Abstract
With an understanding of the molecular changes that accompany cell transformation, cancer drug discovery has undergone a dramatic change in the past few years. While most of the emphasis in the past has been placed on developing drugs that induce cell death based on mechanisms that do not discriminate between normal and tumor cells, recent strategies have emphasized targeting specific mechanisms that have gone awry in tumor cells. However, the identification of cancer-associated mutations in oncogenes and their amplification in tumors has suggested that inhibitors against such proteins might represent attractive substrates for targeted therapy. In the clinic, the success of imatinib (Gleevec®, STI571) and trastuzumab (Herceptin®), both firsts of their kind, spurred further development of new, second-generation drugs that target kinases in cancer. This review highlights a few important examples each of these types of therapies along with some newer agents that are in various stages of development. Second-generation kinase inhibitors aimed at overriding emerging resistance to these therapies are also discussed.
Keywords: kinase inhibitor, targeted therapy, monoclonal antibody, small molecule inhibitors
One of the most important breakthroughs in cancer research came from the discovery that oncogenes of acute transforming viruses are in fact derived form normal cellular DNA and that this genetic information is transduced by acute transforming viruses via genetic recombination. Studies within the last few decades have also shown that tumor cells often gain a growth advantage through amplification or mutation of various oncogenes, the end result of which appears to be a profound enhancement in the amplitude of the growth and survival signals. Some examples of these changes include, amplification of the epidermal growth factor (EGF; also known as ErbB1) and ErbB2 receptors in lung and breast tumors, mutation of the ras oncogene in a wide variety of human tumors and chromosomal translocations such as the Philadelphia Chromosome, which results in the activation of the Abl tyrosine kinase.
With an understanding of these molecular changes that accompany cell transformation, cancer drug discovery has undergone a dramatic change in the past few years. The elucidation of signaling pathways that are deregulated in tumor cells as well as the identification of mutations in both oncogenes and growth suppressor genes has suggested multiple targets and revealed approaches for the development of new classes of drugs including antibodies to receptors and small molecule inhibitors to mutant kinases. The most successful of these types of agents, by far, is Gleevec® (imatinib, STI57; Novartis), and it is because of the tremendous success that this drug has had in the clinic that additional kinase inhibitors have been and are being developed. Because the past five years have seen a large amount of research performed in the area of rational drug design, it has not been possible to review all of the approaches that are currently being developed. We have therefore limited this review to the discussion of a few rationally designed targeted therapies that have received approval of the United States Food and Drug Administration (FDA) and exemplify the utility and problems associated with this line of research.
BCR-ABL ONCOGENE TARGETED THERAPY
The Philadelphia Chromosome
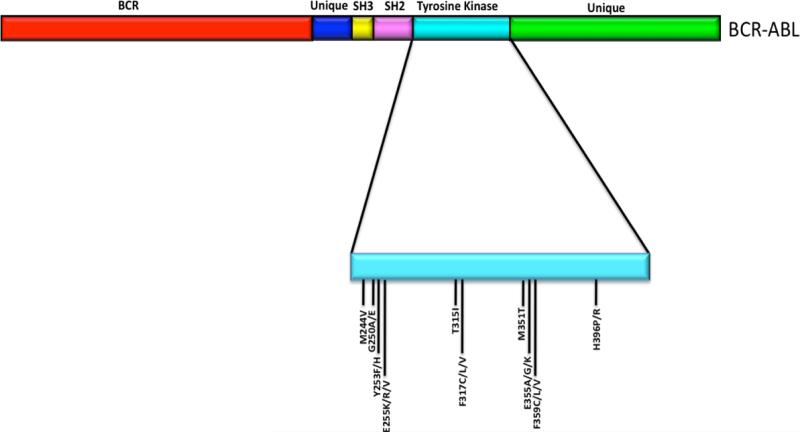
The Philadelphia chromosome (Ph) was discovered in 1960 by Nowell and Hungerford, who analyzed samples derived from 7 patients suffering from what was known at that time as chronic granulocytic leukemia. Each patient harbored a similar “minute chromosome,” and none showed any other chromosomal abnormality (Nowell and Hungerford, 1960). We now know that this abnormal Ph chromosome results from a reciprocal translocation between chromosomes 9 at band q34 and 22 at band q11. More importantly, this translocation fuses the breakpoint cluster region (Bcr) gene with the Abl gene and creates the BCR-ABL oncogene (Heisterkamp et al., 1985) (figure 2) whose expression is responsible for greater than 90% of chronic meylogenous leukemias (CML) (reviewed in Shah and Sawyers 2003).
Figure 2.
Schematic representation of the BCR-ABL protein. The positions of 10 of the most common mutations in the kinase domain that confer imatinib resistance are shown. (Note: not drawn to scale).
Imatinib
Until recently, CML was treated with a variety of chemo- and biotherapeutic drugs (reviewed in Hehlmann, 2003). Because the BCR-ABL protein is active in the majority of CML cases, it has been possible to synthesize small molecules that inhibit BCR-ABL kinase activity in leukemic cells without adversely affecting the normal cell population. Gleevec® (STI571, imatinib mesylate; Novartis) (figure 1) is a small molecule that binds to the kinase domain of BCR-ABL when the protein is in its closed, inactive conformation (Druker et al., 1996).
[C allout] Gleevec® (STI571, imatinib mesylate; Novartis) (figure 1) is a small molecule that binds to the kinase domain of BCR-ABL when the protein is in its closed, inactive conformation.
In this conformation, the catalytic central domain is blocked by the regulatory activation (A) loop and mutations within this loop have been shown to prevent the kinase from adopting an inactive conformation (reviewed in Apperley 2007).
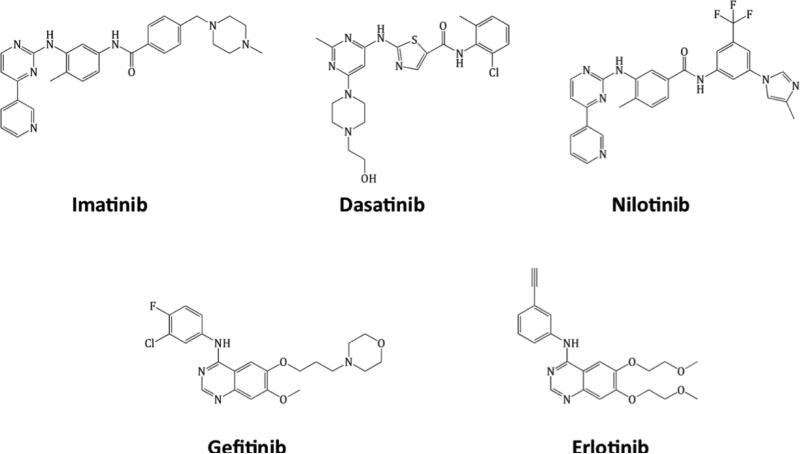
Figure 1.
