Summary
Formins are a large family of actin assembly-promoting proteins with many important biological roles [1-3]. However, it has remained unclear how formins nucleate actin polymerization. All other nucleators are known to recruit actin monomers as a central part of their mechanisms [3-5]. However, the actin-nucleating FH2 domain of formins lacks appreciable affinity for monomeric actin [6, 7]. Here, we found that yeast and mammalian formins bind actin monomers, but this activity requires their C-terminal DAD domains. Further, we observed that the DAD works in concert with the FH2 to enhance nucleation without affecting the rate of filament elongation. We dissected this mechanism in mDia1, mapped nucleation activity to conserved residues in the DAD, and demonstrated that DAD roles in nucleation and autoinhibition are separable. Further, DAD enhancement of nucleation was independent of contributions from the FH1 domain to nucleation [8]. Together, our data show that: (i) the DAD has dual functions in autoinhibition and nucleation, (ii) the FH1, FH2 and DAD form a tri-partite nucleation machine, and (iii) formins nucleate by recruiting actin monomers, and therefore are more similar to other nucleators than previously thought.
Keywords: Actin, formin, DAD domain, nucleation, diaphanous
Results and Discussion
The DAD domain of mDia1 enhances actin nucleation
In earlier studies, a reported lack of G-actin binding affinity for the formin FH2 domain led to the hypothesis that formins might instead nucleate by stabilizing spontaneously formed actin dimers and/or trimers [6]. However, all other known actin nucleators actively recruit actin monomers [4, 5]. This has left open the possibility that efficient nucleation by formins may involve actin-binding sequences located outside of the FH2 domain. Previous studies suggested that the FH1 domain enhances both nucleation and elongation by recruiting profilin-actin [6, 8-10]. However, the potential roles in nucleation of sequences C-terminal to the FH2 have remained unclear. WH2-like sequences have been identified in the C-terminal regions of several formins (FRL2, FRL3, and INF2), suggesting possible actin interactions [11, 12]. Further, the C-terminus of INF2 promotes actin assembly [11]. However, INF2 also severs filaments, making it difficult to assess whether the C-terminus contributed specifically to nucleation. Here, we investigated this issue by comparing in several different formins the activities of FH1-FH2 constructs with and without additional C-terminal sequences.
We initially focused on mDia1, since it neither severs nor bundles filaments [11, 13]. We investigated DAD contributions to nucleation by comparing the actin assembly-promoting activities of freshly purified mDia1 polypeptides consisting of the FH1-FH2 region with and without C-terminal DAD-containing region (Fig. 1A). These two formin constructs are referred to herein as C and C-ΔDAD, respectively (schematic, Fig. 1A). The activities of C and C-ΔDAD polypeptides were compared in pyrene-actin assembly assays over a range of concentrations in the presence and absence of profilin (Fig. 1B and 1C). The C polypeptide had significantly higher activity than C-ΔDAD (example raw curves in Fig. 1D and 1E). This difference was substantial in the absence of profilin, but even more pronounced in the presence of profilin. Earlier studies on mDia1 C and C-ΔDAD polypeptides did not note any differences in their activities ([8], mDia1 C;[14], mDia1 C-ΔDAD); however, the activities of the two constructs had been quantified in separate studies and thus not compared directly, and not in the presence of profilin where differences are greatest. In addition, we compared polypeptides immediately after purification, avoiding freeze/thawing, which we found can diminish DAD contributions.
Figure 1. Contributions of mDia1 C-terminal sequences to actin nucleation.

A) Schematic and Coomassie-stained gels of purified C and C-ΔDAD mDia1 polypeptides. B,C) Actin (2 μM, 5% pyrene labeled) was assembled in the presence of different concentrations of mDia1 C and C-ΔDAD constructs with and without 4 μM profilin. Rates of assembly were determined from the slopes of the curves and graphed as Fluorescence Arbitrary Units (FAU) per unit time (sec). D, E) Raw curves comparing effects of 10nM mDia1 C and C-ΔDAD polypeptides in the presence (E) and absence (D) of 4μM profilin. F) Time-lapse TIRF microscopy of actin filament elongation comparing barbed end growth rates for reactions containing actin and profilin alone and with mDia1 C or mDia1 C-ΔDAD (see Supplementary Movies). Panels show actin filaments imaged at the indicated time points after initiation of polymerization. Arrows in each panel are color-coded (blue, barbed end; red, pointed end). G) Average rates of barbed end elongation (n = 10 filaments). H) Effects of Cof1 and mDia1 C (250 nM and 125 nM, respectively) on rate of disassembly of 2 μM F-actin (10% pyrene-labeled) induced at time zero by Vitamin D-binding protein.
