, and recovered proteins were analysed by SDS–PAGE and autoradiography. In a preparative pull-down experiment, ∼108 MA104 cells were solubilized in 10 ml of lysis buffer incubated with 1 ml of beads containing ∼2 mg of GST–C90 as above. The beads were washed and bound proteins eluted by a stepwise increase in NaCl concentration. Fractions were collected manually, separated by one- or two-dimensional gel electrophoresis and identified by staining with Coomassie Blue. Bands or spots corresponding to proteins recovered by preparative pull-down experiments from one- or two-dimensional gels were excised and subjected to in-gel trypsin digestion as described previously (Xu et al., 1998). The peptide mixtures were separated by reverse-phase HPLC (C18 column, Phenomenex) and fractions were collected manually. Well-resolved peaks were sequenced in a Perkin Elmer (Procise Model 492) sequenator.
In vitro polymerization of microtubules
Bovine tubulin was purified by two cycles of assembly–disassembly and phosphocellulose chromatography (Williams and Lee, 1982) and stored in MG buffer (50 mM MES pH 6.8, 0.5 mM EGTA, 0.5 mM MgCl2, 3.5 mM glycerol, 0.5 mM GTP) at –80°C until use. For C90 binding experiments, purified tubulin (20 µM) was polymerized in 150 µl of MG buffer supplemented with 6 mM MgCl2, 500 µM GTP and 1.5 M taxol (Sigma) for 15 min at 37°C in a series of tubes. An equal amount of 32P-labelled C90 (mixed with varying quantities of unlabelled protein) was added to the polymerized microtubules for 30 min. Complexes were recovered by centrifugation in an airfuge and the bound radioactivity (minus radioactivity from microtubule-free controls) determined by scintillation counting.
Immunofluorescent labelling and confocal laser scanning microscopy
Transfected Cos-7 cells were trypsinized 24 h after transfection and seeded onto coverslips in a 24-well culture dish. At 48 h after DNA transfection or 8 h after RNA transfection, cells were fixed by 3% paraformaldehyde and permeablized by 0.2% Triton X-100. The cells were subsequently incubated with the corresponding primary antibodies in phosphate-buffered saline containing 2% bovine serum albumin, and stained with fluorescein isothiocyanate (FITC)- or rhodamine-conjugated secondary antibodies. Specimens were observed using a Leica 4d TCS confocal laser scanning microscope.
Acknowledgments
Acknowledgements
We are grateful to Drs Hans-Peter Hauri (ERGIC53), Carolyn Machamer (VSV-G), Wanjin Hong (Sec13p) and Harry Greenberg (NSP4) for generously providing antibodies used in this study. Dr Judith O’Brien provided constructs encoding some GST–NSP4 fusion proteins, Hillary Holloway assisted with confocal microscopy, and Dr Joerg Kistler provided critical comments on the manuscript. We thank Dr Jennifer Lippincott-Schwartz for suggesting experiments using nocodazole. This research was funded by a Project Grant from the Health Research Council of New Zealand.