Structures of imatinib, nilotinib, dasatinib, gefitinib and erlotinib.
As with most kinase inhibitors that are ATP mimetics, imatinib inhibits several tyrosine kinases, including but not limited to platelet-derived growth factor receptor (PDGFR) a and b, c-Kit, Lck, fms, FGFR-1, VEGFR-1, 2, 3 colony stimulating factor-1 receptor and c-raf (reviewed in Deininger et al., 2005; Mashkani et al., 2010). NQO2 oxidoreductse is also inhibited by the drug, even though it is not a kinase (Rix et al., 2007). Imatinib, however, is most active against c-ABL and more so, its oncogenic forms. BCR-ABL+ cells that are exposed to this drug do not proliferate and have been shown to undergo apoptosis, while normal, IL-3-dependent cells remain virtually unaffected (Druker et al., 1996; Deininger et al., 1997).
In the clinic, the Phase I trials aimed at assessing the safety of imatinib were remarkably successful. In those CML patients who were previously treated with interferon (IFN)-α but failed to respond, almost all of the patients who were treated with 300mg imatinib or greater achieved complete hematological responses. Furthermore, complete cytogenetic responses were observed in 13% of these patients. In Phase 2 studies evaluating the efficacy of imatinib as a single agent, the results mirrored those obtained in Phase I trials, whereby a significant number of patients in various stages of disease showed cytogenic responses. Phase 3 trials and those assessing the effectiveness of imatinib in combination with other cytotoxic agents and/or those aimed at improving the efficacy of imatinib monotherapy were equally successful, whereby imatinib proved to be the most effective treatment for various stages of leukemic disease. As a result, imatinib is now considered as a first-line therapy for the majority of CML cases due to its high efficacy level and relatively mild side effects (reviewed in Deininger et al., 2005).
Imatinib Resistance in CML
In spite of the fact that the majority of patients receiving imatinib respond to treatment at both the hematological and cytogenetic levels, relapse occurs in a subset of patients with chronic disease, and this number jumps significantly to nearly 100% in those patients whose disease is in the advanced stages (reviewed in Shah and Sawyers, 2003). Several studies have attempted to address the mechanism(s) by which CML cells acquire imatinib resistance.
Non-mutation-dependent mechanisms
Elevated levels of BCR-ABL are sometimes expressed as a result of genomic amplification of the BCR-ABL locus (le Coutre et al., 2000; Mahon et al., 2000; Weisberg and Griffin, 2000) and amplification of the locus and/or multiple copies of the Philadelphia chromosome have also been reported in a small number of patients (Gorre et al. 2001; Hochhaus et al., 2002). Studies have also revealed that imatinib resistance can be mediated by increased levels of the Pgp multi-drug resistance protein (MDR1; ABCB1) (Mahon et. al., 2000), although patient derived samples do not tend to support such a mechanism in a clinical setting.
Other studies have examined signaling pathways and proteins that are activated downstream of BCR-ABL. In some advanced stages of CML, levels of Hck and Lyn, members of the Src family of tyrosine kinases, are increased and constitutively phosphorylated (Donato et al., 2003 and 2004;Wu et al., 2008a). Additional BCR-ABL independent mechanisms that mediate imatinib resistance include (but are not limited to), modulation of Janus Kinase 2 (JAK2) (Wang et al., 2007), the activation of the Akt/PI-3 kinase/mtor pathway (Burchert et al., 2005), activation of RUNX transcription factors (Miething et al., 2007), activation of MAP kinase activity (Chu et al., 2004) and inactivation/mutation of p53 (Wendel et al., 2006; reviewed in Burchert, 2007).
Mutation of BCR-ABL
In spite of these studies, the majority of reports indicate that the mechanism that accounts for the majority of imatinib resistant leukemias, in vivo, is mutation of the BCR-ABL gene itself.
[Callout] the majority of reports indicate that the mechanism that accounts for the majority of imatinib resistant leukemias, in vivo, is mutation of the BCR-ABL gene itself.
Mutation within the kinase domain is most common (figure 2). The first study that analyzed the frequency and of these mutations was published in 2001 by Gorre et al., and examined cells derived from 11 patients in the advanced stages of CML who were being treated with imatinib and had initially responded well to treatment but had relapsed. Although genomic amplification was detected in three of the samples, a specific mutation within the BCR-ABL kinase domain was present in six cases. Specifically, this mutation resulted in the substitution of a threonine residue with isoleucine at amino acid position 315 (T315I). This substitution, based on the crystal structure of the Abl kinase domain complexed with an imatinib derivative, is thought to decrease the binding affinity of imatinib to the BCR-ABL protein whereby the isoleucine substitution prevents hydrogen bond formation between BCR-ABL and imatinib (Schindler et al., 2000).
Since that initial report, more than 50 mutations in the kinase domain of the BCR-ABL gene have been identified in imatinib resistant leukemic cells. The list of mutations has been extensively summarized and reviewed in 2007 by Apperley (Apperley, 2007). Although the exact mechanism(s) that drive the mutation process are at present unclear, it is widely believed that many (if not all) result from the “selection pressure” exerted by exposure to imatinib. Studies also indicate that they may also arise due to genetic instability induced by BCR-ABL (Jiang et al., 2007a and 2007b) and inhibition of the mismatch (MMR) and nucleotide excision repair (NER) processes (Canitrot et al., 2003; Sliwinski et al., 2008; Stoklosa et al., 2008).
The imatinib-resistant mutations that arise in BCR-ABL can be classified as those that potentially interfere with the ability of imatinib to interact directly with the BCR-ABL kinase domain and those that are predicted to destroy or hinder the ability of the kinase domain to adopt a conformation that is required for imatinib binding (reviewed in Shah and Sawyers, 2003; Sawyers, 2004). The number of mutations that occur with the highest frequency tend to be clustered within the P-loop, the imatinib-binding site, the catalytic domain and the activation loop.