Since bulk polymer assembly assays do not discern between formin effects on nucleation and elongation, we used time-lapse total internal reflection fluorescence (TIRF) microscopy to compare C and C-ΔDAD mDia1 effects on elongation rates of individual filaments (Fig. 1F). In the presence of profilin, both mDia1 C and C-ΔDAD accelerated elongation by >5 fold (movies S1-S3), as previously reported for mDia1 C [9]. Quantification of elongation rates revealed that there was no significant difference between C and C-ΔDAD polypeptides (Fig. 1G), indicating that the differences in their actin assembly activities must be due to differences in nucleation. Consistent with these observations, C and C-ΔDAD polypeptides showed similar effects on barbed end growth in seeded elongation assays with and without capping protein (Fig. S1A and S1B). In addition, mDia1 C showed no filament severing activity (Fig. 1H), as previously reported [11]. Taken together, these data show that the C-terminus of mDia1 makes a substantial contribution to actin nucleation without affecting rate of elongation or protection from capping protein.
Similar differences in the actin assembly activities of C and C-ΔDAD polypeptides were observed for three other formins, Bni1, Bnr1, and Daam1 (Fig. S2), suggesting that the role of the C-terminus in nucleation could be conserved. Differences were most striking for Daam1, where loss of the C-terminus caused a >30 fold decrease in assembly activity (Fig. S2D). None of these formins exhibited severing activity, indicating that the C-terminus in each case contributes to de novo actin assembly (Fig. S2E). However, further analysis will be required to determine whether these DAD contributions to actin assembly are due to effects on nucleation and not elongation as in mDia1.
Identification of residues in DAD that mediate actin nucleation
Next, we mapped the nucleation activity of the mDia1 C-terminus. Our mDia1 C and C-ΔDAD polypeptides differed by 76 residues (549-1255 and 549-1179, respectively). We first tested whether sequences C-terminal to the DAD motif contribute to nucleation. For this, we generated a new truncation, mDia1 C-ΔCT (549-1200), and compared its activity to mDia1 C. We observed no difference in their activities over a range of concentrations (Fig. S3A), suggesting that nucleation activity in the mDia1 C-terminus stems from sequences in the DAD domain itself.
Next, we used site directed mutagenesis to dissect the nucleation activity. Structural studies on DAD have shown that it consists of a short amphipathic helix followed by an unstructured sequence rich in basic residues [15-17]. In addition, mutational and structural analyses have identified specific residues in DAD that mediate autoinhibitory interactions with the N-terminal DID [15, 16, 18, 19]. From an alignment of DAD sequences, we identified conserved residues (Fig. 2A, black open circles). We designed five alleles (Fig. 2A, m1-m5) that collectively mutate 11 residues, including some known to be important for autoinhibition (Fig. 2A, red dots). We purified wild type and mutant mDia1 C polypeptides and compared their actin assembly activities in the absence and presence of profilin (Fig. 2B and 2C). One mutant (m5) showed a severe loss of nucleation activity, comparable to deleting the entire DAD (raw curves in Fig. 2D and 2E). Another mutant (m4) showed a partial loss of activity. The remaining three mutants (m1, m2, m3) had activities similar to wild type mDia1 C. These results suggest that one or both of the residues mutated in m5 (K1198E and R1199E) are critical for DAD nucleation activity, and that the residues mutated in m4 (Q1190A and S1191A) make a smaller contribution.
Figure 2. Mapping the actin nucleation activity of the DAD domain.