References
- Andersson H., Kappeler,F. and Hauri,H.P. (1999) Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J. Biol. Chem., 274, 15080–15084. [DOI] [PubMed] [Google Scholar]
- Ball J.M., Tian,P., Zheng,C.Q., Morris,A.P. and Estes,M.K. (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science, 272, 101–104. [DOI] [PubMed] [Google Scholar]
- Bannykh S.I., Nishimura,N. and Balch,W.E. (1998) Getting into the Golgi. Trends Cell Biol., 8, 21–25. [DOI] [PubMed] [Google Scholar]
- Barlowe C. (1998) COPII and selective export from the endoplasmic reticulum. Biochim. Biophys. Acta, 1404, 67–76. [DOI] [PubMed] [Google Scholar]
- Bergmann C.C., Maass,D., Poruchynsky,M.S., Atkinson,P.H. and Bellamy,A.R. (1989) Topology of the non-structural rotavirus receptor glycoprotein NS28 in the rough endoplasmic reticulum. EMBO J., 8, 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J.E. (1989) Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell Biol., 32, 85–110. [DOI] [PubMed] [Google Scholar]
- Both G.W., Siegman,L.J., Bellamy,A.R. and Atkinson,P.H. (1983) Coding assignment and nucleotide sequence of simian rotavirus SA11 gene segment 10: location of glycosylation sites suggests that the signal peptide is not cleaved. J. Virol., 48, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V. and Ganem,D. (1991) The role of envelope proteins in hepatitis B virus assembly. Proc. Natl Acad. Sci. USA, 88, 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J.C., McGraw,T.E., Gruber,D., Nguyen,H.L. and Sheetz,M.P. (1997) Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J. Cell Sci., 110, 3055–3064. [DOI] [PubMed] [Google Scholar]
- Burkhardt J.K. (1998) The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim. Biophys. Acta, 1404, 113–126. [DOI] [PubMed] [Google Scholar]
- Burkhardt J.K., Echeverri,C.J., Nilsson,T. and Vallee,R.B (1997) Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol., 139, 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquerel L., Duvet,S., Meunier,J.C., Pillez,A., Cacan,R., Wychowski,C. and Dubuisson,J. (1999) The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J. Virol., 73, 2641–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N.B. and Lippincott-Schwartz,J. (1995) Organization of organelles and membrane traffic by microtubules. Curr. Opin. Cell Biol., 7, 55–64. [DOI] [PubMed] [Google Scholar]
- Collins J. et al. (1988) Intestinal enzyme profiles in normal and rotavirus-infected mice. J. Pediatr. Gastroenterol. Nutr., 7, 264–272. [DOI] [PubMed] [Google Scholar]
- Davidson G.P., Gall,D.G, Petric,M., Butler,D.G. and Hamilton,J.R. (1977) Human rotavirus enteritis induced in conventional piglets. Intestinal structure and transport. J. Clin. Invest., 60, 1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G., Ebneth,A. and Mandelkow,E.M. (1998) MAPs, MARKs and microtubule dynamics. Trends Biochem. Sci., 23, 307–311. [DOI] [PubMed] [Google Scholar]
- Ebneth A., Godemann,R., Stamer,K., Illenberger,S., Trinczek,B. and Mandelkow,E. (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J. Cell Biol., 143, 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M.K. (1996) Rotaviruses and their replication. In Fields,B.N. et al. (eds), Virology, 3rd edn. Lippincott-Raven Publishers, Philadelphia, PA, pp. 1625–1655. [Google Scholar]
- Gabel C.A. and Bergmann,J.E. (1985) Processing of the asparagine-linked oligosaccharides of secreted and intracellular forms of the vesicular stomatitis virus G protein: in vivo evidence of Golgi apparatus compartmentalization. J. Cell Biol., 101, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert P.A., Shaw,K.L., Ritter,G.D.,Jr and Mulligan,M.J. (1997) A sorting motif localises the foamy virus glycoprotein to the endoplasmic reticulum. J. Virol., 71, 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante C., Ramos-Morales,F., Fedriani,C., Bornens,M. and Rios,R.M. (1999) GMAP-210, a cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J. Cell Biol., 145, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin C., Schindler,R. and Hauri,H.P. (1995) Targeting of protein ERGIC-53 to the ER/ERGIC/cis-Golgi recycling pathway. J. Cell Biol., 131, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.R., Nilsson,T. and Peterson,P.A. (1990) Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J., 10, 3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H.C. (1998) Microtubule dynamics in living cells. Curr. Opin. Cell Biol., 10, 35–44. [DOI] [PubMed] [Google Scholar]