[Callout] The number of mutations that occur with the highest frequency tend to be clustered within the P-loop, the imatinib-binding site, the catalytic domain and the activation loop.
In patients, it is clear that certain mutations are present in particular phases of CML. However, it is at present unclear whether some or any of the mutations are causative of disease or whether they merely correlate with certain phases (reviewed in Apperley, 2007).
Second-generation BCR-ABL Inhibitors
Because of the frequency of mutations, efforts have been focused on the identification of novel, second-generation BCR-ABL inhibitors that are active against imatinib resistant mutants of the protein. The most successful alternatives to imatinib to date are second-generation inhibitors of BCR-ABL that inhibit the protein at lower concentrations than imatinib. Some agents, such as dasatinib, are unrelated to imatinib and are also inhibitory to other tyrosine kinases, making them active against a variety of imatinib-resistant tumors. Nilotinib, on the other hand, was developed as a rationally designed alternative to imatinib that exploited imatinib's affinity and selectivity for the Abl kinase domain.
Dasatinib
Originally termed BMS-354825 (Lombardo et al., 2004; Shah et al, 2004), dasatinib (Sprycel®; Bristol-Myers Squibb) is an orally bioavailable inhibitor of BCR-ABL and was the first drug to be approved for the treatment of imatinib-resistant Ph+ leukemias (figure 1). The drug has a shorter half-life than imatinib and no active metabolites (reviewed in le Coutre et al., 2010). Although structurally unrelated to imatinib, dasatinib inhibits some of the same target proteins such as BCR-ABL (albeit at lower concentrations than imatinib), c-Kit and PDGFR. It is also inhibitory to other kinases such as Src family tyrosine kinases, the Tec kinases Btk and Tec and the ephrin A (EphA) receptor tyrosine kinase (Lombardo et al., 2004; Nam et al., 2005, Schittenhelm et al., 2006; Hantschel et al., 2007). Dasatinib does not share structural conformation requirements for BCR-ABL with imatinib and therefore binds to both active and inactive conformations of the protein (Tokarski et al., 2006). As a result, the drug has been successful in inhibiting nearly all imatinib-resistant forms of the protein, with the exception of T315I (Shah et al., 2004, O'Hare et al., 2005a, reviewed in Ramirez and DiPersio 2008).
In the clinic, dasatinib has been successful in treating patients who were intolerant to imatinib or whose disease was resistant to imatinib therapy. In clinical trials, the drug has shown the greatest efficacy in patients in the chronic phase of CML. Nonetheless, cases of dasatinib resistance have begun to emerge. Specifically, sequencing of BCR-ABL+ clones isolated from patients receiving dasatinib after failing or showing intolerance to imatinib revealed a predominance of mutations at the T315 and F317 residues. In addition to the T315I mutation, a second mutation at this residue, T315A, has also been detected in one patient (Soverini et al., 2007; Khorashad et al., 2008). Interestingly, this same mutation was isolated during a mutagenesis screen aimed at isolating dasatinib resistant forms of BCR-ABL (Burgess et al., 2005). At the F317 residue, four different mutations have been identified (F317I/L/V/C), although the F317L mutation has been documented in the greatest percentage of cases. These studies suggest that these two residues may be key in the ability of dasatinib to inhibit BCR-ABL (Soverini et al., 2006 and 2007). It is interesting to note that the F317L was tested as part of the panel of imatinib resistant mutants in in vitro cell based assays as well as in animal tumor model systems (Shah et al., 2004; O'Hare et al., 2005a). While it has been noted that higher amounts of dasatinib were required to inhibit kinase activity and cellular proliferation, the levels were not such that they substantially exceeded those for other imatinib-resistant mutations that do not arise in dasatinib sensitive patients. Other studies have identified mutations at several additional residues (Shah et al., 2007; Soverini et al., 2007; reviewed in Lee et al., 2008) and compound mutations have also been observed in patients undergoing sequential imatinib and dasatinib therapies. One of these mutations, V299L, is of interest as it occurs at a residue that directly binds to dasatinib. This mutation, however, is sensitive to both imatinib and nilotinib (Shah et al., 2007), further underscoring the differences between these drugs.
Nilotinib
Nilotinib (Tasigna®, AMN107; Novartis) (Weisberg et al, 2005) is structurally related to imatinib, has a similar half-life and has a greater affinity (approximately 20-fold) for wild-type BCR-ABL (reviewed in O'Hare et al, 2005b; le Coutre et al., 2010). Because of the structural similarities between the two drugs (figure 1), in addition to inhibiting BCR-ABL, nilotinib is also inhibitory to c-Kit, PDGFR and the TEL-PDGFRα and FIP1L1-PDGFRα fusion proteins (Stover et al., 2005; reviewed in Lee et al., 2008). Also similar to imatinib, nilotinib binds to BCR-ABL when it is in an inactive conformation; however, its design allows it to bind more tightly, thereby enhancing its inhibitory activity (Manley et al., 2005). As a result, most of the imatinib-resistant forms of BCR-ABL tested in in vitro cell-based assays are also 20-fold more sensitive to this drug, although studies using certain imatinib sensitive cell lines have shown that the increased potency may be as high as 60-fold (Weisberg et al., 2005; Golemovic et al., 2005). Nilotinib does, however, when compared to wild-type BCR-ABL, show a reduced ability to inhibit P-loop mutations and this is correlated with a response to the drug in the clinic (Kantarjian et al., 2006). The only exception is the T315I mutation, which is completely insensitive to nilotinib (Weisberg et al, 2005; reviewed in Deininger, 2008).