A) Sequence alignment of formin DAD domains from Mus musculus (mDia1), Saccharomyces cerevisiae (Bni1 and Bnr1), and Homo sapiens (Daam1). Residues conserved in DAD are shaded in grayscale (darker shading indicating a higher degree of conservation). Open black circles indicate residues mutated in the m1-m5 alleles of mDia1 C: m1 (E1175A, D1177A, E1178A), m2 (M1182A, L1185A), m3 (D1182A, L1186A), m4 (Q1190A, S1191A) and m5 (K1198E, R1199E). Red dots indicate residues known to mediate autoinhibition [15, 16, 18, 19]. B,C) Pyrene-actin (2 μM, 5% labeled) was assembled with different concentrations of wild type or mutant mDia1 C polypeptides in the absence (B) or presence (C) of 5 μM profilin. Rates of assembly determined from the slopes of assembly curves. D,E) Raw curves for mDia1 C and mDia1 C(m5) in the presence of 5 μM profilin. F) Pyrene-actin was assembled as in C, using 2 nM wild type and mutant mDia1 C polypeptides and variable concentrations of N-mDia1. Rates of assembly were determined from the raw curves and graphed.
We also investigated the relationship between nucleation and autoinhibition mediated by DAD, by comparing the activities of wild type and mutant mDia1 C polypeptides (2 nM) in the presence of different concentrations of a DID-containing fragment (mDia1 N) (Fig. 2F). The activity of wild type mDia1 C was inhibited in trans by mDia1 N in a concentration-dependent manner, reaching 50% inhibition at 2-3 nM mDia1 N, as previously reported [8]. Inhibition of m1, m4 and m5 mutant mDia1 C polypeptides was similar to wild type, whereas m2 and m3 mutant polypeptides were refractory to inhibition. These results are consistent with m2 and m3 having weakened DAD-DID interactions, as predicted [17]. Taken together, these observations show that m4 and m5 impair nucleation without affecting autoinhibition, whereas m2 and m3 impair autoinhibition without affecting nucleation. Thus, DAD functions in autoinhibition and nucleation are separable.
DAD domain binding to actin monomers
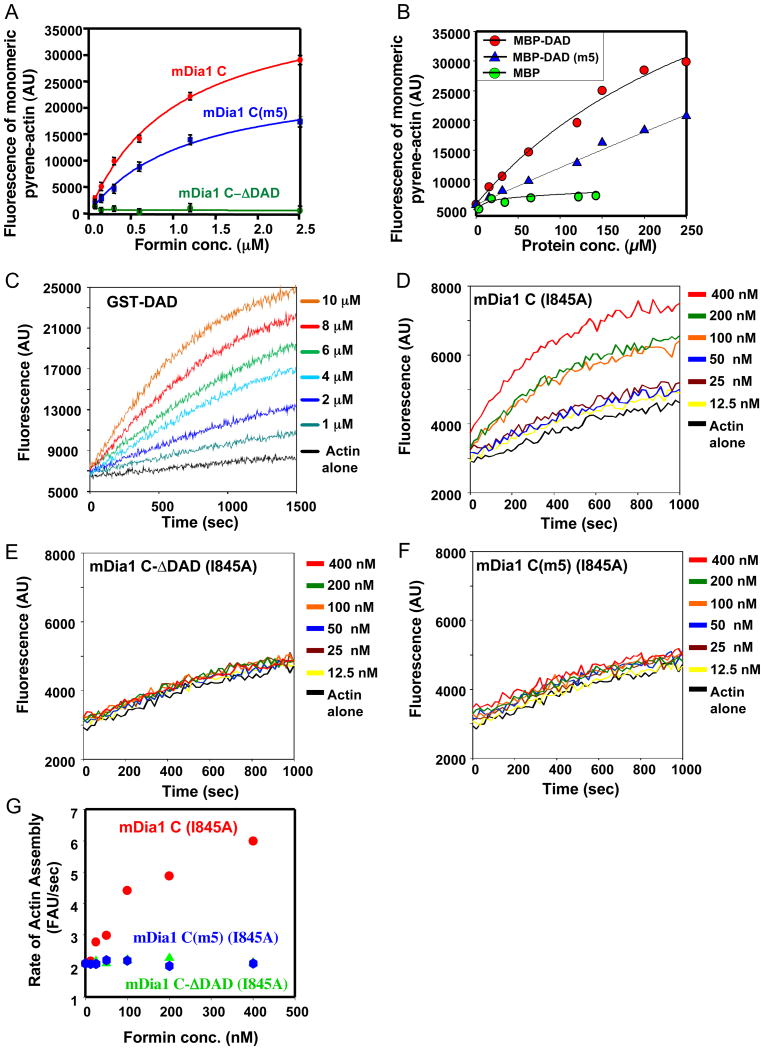
The simplest mechanism to explain how DAD might enhance nucleation is by directly binding actin. To test this, we compared the abilities of mDia1 C and C-ΔDAD polypeptides to affect the fluorescence signal of pyrene-G-actin under conditions that prevent polymerization (250 nM actin and 400 nM Latrunculin B). Under these conditions, mDia1 C induced a concentration-dependent increase in pyrene-actin signal, suggesting binding. In contrast, C-ΔDAD had no significant effect on pyrene-actin fluorescence (Fig. 3A), suggesting that DAD is required for binding to G-actin. Mutant mDia1 C(m5) showed altered effects on pyrene-actin fluorescence compared to wild type mDia1 C, suggesting that this mutation changes some aspect of DAD interactions with G-actin. However, the data did not suggest that the m5 mutation changed the affinity of actin binding. The observation that DAD is critical for C polypeptide binding to G-actin agrees with previous studies showing that FH2 (lacking DAD) has little if any affinity for G-actin [6, 7].