- Jourdan N., Maurice,M., Delautier,D., Quero,A.M., Servin,A.L. and Trugnan,G. (1997) Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol., 71, 8268–8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan N., Brunet,J.P., Sapin,C., Blais,A., Cotte-Laffitte,J., Forestier,F., Quero,A.M., Trugnan,G. and Servin,A.L. (1998) Rotavirus infection reduces sucrase–isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of the cytoskeleton. J. Virol., 72, 7228–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein D.R., Kappeler,F. and Hauri,H.P. (1998) A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J., 17, 6168–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Ferguson,M. and Chen,L.B. (1989) Construction of the endoplasmic reticulum. J. Cell Biol., 109, 2045–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. (1998) Cytoskeletal proteins and Golgi dynamics. Curr. Opin. Cell Biol., 10, 52–59. [DOI] [PubMed] [Google Scholar]
- Machamer C.E. and Rose,J.K. (1988) Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J. Biol. Chem., 263, 5955–5960. [PubMed] [Google Scholar]
- Newton K., Meyer,J.C., Bellamy,A.R. and Taylor,J.A. (1997) Rotavirus nonstructural glycoprotein NSP4 alters plasma membrane permeability in mammalian cells. J. Virol., 71, 9458–9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N., and BalchW.E. (1997) A di-acidic signal required for selective export from the endoplasmic reticulum. Science, 277, 556–558. [DOI] [PubMed] [Google Scholar]
- Nishimura N., Bannykh,S., Slabough,S., Matteson,J., Altschuler,Y., Hahn,K. and Balch,W.E. (1999) A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J. Biol. Chem., 274, 15937–15946. [DOI] [PubMed] [Google Scholar]
- Owen K.E. and Kuhn,R.J. (1997) Alphavirus budding is dependent on the interaction between the nucleocapsid and hydrophobic amino acids on the cytoplasmic domain of the E2 envelope glycoprotein. Virology, 230, 187–196. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Scheel,J., Horstmann,H., Hauri,H.P., Griffiths,G. and Kreis,T.E. (1993) β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell, 74, 71–82. [DOI] [PubMed] [Google Scholar]
- Presley J.F., Cole,N.B., Schroer,T.A., Hirschberg,K., Zaal,K.J. and Lippincott-Schwartz,J. (1997) ER-to-Golgi transport visualized in living cells. Nature, 389, 81–85. [DOI] [PubMed] [Google Scholar]
- Rogalski A.A., Bergmann,J.E. and Singer,S.J. (1984) Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J. Cell Biol., 99, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel K., West,J.T.,Jr, Mulligan,M.J. and Hunter,E. (1998) Retention of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum does not redirect virus assembly from the plasma membrane. J. Virol., 72, 7523–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales S.J., Pepperkok,R. and Kreis,T.E. (1997) Visualisation of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell, 90, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Fransen,J.A., Bachi,T., Ginsel,L. and Hauri,H.P. (1988) Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol., 107, 1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D., Scales,S., Kreis,T.E. and Pepperkok,R. (1999) Segregation of COPI-rich and anterograde-cargo-rich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr. Biol., 9, 821–824. [DOI] [PubMed] [Google Scholar]
- Strauss J.H., Strauss,E.G. and Kuhn,R.J. (1995) Budding of alphaviruses. Trends Microbiol., 3, 346–350. [DOI] [PubMed] [Google Scholar]
- Suomalainen M., Liljestrom,P. and Garoff,H. (1992) Spike protein– nucleocapsid interactions drive the budding of alphaviruses. J. Virol., 66, 4737–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.L., Low,S.H., Hauri,H.P. and Hong,W. (1995) Segregation of ERGIC53 and the mammalian KDEL receptor upon exit from the 15°C compartment. Eur. J. Cell Biol., 68, 398–410. [PubMed] [Google Scholar]
- Tang B.L., Peter,F., Krijnse-Locker,J., Low,S.H., Griffiths,G. and Hong,W. (1997) The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol. Cell. Biol., 17, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.A., O’Brien,J.A. and Yeager,M. (1996) The cytoplasmic tail of NSP4, the endoplasmic reticulum-localized non-structural glycoprotein of rotavirus, contains distinct virus binding and coiled coil domains. EMBO J., 15, 4469–4476. [PMC free article] [PubMed] [Google Scholar]
- Tian P., Yanfang,H., Schilling,W.P., Lindsay,D.H. and Estes,M.K. (1994) Rotavirus NSP4 affects intracellular calcium levels. J. Virol., 68, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.C. and Lee,J.C. (1982) Preparation of tubulin from brain. Methods Enzymol., 85, 376–385. [DOI] [PubMed] [Google Scholar]
- Xu A., Bellamy,A.R. and Taylor,J.A. (1998) BiP (GRP78) and endoplasmin (GRP94) are induced following rotavirus infection and bind transiently to an endoplasmic reticulum-localised virion component. J. Virol., 72, 9865–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. and Storrie,B. (1998) Scattered Golgi elements during microtubule disruption are initially enriched in trans-Golgi proteins. Mol. Biol. Cell, 9, 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]