Overcoming T315I Resistance
The T315 residue is commonly referred to as the “gatekeeper” due to the fact that the T315I mutation confers complete resistance to imatinib, dasatinib and nilotinib. It has become clear that the use of ATP mimetics that have the ability to target the kinase domain drives the emergence of such mutations and hence, compounds that inhibit the T315I conformation directly are invaluable. Use of x-ray crystallography in conjunction with structure-guided optimization has led to the isolation of SGX393, a compound that inhibits both T315I and T315A. It does not, however, inhibit certain other P-loop mutations. Fortunately, some of these mutations are inhibited by either nilotinib and/or dasatinib, suggesting that this agent, should it be used in a clinical setting, could be used in combination therapy (O'Hare et al., 2008). Other drugs with the ability to inhibit T315I directly that are undergoing clinical trials are inhibitors of the Aurora kinases and include: MK-0457, XL228, PHA-739358 and KW-2449 (reviewed in Quintas-Cardama et al., 2007; Quintas-Cardama and Cortes, 2008). In addition to T315I inhibitors, it may also be possible to target signaling pathways that are either downstream or parallel to BCR-ABL. There are numerous compounds at various stages of development that could possibly fill such a need, including (but not limited to) Src family kinase inhibitors, inhibitors of HSP90, inducers of apoptosis, activators of protein phosphatases and histone deacetylase (HDAC) inhibitors (reviewed in Quintas-Cardama et al., 2007; Quintas-Cardama and Cortes, 2008) and even an allosteric BCR-ABL inhibitor that binds to the kinase at its myrostoylation site (Zhang et al., 2010).
EPITHELIAL GROWTH FACTOR RECEPTOR TARGETED THERAPY
Epithelial Growth Factor Receptor and Cancer
The significance of the epidermal growth factor receptor (EGFR) in cancer was suggested years before it was shown that the protein was altered or aberrantly expressed in tumors. During the early 1980s, studies of the avian erythroblastosis virus led to the identification of viral, transforming versions of the EGFR (Anderson et al., 1980; Vennstrom and Bishop, 1982; Yamamoto et al., 1983), and subsequent studies showed that introduction of the EGFR gene into NIH-3T3 cells results in ligand-dependent transformation (Velu et al., 1987; Hudziak et al., 1987). In certain types of tumors, such as gliomas, the EGFR locus is amplified, resulting in high levels of the receptor. Other tumor types express mutant, constitutively active forms of the protein (reviewed in Pao et al., 2004a). Activation of the EGFR stimulates proliferation, metastasis, angiogenesis and other phenotypes that are beneficial for tumor growth. EGFR is expressed in a variety of cell types and tumors, including (but not limited to) those of the bladder, lung, head and neck, breast, colon and pancreas. In certain types of malignancies, such as head and neck cancers, the level of EGFR expression is of prognostic value where elevated levels are correlated with decreased survival, whereas in others, this link is not as well defined (reviewed in Pao et al., 2004a).
Gefitinib and Erlotinib: Small Molecule Epithelial Growth Factor Receptor -targeted Inhibitors
Gefitinib (ZD1839, Iressa®; AstraZeneca) (figure 1) belongs to a class of compounds termed anilinoquinazolinones and is an inhibitor of EGFR enzymatic activity in the low nanomolar range (Ward et al., 1994). Subsequent studies also revealed that gefitinib effectively inhibited the proliferation of a variety of established tumor cell lines (Sirotnak, 2003), although this did not necessarily correlate with EGFR expression.
Gefitinib, as well as most targeted kinase inhibitors developed to date, acts by reversibly competing with adenosine triphosphate (ATP) for binding to the EGFR, thereby inhibiting its autophosphorylation and activity (Wakeling wt al., 2002).
[Callout] Gefitinib, as well as most targeted kinase inhibitors developed to date, acts by reversibly competing with adenosine triphosphate (ATP) for binding to the epithelial growth factor receptor (EGFR), thereby inhibiting its autophosphorylation and activity.
Cells that have been exposed to this drug undergo cell cycle arrest and apoptosis (Magne et al., 2002; Di Gennaro et al., 2003) and express lower levels of pro-angiogenic proteins such as VEGF (Ciardiello et al., 2001; Hirata et al., 2002). EGF has been shown to regulate the expression of VEGF, IL-8 and other angiogenic factors. It is therefore not surprising that gefitinib has the ability to regulate tumor growth by controlling the neovasculature (reviewed in Ono and Kuwano, 2006).
Response to Gefitinib Treatment
In the United States, Gefitinib was initially approved for the treatment of non-small lung cancer (NSCLC) in patients who had failed or were not able to receive standard chemotherapeutic regimens. In general, non-smokers, women and Asians have an overall more favorable response to EGFR kinase inhibition (reviewed in Fukui and Mitsudomi, 2008). However, overall response to gefitinib has been shown to vary considerably in these and other patient cohorts, thereby prompting research to identify biomarkers that could account for this variability and predict sensitivity. Sequence analysis of the EGFR in primary NSCLC tumors derived from patients in Japan and the United States (Lynch et al., 2004; Paez et al., 2004) identified mutations within the tyrosine kinase domain that correlated with sensitivity to gefitinib.
[Callout] Sequence analysis of the EGFR in primary non-small lung cancer (NSCLC) tumors derived from patients in Japan and the United States (Lynch et al., 2004; Paez et al., 2004) identified mutations within the tyrosine kinase domain that correlated with sensitivity to gefitinib.