Figure 3. DAD domain binding to actin monomers.
A) Fluorescence signal for pyrene-G-actin (250 nM, 100% label) in the presence of variable concentrations of mDia1 C, C(m5), and C-ΔDAD polypeptides in HEKG5 (25 mM HEPES (pH 8), 1 mM EDTA, 50 mM KCl, and 5% glycerol). B) Binding of wild type and mutant m5 mDia1 MBP-His6-DAD-containing fragments to pyrene-G-actin, as in A. Buffer conditions were 25 mM Tris (pH 8), 200 mM NaCl, 50 mM L-Arginine. C) Pyrene-actin (2 μM, 5% pyrene labeled) was assembled in the presence of different of concentrations of GST-mDia1 DAD (1175-1200). D-F) Pyrene-actin was assembled (as in C) in the presence of a range of concentrations of mDia1 C (I845A) polypeptide (D), mDia1 C-ΔDAD (I845A) (E), or mDia1 C(m5) (I845A) (F). G) Rates of assembly determined from the slopes of the curves.
Next we asked whether a DAD peptide alone (no FH2) is sufficient to bind G-actin by testing interactions between pyrene-G-actin and mDia1 DAD (1175-1200) fused to maltose binding protein (MBP). MBP-DAD induced a concentration-dependent increase in pyrene-G-actin fluorescence, consistent with G-actin binding, whereas MBP alone had no effect (Fig. 3B). Further addition of profilin, even as high as 200 μM, did not alter MBP-DAD interactions with pyrene-G-actin (Fig. S3F), suggesting that DAD and profilin do not compete for actin binding. Mutant MBP-DAD(m5) showed altered effects on fluorescence, again consistent with this mutation altering some aspect of the DAD interaction with G-actin.
The DAD regions of Bni1 and Bnr1 were also critical for G-actin binding. This was evident from a comparison of the effects of Bni1 and Bnr1 C and C-ΔDAD polypeptides on pyrene-G-actin fluorescence (Fig. S3B and S3C). In addition, DAD peptides of Bni1 (1750-1858) and Bnr1 (1274-1342) induced concentration-dependent increases in pyrene-G-actin fluorescence (Fig. S3D and S3E). Although the binding affinities of DAD peptides for G-actin were very low, our nucleation data argue that these actin-binding sites make important contributions to nucleation in the context of an intact formin, i.e. where two DAD domains are physically tethered to an FH2 dimer. In addition, note that our data argue that the FH2 makes a critical contribution to G-actin binding. This is reflected in the major difference in G-actin binding affinities between DAD and FH2-DAD. Thus, while DAD alone is sufficient for weak autonomous interactions with G-actin, binding is strengthened substantially by inclusion of the FH2, even though FH2 alone shows no detectable binding.