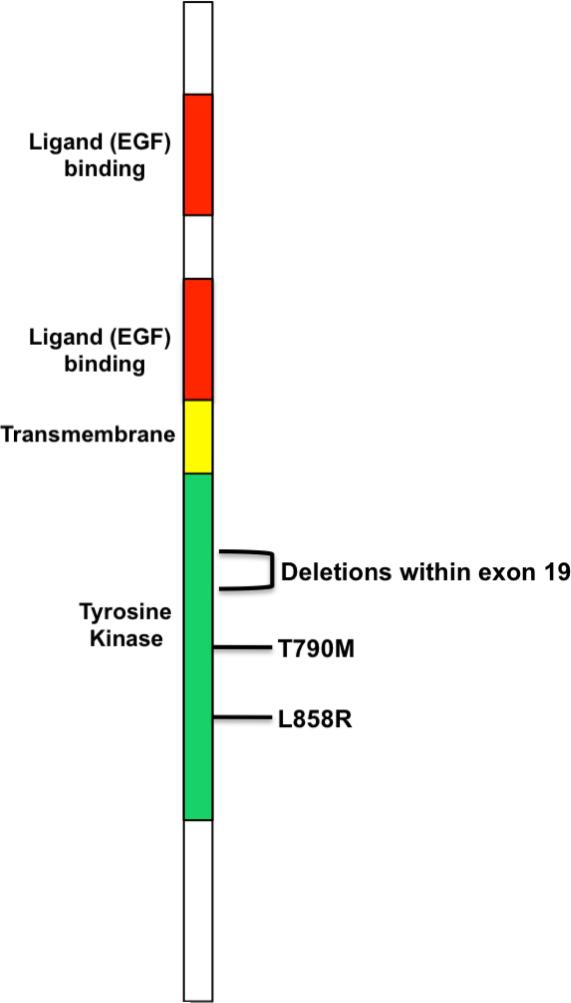
For the most part, the abnormalities are in-frame deletions in amino acids 747-750 (del746-750) or a point mutation at position 858 (L858R) (Lynch et al., 2004; Paez et al., 2004; Pao et al., 2004b). These studies also showed that such mutations, which result in increased levels of ligand-induced receptor autophosphorylation, are more common in non-smokers, women, Japanese patients and those with the adenocarcinoma form of the disease. Since these initial studies, other mutations in the forms of in-frame deletions and insertions, point mutations and duplications within exons 19-22 of the EGFR gene that confer some level of response to gefitinib have been identified. However, the “classical” L858R (exon 21) and the del746-750 (exon 19) mutations still account for roughly 90% of the alterations in this region (reviewed in Irmer et al., 2007). Overall, NSCLC patients treated with gefitinib (or erlotinib) who harbor the del746-750 mutation have a longer median survival when compared to those with the L858R mutation (Riely et al., 2006; Jänne and Johnson., 2006). In addition, immunohistochemical staining of tumor specimens for proteins that are downstream of the EGFR revealed that patients with tumors that stained positive for phosphorylated Akt (p-Akt) responded better to gefitinib treatment than those which stained negative; however, there was no such correlation for phosphorylated MAP kinase (Cappuzzo et al., 2004). Similar results were obtained using human NSCLC cell lines, although these studies also revealed an association between p-Akt and ERK1/2 phosphorylation in gefitinib-resistant cells (Ono et al., 2004; Han et al., 2005).
Erlotinib
Erlotinib (Tarceva®, OSI-774, Genentech) (figure 1) belongs to the class of quinazolinones and is a selective but reversible inhibitor of EGFR enzymatic activity in the low nanomolar range (Pollack et al., 1999).
[Callout] Erlotinib (Tarceva®, OSI-774, Genentech) (figure 1) belongs to the class of quinazolinones and is a selective but reversible inhibitor of EGFR enzymatic activity in the low nanomolar range.
It also inhibits the constitutively active EGFRvIII variant that contains an in-frame deletion in exons 2-7 of the EGFR gene (Lal et al., 2002). Erlotinib is currently approved for the treatment of advanced or metastatic resistant NSCLC patients and for use in combination therapy with gemcitabine in treating advanced, unresectable or metastatic pancreatic cancer (reviewed in Bareschino et al., 2007; Gridelli et al., 2007). In in vitro assays, while the degree to which wild-type EGFR and the delL474-S752 mutant were inhibited by both gefitinib and erlotinib was similar, the L858R exhibited an approximate 10-fold increase in sensitivity to both drugs. It is therefore not surprising that in the clinic, patients whose tumors harbor mutant forms of the EGFR respond to treatment with erlotinib, which is similar to what has been observed with gefitinib (Pao et al., 2004b; reviewed in Gridelli et al., 2007).
Gefitinib and Erlotinib Resistance
Secondary EGFR Mutations
In spite of the success that these drugs have had in treating NSCLCs with EGFR activating mutations, resistance emerges in a percentage of patients who have initially responded to treatment. Biochemical analysis and structural modeling have resulted in the identification of a second point mutation at amino acid position T790 (T790M) in a patient who had initially responded to gefitinib treatment but who had relapsed (Kobayashi et al., 2005). At that time, resistance was also predicted to occur in patients treated with erlotinib (Kobayashi et al., 2005), and as expected, cases of erlotinib-resistant disease that harbor this mutation have emerged in the clinic (Pao et al., 2005a). The same mutation has also been identified in a family with several cases of inherited NSCLC and is thought to act in cis with a second somatic T790M mutation (Bell et al., 2005). In cis cooperation has also been observed with both the L858R and del746-750 EGFR mutations as is evidenced by increased transformation potential and ligand-independent signaling (Godin-Heymann et al., 2007). Termed as EGFR's “gatekeeper,” T790 is analogous to T315 of BCR-ABL as it is necessary to maintain proper conformation of the ATP-binding cleft and because of its ability to confer resistance to gefitinib and erlotinib. However, T790M does differ from T315I in that it does not have increased enzymatic activity in vitro (Pao et al., 2005a). At a basic mechanistic level, T790M increases the affinity of the EGFR kinase domain for ATP (Yun et al., 2008) and this single mutation accounts for approximately 50% of all resistance to both gefitinib and erlotinib (Kosaka et al., 2006; Balak et al., 2006). In addition to T790M, other mutations that arise in TKI-resistant tumors inhibition have been isolated in rare instances (Balak et al., 2006).
Non-EGFR Mutation Dependent m\Mechanisms
In addition to mutation of the EGFR, mutations in RAS family genes are present in approximately 15-30% of lung adenocarcinomas in patients with a history of cigarette smoking. Most of these mutations are located in exons 12 and 13 of the K-ras gene and are associated with resistance to gefitinib and erlotinib (Pao et al., 2005b).
Activation of parallel signaling pathways or those that are downstream of the EGFR can also result in resistance to gefitinib and erlotinib. MET locus amplification was originally identified in a gefitinib-resistant cell line and the resultant increased levels of MET protein signal through Akt via ErbB3. Subsequent analysis revealed that the same pathway was activated in the majority of gefitinib and erlotinib resistant tumors with MET amplification and that this amplification occurs in the presence or absence of the T790M mutation (Engelman et al., 2007; Bean et al., 2007). Other proteins/pathways whose activation is associated with EGFR kinase inhibition include but are not limited to PI-3 kinase/Akt (Engelman et al., 2006), the insulin-like growth factor receptor (IGF-1R) (Morgillo et al., 2006; Guix et al., 2008), s-Src (in conjunction with c-Met) (Mueller et al., 2008) and MAPK (Morgillo et al., 2006).
Overcoming T790M Resistance
Overcoming resistance to the T790M mutation is of critical importance in the area of EGFR kinase inhibition. This mutation is a “generic” mutation and biochemical assays and x-ray crystallization predict that it will confer resistance or at least reduce the inhibitory properties of most if not all ATP-competitive inhibitors (Yun et al., 2008). Both erlotinib and gefitinib are reversible inhibitors of the EGFR and T790M reduces the affinity of the EGFR for both. Second-generation irreversible EGFR inhibitors, such as HKI-272 (Rabindran et al., 2004; Kwak et al., 2005) have shown efficacy in mouse models and are predicted to overcome this resistance through their ability to form covalent bonds with the EGFR protein (Li et al., 2007; Yun et al., 2008). While these results are encouraging, resistance to HKI-272 has recently been shown in in vitro cell culture systems (Godin-Heymann et al., 2008) and it is predicted that other irreversible EGFR inhibitors will be required to overcome the effects of T790M.
ANTIBODY-BASED EPITHELIAL GROWTH FACTOR RECEPTOR /HER-2/NEU/ERBB-2 TARGETED THERAPY
HER-2 and Cancer
Heregulin-2 (HER-2), also known as ErbB-2 and Neu, is a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (Schechter et al., 1984; King et al., 1985). ErbB-2 was first shown to be an oncogene gene in 1987 using NIH-3T3 transformation assays (Di Fiore et al., 1987; Hudziak et al., 1987). Soon after, MMTV (mouse mammary tumor virus)-driven HER-2 expression was shown to cause mammary tumors in transgenic mice (Bouchard et al., 1989), providing early experimental evidence that suggested a role for this gene in the genesis and progression of breast cancer.
Trastuzumab
In human cancers, particularly those of the breast, amplification of HER-2 results in its overexpression (approximately 25-30%) and is correlated with a poor prognosis, tumor grade, likelihood of relapse and rate of survival (Slamon et al., 1987; Berger et al., 1988; Slamon et al., 1989). High levels of HER-2 expression have also been observed in other tumor types and like those of the breast, such expression is also correlated with poorer outcomes (reviewed in Kruser and Wheeler, 2010). It is believed that this overexpression results in constitutive activation via the formation of HER-2 homodimers (reviewed in Sliwkowski et al., 1999). As a result, many groups focused their efforts on the development of an antibody that could bind to the extracellular domain of HER-2 and block the adverse effects of its overexpression. While several murine monoclonal antibodies were capable of inhibiting the proliferation of cell lines and human breast cancer xenografts that overexpressed the receptor, the most promising candidate in this regard proved to be Genentech's MAb 4D5 (reviewed in Harries and Smith, 2002). The humanized, chimeric version of this antibody, termed trastuzumab, or Herceptin® (Genentech), actually has a higher affinity for HER-2 than its parent and shares its ability to suppress the proliferation of HER-2+ cells and xenografts as opposed to cells that do not overexpress the receptor (Carter et al., 1992; reviewed in Harries and Smith, 2002; Nahta and Esteva, 2003).
[Callout] The humanized, chimeric version of this antibody, termed trastuzumab actually has a higher affinity for HER-2 than its parent and shares its ability to suppress the proliferation of HER-2+ cells and xenografts as opposed to cells that do not overexpress the receptor
Although the exact mechanism by which trastuzumab acts is not completely defined, studies have shown that exposure to the antibody, which binds to the extracellular domain of HER-2, inhibits dimerzation of the receptor and thereby attenuates HER-2 signaling (Junttila et al., 2009; reviewed in Carter et al., 2009). As a result, the proteins that are activated downstream of HER-2 that promote proliferation, such as phosphatidylinositol-3 (PI-3) kinase, are effectively inhibited as well (Yakes et al., 2002). Those cells that have ceased proliferating become arrested in the G1 phase of the cell cycle via increased levels of p27Kip1/cdk-2 complexes (Ye et al., 1999; Lane et al., 2000; Le et al., 2000; Neve et al., 2000; Yakes et al., 2002), effects which are enhanced by extending the exposure time to the antibody and increasing the dosage (Marches and Uhr, 2004). Trastuzumab also antagonizes tumor growth by inhibiting the expression of proangiogenic factors such as vascular endothelial growth factor (VEGF) (Petit et al., 1997) and inducing others that are anti-angiogenic such as thrombospondin 1 (TSP1) (Izumi et al., 2002). Finally, proteolytic cleavage of the extracellular domain of HER-2 is also inhibited by trastuzumab (Molina et al., 2001).
Resistance
In the clinic, when used as a single agent, trastuzumab is effectively used in metastatic breast cancer patients who have undergone previous treatment regimens with as well as those who were untreated, resulting in overall responses of 20-35% and 11-18%, respectively. These percentages improve when the drug is used in combination therapy (Cobleigh et al., 1999, Vogel et al., 2002; reviewed in Roy and Perez, 2009). Based on this and other data obtained in clinical trials, patients with HER-2-positive early-stage breast cancer are placed on a 1-year course of trastuzumab therapy. Combination therapy is also indicated for those patients with HER-2-positive metastatic disease. In spite of these response rates, resistance to trastuzumab emerges in a percentage of patients who have been treated with the drug within a period of one year (reviewed in Kruser and Wheeler, 2010). The emergence of resistance over the course of treatment, taken together with the fact that some tumors are inherently insensitive to trastuzumab underscores the need to understand the pathways that are associated with a positive response to the drug.
At a mechanistic level, some of the first in vitro studies with breast tumor cell lines revealed that increased levels of insulin-like growth factor-1 receptor (IGF-1R) antagonize the effects of trastuzumab (Lu et al., 2001). These cells, which overexpressed the IGF-1R gene, also showed reduced levels of p27Kip1, a phenotype that was also observed in resistant forms of the SKBR3 cell line that overexpress HER2 (Nahta et al., 2004). In these cells, IGF-1R leads to the upregulation of the Skp2 ubiquitin ligase that mediates degradation of p27 (Lu et al., 2004; Nahta et al., 2004 and 2005).