Dimerized DAD domain is sufficient to nucleate actin assembly
We next asked whether DAD is sufficient to promote actin nucleation in the absence of FH2. While no nucleation was observed for MBP-DAD at concentrations as high as 50 μM (Fig. S4B), significant nucleation effects were observed for GST-DAD at concentrations as low as 1 μM (Fig. 3C). This difference in activity between MBP and GST fusions suggested that nucleation might arise due to GST dimerization, tethering two DAD domains together. This led us to also ask whether DAD domains can support nucleation in the context of an intact dimeric formin when the actin binding/ nucleation activity of the FH2 is disrupted. To test this idea, we introduced into mDia1 C, mDia1 C-ΔDAD, and mDia1 C(m5) the I845A mutation, which severely disrupts actin binding and nucleation by the FH2 [7, 13]. Compared to wild type mDia1 C, mDia1 C (I845A) showed drastically reduced nucleation activity, requiring >100 fold higher concentration to produce similar levels of activity (Fig. 3D). Combining I845A with a truncation of DAD abolished the residual nucleation activity, as did combining m5 and I845A (Fig. 3G and raw curves Fig. 3E and 3F). These data suggest that when two DAD domains are physically tethered to the FH2 dimer they support a modest level of nucleation activity, which is also consistent with our GST-DAD results.
FH1 and DAD domains make separate contributions to nucleation
Previous studies have suggested that in the presence of profilin the FH1 domain of mDia1 enhances nucleation by the FH2 [8, 10]. We purified mDia1 C-ΔFH1 (Fig. 4A), and observed that indeed it had much weaker actin assembly activity compared to mDia1 C in the presence of profilin over a wide range of formin concentrations (Fig. 4B, 4C, and 4D). Specifically, almost a 30-fold higher concentration of mDia1 C-ΔFH1 was required to support the same activity level as mDia1 C (compare the light blue curve in Fig. 4B and the dark blue curve in Fig. 4C). Even accounting for a 5-fold elongation effect, this suggests that the FH1 makes a substantial contribution to nucleation. Consistent with this view, we directly visualized an increase (>10 fold) in the number of filaments assembled by mDia1 C compared to mDia1 C-ΔFH1 (Fig. S4D and S4E). As expected, no difference in the activities of mDia1 C-ΔFH1 and mDia1 C was observed in the absence of profilin (Fig. 4E, raw curves in Fig. S4C). These data confirm that in the presence of profilin the FH1 contributes to nucleation. On the other hand, DAD enhanced nucleation both in the presence of profilin, at a range of profilin concentrations (Fig. 4F), and in the absence of profilin (Fig. 1B). Further, at very high profilin concentrations (50 μM and above) that inactivate FH1 contributions to actin assembly by competitively blocking profilin-actin recruitment, DAD is instrumental for nucleation (Fig. 4G).
Figure 4. FH1 domain contributions to actin nucleation.
A) Schematic and Coomassie stained gel of mDia1 C (553-1255) and mDia1 C-ΔFH1 (739-1255). B, C) Pyrene-actin (2 μM, 5% labeled) was assembled in the presence of 5 μM profilin and different concentrations of mDia1 C (553-1255) (B) or mDia1 C-ΔFH1 (739-1255) (C). Curves are color-coded by concentration of formin. D, E) Rates of assembly were determined from the slopes of the curves in B and C, and in Fig. S4C. F, G) Pyrene-actin was assembled in the presence of varying concentrations of profilin and 75 nM mDia1 C or C-ΔDAD. F) Rates of assembly were determined from the slopes of the curves. G) Representative raw curves for 75 nM mDia1 C and C-ΔDAD in the presence of 20 μM profilin, compared to 2 μM actin without profilin.
Conclusions
The DAD domain was first defined as a sequence in the C-terminus of diaphanous-related formins that mediates autoinhibition through intramolecular interactions with the formin N-terminus [18]. Since then, this motif has been identified in a large number of formins, including members of at least 4 out of the 7 formin subfamilies in diverse organisms [20, 21]. However, recent findings suggest that not all formins carrying DAD domains are autoinhibited, including S. pombe Cdc12, D. melanogaster Cappucino, and mammalian Delphilin, FRL2 and INF2 [22]. This observation raises the possibility that the DAD has been maintained in these formins to perform other functions. Our data show that the DAD domain of mDia1 binds to actin monomers and strongly enhances the nucleation activity of the FH2 domain. This function may extend to other formins, as we found that the DAD regions of S. cerevisiae Bni1 and Bnr1 are required for G-actin binding and enhance actin assembly in these formins (Fig. S3B-D). Thus, our view of the formin nucleation mechanism has changed substantially from the FH2 acting alone, to one in which the FH1, FH2 and DAD domains act in concert as a tripartite nucleation apparatus, combining G-actin and profilin-G-actin recruitment sites with high affinity filament end-capturing activity.