Another protein that has been shown to mediate a clinical response to trastuzumab is PTEN, a lipid phosphatase that inhibits proliferation by inhibiting PI 3-kinase-mediating signaling (reviewed in Leslie and Downes, 2004). Studies by Nagata et al. (Nagata et al., 2004) have shown that treatment with trastuzumab reduces PTEN phosphorylation and increases its membrane localization and activity within 10 minutes of administration. Patients with breast cancers that do not express PTEN had poorer responses to treatment than those with normal PTEN levels and that PTEN-deficient tumors regain their responsiveness to trastuzumab when treated with PI-3 kinase inhibitors (Nagata et al., 2004). It was later demonstrated that trastuzumab inhibits the ErbB-3/PI-3K/Akt pathway in vitro in a variety of tumor types and that tumors that harbor activating mutations in PI-3 kinase (PI3KCA) are insensitive to the drug. As PI-3 kinase is normally downregulated in response to trastuzumab, these results indicate that trastuzumab resistance can be overcome with co-administering such inhibitors (Junttila et al., 2009).
Resistance to trastuzumab is also observed tumors that express variants of HER-2 that lack the trastuzumab-binding site in the extracellular domain, as well as high levels of transforming growth factor-α (TGF-α) and MUC-4, both of which inhibit binding of trastuzumab to the receptor (reviewed in Kruser and Wheeler, 2010). In cases such as these where trastuzumab simply cannot bind to the receptor, use of second-generation EGFR/ErbB-2 small molecule inhibitors, such as lapatinib (Glaxo-Smith-Kline), may be of particular interest.
Other EGFR-Family Targeted Monoclonal Antibodies
Cetuximab (IMC, Erbitux®, Bristol-Meyers Squibb. Merck, Imclone Systems) is a chimeric monoclonal IgG1 antibody that was approved in 2004 as either a monotherapy or as part of a combination therapy regimen with irinotecan for metastatic colorectal cancer patients and induces receptor internalization and antibody-based cytotoxicity (Bleeker et al., 2004). Because subsequent clinical trials showed that the drug was efficacious against other solid tumors, cetuximab was later approved for use in combination therapy with radiation or as a single agent in patients who previously underwent platinum-based chemotherapy (reviewed in Lurje and Lenz, 2009). Interestingly, unlike many EGFR-targeted antibodies, cetuximab also has demonstrable activity against the mutant vIII variant of the receptor that is expressed in NSCLC, inhibiting its kinase activity and internalization (Perez-Torres et al, 2006; Doody et al., 2007; Steiner et al., 2007). This property suggests that cetuximab could be useful in a greater variety of tumor types, including those that express mutant L858R/T790M EGFRs, that are resistant to small molecule kinase inhibitors (Perez-Torres et al, 2006).
Another anti-EGFR monoclonal antibody currently approved for the treatment of metastatic colorectal cancer is panitumumab (ABX-EGF-Vectibix®, Amgen), a human IgG2 monclonal antibody that binds to the extracellular domain of the EGFR and inhibits its activity and internalization. Panitumumab was the first recombinant human antibody approved for the treatment of EGFR-positive metastatic colorectal cancers that were refractory to previous chemotherapy regimens that included either 5-fluorouracil, irinotecan and oxaliplatin (reviewed in Wu et al., 2008b). Unfortunately, patients with mutations in KRAS do not benefit from cetuximab or panitumumab treatment (Amado et al, 2008), underscoring the need to use these agents in patients with wild-type KRAS or to use other small molecule inhibitors that suppress Ras-mediated signaling pathways.
CONCLUSION
Targeted therapies represent some of the best discoveries that have resulted from the convergence of basic and clinical research efforts. The development of imatinib and trastuzumab, both firsts of their kind, have revolutionized the treatment of cancer and spawned the development of second-generation inhibitors. While it is clear that we are witnessing a change in the paradigm of cancer therapy, resistance to these inhibitors presents a challenge to researchers and physicians alike. The emergence mutations in the ATP-binding domains of tyrosine kinases and the activation of downstream and parallel signaling pathways in drug-resistant tumors highlights the difficulty in generating inhibitors that can effectively overcome resistant forms of the disease and reminds us that there is, unfortunately, always a need to develop novel targeted therapies.
Figure 3.
Schematic representation of the EGFR. The positions of the most commonly identified mutations are shown. (Note: not drawn to scale).
Acknowledgments
Funding Sources: This work was supported by grants from the National Institutes of Health (1R01CA109820-04 and 5 R01 HL080666-03) to EPR.
REFERENCES
- Amado RG, Wolf M, Peters M. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Hayward WS, Neel BG, Hanafusa H. Avian erythroblastosis virus produces two mRNAs. J. Virol. 1980;36:676–683. doi: 10.1128/jvi.36.3.676-683.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperley JF. Part II: management of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1116–1128. doi: 10.1016/S1470-2045(07)70379-0. [DOI] [PubMed] [Google Scholar]
- Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin. Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- Bareschino MA, Schettino C, Troiani T, Martinelli E, Morgillo F, Ciardiello F. Erlotinib in cancer treatment. Annal. Oncol. 2007;18:vi35–vi41. doi: 10.1093/annonc/mdm222. [DOI] [PubMed] [Google Scholar]
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA. 104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat. Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- Berger MS, Locher GW, Saurer S, et al. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res. 1988;48:1238–1243. [PubMed] [Google Scholar]
- Bleeker WK, Lammerts van Bueren JJ, van Pjik HH. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol. 2004;173:4699–4707. doi: 10.4049/jimmunol.173.7.4699. [DOI] [PubMed] [Google Scholar]
- Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the mmtv/c-meu oncogene. Cell. 1989;57:931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Burchert A. Roots of imatinib resistance: a question of self-renewal? Drug Resust. Updat. 2007;10:152–161. doi: 10.1016/j.drup.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Burchert A, Wang Y, Cai D, et al. Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia. 2005;19:1774–1782. doi: 10.1038/sj.leu.2403898. [DOI] [PubMed] [Google Scholar]
- Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc. Natl. Acad. Sci. 2005;102:3395–3400. doi: 10.1073/pnas.0409770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canitrot Y, Falinski R, Louat T, et al. p210 BCR/ABL kinase regulates nucleotide excision repair (NER) and resistance to UV radiation. Blood. 2003;102:2632–2637. doi: 10.1182/blood-2002-10-3207. [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Magrini E, Ceresoli, et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 2004;96:1133–1141. doi: 10.1093/jnci/djh217. [DOI] [PubMed] [Google Scholar]