Another central conclusion we draw from our results is that the DAD performs dual roles in actin nucleation and autoinhibition. This suggests two interesting mechanistic parallels between formins and N-WASp-Arp2/3 complex. First, formins and Arp2/3 both nucleate actin assembly by combining an actin monomer recruitment domain (DAD in formins; VCA in N-WASp) and a filament end-capturing unit (FH2 dimer in formins; actin-related protein dimer in Arp2/3). In each nucleating system, the end-capturing unit is pivotal for nucleation, and the monomer recruitment domain strongly enhances the activity. Second, in each case, the monomer recruitment domain can have dual roles in nucleation and autoinhibition. This general strategy of masking sites important for activity in the autoinhibited state, which become available upon release/activation, is common to autoinhibited proteins with a variety of cellular functions [23].
Finally, our data also indicate that the formin nucleation mechanism is more similar to those of other actin assembly factors than previously thought. Spire, Cobl, Lmod, JMY, and APC each recruit 2-4 actin monomers using tandem arrays of actin-binding motifs (often WH2 domains) to form prenucleation complexes [3]. Further, the nucleation activity of Arp2/3 depends strongly on its association with N-WASp and the ability of N-WASp to recruit monomers. Until now, formins have been proposed to use a distinct mechanism of nucleation, involving capture of spontaneously formed actin dimers and/or trimers. However, such a mechanism does not adequately explain how nucleation would occur in vivo, where an abundance of actin monomer-binding proteins are thought to suppress spontaneous dimer and trimer formation. Instead, we have shown that formins recruit actin monomers to enhance nucleation through interactions of their DAD domains with G-actin. Thus, actin monomer recruitment appears to be a universal property of all currently known actin nucleators.
Experimental Procedures
Actin assembly and disassembly assays
Purification of all proteins is described in Supplemental Experimental Procedures. Gel filtered, monomeric actin (2 μM final; 5% pyrene labeled) in G-buffer (10 mM Tris (pH 8.0), 0.2 mM ATP, 0.2 mM CaCl2, and 0.2 mM DTT) was converted to Mg-ATP-actin immediately before each reaction. Actin was mixed with 15 μl of proteins or control buffer (HEKG5) and 3 μl of 20× initiation mix (40 mM MgCl2, 10 mM ATP, 1 M KCl). For reactions containing profilin, S. cerevisiae profilin was used with Bni1 and Bnr1, and human platelet profilin was used with mDia1 and Daam1. Pyrene fluorescence was monitored for 10 min at excitation 365 nm and emission 407 nm at 25°C in a fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ) or an Infinite M200 plate reader (Tecan, Männedorf, Switzerland). Rates of assembly were calculated from slopes of the curves at 50% polymerization, except when reactions failed to reach 50% polymerization during the 10 min; in these cases, slopes were measured at the steepest points in the curves. Disassembly assays were performed, using preassembled F-actin (10% pyrene-labeled). Briefly, the preassembled F-actin (2 μM) was incubated with formin constructs or Cof1 for 300 sec, then 4μM Vitamin D-binding protein (human plasma Gc-globulin, Sigma-Aldrich, St. Louis, MO) was added to initiate disassembly.
G-actin binding assays
Pyrene-G-actin (250 nM, 100% labeled) was incubated for 15 min at room temperature in the presence of 500 nM latrunculin B with mDia1 C, mDia1 C-ΔDAD, or mDia1 C(m5) in HEKG5 (25 mM HEPES (pH 7.5), 50 mM KCl, 1 mM EDTA, and 5% glycerol) buffer, then pyrene flourescence was measured as above. For reactions containing wild type and mutant m5 mDia1 MBP-His6-mDia1-DAD (1175-1200) the buffer was 25 mM Tris (pH 8.0), 200 mM NaCl, and 50 mM L-Arginine. Salt composition of the buffer was varied from 0-200 mM NaCl with no alteration in MBP-mDia1 DAD effects on pyrene fluorescence.
TIRF microscopy
Freely diffusing F-actin filaments were polymerized in BSA coated chambers. Reactions contained 1 μM monomeric actin (30% labeled), 3 μM human profilin, and 2 nM mDia1 C or C-ΔDAD polypeptides. To induce actin polymerization, protein mixtures were diluted into freshly prepared fluorescence buffer containing 10 mM imidazole-HCl (pH 7.8), 50 mM KCl, 1 mM MgCl2, 100 mM DTT, 3 mg/ml glucose, 20 mg/ml catalase, 100 mg/ml glucose oxidase, and 0.5% methylcellulose. Elongation of the barbed end of filaments was monitored as increase in length over time, as described previously [24]. Samples were imaged at 20 sec intervals on an Olympus IX-71 inverted microscope equipped with a 60×, 1.45 NA Plan apo objective (Olympus, Melville, NY), and modified as described [25] to receive TIRF illumination. Samples were illuminated with an Argon/Krypton laser (CVI Melles Griot, Alburquerque, USA) emitting at 488 nm. Images were acquired with a Hamamatsu ORCA-ER camera (Hamamatsu Photonics Deutschland GmbH) running MetaMorph version 6.2r6 software (Universal Imaging Corp., Media, PA).
Supplementary Material
Acknowledgments
We thank Melissa Chesarone for critical reading of the manuscript. This work was supported in part by grants from the NIH (GM083137 and GM063691) to B.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 2.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Chesarone MA, Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez R. Actin filament nucleation and elongation factors--structure-function relationships. Crit Rev Biochem Mol Biol. 2009;44:351–366. doi: 10.3109/10409230903277340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qualmann B, Kessels MM. New players in actin polymerization--WH2-domain-containing actin nucleators. Trends Cell Biol. 2009;19:276–285. doi: 10.1016/j.tcb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Pring M, Evangelista M, Boone C, Yang C, Zigmond SH. Mechanism of formin-induced nucleation of actin filaments. Biochemistry. 2003;42:486–496. doi: 10.1021/bi026520j. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–1340. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 9.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Paul AS, Pollard TD. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr Biol. 2008;18:9–19. doi: 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhabra ES, Higgs HN. INF2 Is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem. 2006;281:26754–26767. doi: 10.1074/jbc.M604666200. [DOI] [PubMed] [Google Scholar]
- 12.Vaillant DC, Copeland SJ, Davis C, Thurston SF, Abdennur N, Copeland JW. Interaction of the N- and C-terminal autoregulatory domains of FRL2 does not inhibit FRL2 activity. J Biol Chem. 2008;283:33750–33762. doi: 10.1074/jbc.M803156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris ES, Rouiller I, Hanein D, Higgs HN. Mechanistic differences in actin bundling activity of two mammalian formins, FRL1 and mDia2. J Biol Chem. 2006;281:14383–14392. doi: 10.1074/jbc.M510923200. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem. 2005;280:6986–6992. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- 15.Wallar BJ, Stropich BN, Schoenherr JA, Holman HA, Kitchen SM, Alberts AS. The basic region of the diaphanous-autoregulatory domain (DAD) is required for autoregulatory interactions with the diaphanous-related formin inhibitory domain. J Biol Chem. 2006;281:4300–4307. doi: 10.1074/jbc.M510277200. [DOI] [PubMed] [Google Scholar]
- 16.Nezami AG, Poy F, Eck MJ. Structure of the autoinhibitory switch in formin mDia1. Structure. 2006;14:257–263. doi: 10.1016/j.str.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. Embo J. 2005;24:4176–4187. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–2830. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 19.Schonichen A, Alexander M, Gasteier JE, Cuesta FE, Fackler OT, Geyer M. Biochemical characterization of the diaphanous autoregulatory interaction in the formin homology protein FHOD1. J Biol Chem. 2006;281:5084–5093. doi: 10.1074/jbc.M509226200. [DOI] [PubMed] [Google Scholar]
- 20.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16:1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonichen A, Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 23.Pufall MA, Graves BJ. Autoinhibitory domains: modular effectors of cellular regulation. Annu Rev Cell Dev Biol. 2002;18:421–462. doi: 10.1146/annurev.cellbio.18.031502.133614. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn JR, Pollard TD. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys J. 2005;88:1387–1402. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amann KJ, Pollard TD. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc Natl Acad Sci U S A. 2001;98:15009–15013. doi: 10.1073/pnas.211556398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.