- Carter CA, Kelly RJ, Giaccone G. Small-molecule inhibitors of the human epidermal receptor family. Expert Opin. Invest. Drugs. 2009;18:1829–1842. doi: 10.1517/13543780903373343. [DOI] [PubMed] [Google Scholar]
- Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Nat.l Acad. Sci. USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Holtz M, Gupta M, Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood. 2004;103:3167–3174. doi: 10.1182/blood-2003-04-1271. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Caputo R, Bianco R, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin. Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- Deininger MW. Nilotinib. Clin. Cancer Res. 2008;14:4027–4031. doi: 10.1158/1078-0432.CCR-07-5015. [DOI] [PubMed] [Google Scholar]
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90:3691–3698. [PubMed] [Google Scholar]
- Di Fiore PP, Pierce JH, Fleming TP, et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Di Gennaro E, Barbarino M, Bruzzese F, et al. Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 ('Iressa'), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cells. J. Cell. Physiol. 2003;195:139–150. doi: 10.1002/jcp.10239. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64:672–677. doi: 10.1158/0008-5472.can-03-1484. [DOI] [PubMed] [Google Scholar]
- Doody JF, Wang Y, Patel SN, et al. Inhibitory activity of cetuximab on epidermal growth factor receptor mutations in non small cell lung cancers. Mol Cancer Ther. 2007;6:2642–51. doi: 10.1158/1535-7163.MCT-06-0506. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J. Clin. Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Fukui T, Mitsudomi T. Mutations in the epidermal growth factor receptor gene and effects of EGFR-tyrosine kinase inhibitors on lung cancers. Gen. Thorac. Cardiovasc. Surg. 2008;56:97–103. doi: 10.1007/s11748-007-0193-8. [DOI] [PubMed] [Google Scholar]
- Godin-Heymann N, Bryant I, Rivera MN, Ulkus L, Bell DW, Riese DJ, 2nd, Settleman J, Haber DA. Oncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutation. Cancer Res. 2007;67:7319–7326. doi: 10.1158/0008-5472.CAN-06-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin-Heymann N, Ulkus L, Brannigan BW, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol. Cancer Ther. 2008;7:874–879. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- Golemovic M, Verstovsek S, Giles F, et al. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin. Cancer Res. 2005;11:4941–4947. doi: 10.1158/1078-0432.CCR-04-2601. [DOI] [PubMed] [Google Scholar]
- Gorre ME, Mohamed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12:840–849. doi: 10.1634/theoncologist.12-7-840. [DOI] [PubMed] [Google Scholar]
- Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J. Clin. Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Hwang PG, Chung DH, et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int. J. Cancer. 2005;113:109–115. doi: 10.1002/ijc.20550. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Rix U, Schmidt U, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc. Natl. Acad. Sci. USA. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries M, Smith I. The development and clinical use of trastuzumab (Herceptin). Endocrine-Related Cancer. 2002;9:75–85. doi: 10.1677/erc.0.0090075. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph’ translocation. Nature. 1985;315:758–61. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Hehlmann R. Current CML therapy: progress and dilemma. Leukemia. 2003;17:1010–1012. doi: 10.1038/sj.leu.2402951. [DOI] [PubMed] [Google Scholar]
- Hirata A, Ogawa S, Kometani T, et al. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res. 2002;62:2554–2560. [PubMed] [Google Scholar]
- Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- Hudziak RM, Schlessinger J, Ullrich A. Increased expression of the putative grwoth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc. Natl. Acad. Sci. USA. 1987;84:7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmer D, Funk JO, Blaukat A. EGFR kinase domain mutations- functional impact and relevance for lung cancer therapy. Oncogene. 2007;26:5693–5701. doi: 10.1038/sj.onc.1210383. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- Jänne PA, Johnson BE. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin. Cancer Res. 2006;12:4416s–4420s. doi: 10.1158/1078-0432.CCR-06-0555. [DOI] [PubMed] [Google Scholar]
- Jiang X, Saw KM, Eaves A, Eaves C. Instability of BCR-ABL gene in primary and cultured chronic myeloid leukemia stem cells. J. Natl. Cancer Inst. 2007a;99:680–693. doi: 10.1093/jnci/djk150. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhao Y, Smith C, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007b;21:926–935. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- Khorashad JS, Milojkovic D, Mehta P, et al. In vivo kinetics of kinase domain mutations in CML patients treated with dasatinib after failing imatinib. Blood. 2008;111:2378–2381. doi: 10.1182/blood-2007-06-096396. [DOI] [PubMed] [Google Scholar]
- King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin. Cncer Res. 2006;12:5764–5769. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp. Cell Res. 2020;316:1083–1100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc. Natl. Acad. Sci. USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Glazer CA, Martinson HM, F, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. ErbB2 Potentiates Breast Tumor Proliferation through Modulation of p27Kip1-Cdk2 Complex Formation: Receptor Overexpression Does Not Determine Growth Dependency. Mol. Cell. Biol. 2000;20:3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le XF, McWatters A, Wiener J, Wu JY, Mills GB, Bast RC., Jr. Anti-HER2 Antibody and Heregulin Suppress Growth of HER2-Overexpressing Human Breast Cancer Cells through Different Mechanisms. Clin. Cancer Res. 2000;6:260–270. [PubMed] [Google Scholar]
- le Coutre P, Schwarz M, Kim TD. New Developments in Tyrosine Kinase Inhibitor Therapy for Newly Diagnosed Chronic Myeloid Leukemia Clin. Cancer Res. 2010;16:1771–1780. doi: 10.1158/1078-0432.CCR-09-2760. [DOI] [PubMed] [Google Scholar]
- le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758–1766. [PubMed] [Google Scholar]
- Lee F, Fandi A, Voi M. Overcoming kinase resistance in chrinic myeloid leukemia. Int. J. Biochem. Cell Biol. 2008;40:334–343. doi: 10.1016/j.biocel.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem. J. 2004;328(Pt 1):1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer. 2004;108:334–41. doi: 10.1002/ijc.11445. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J. Natl. Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- Lurje G, Lenz HJ. EGFR Signaling and Drug Discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:3129–3139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Mashani B, Griffin R, Ashman LK. Colony stimulating factor-1 receptor as a target for small molecule inhibitors. Bioorg Med Chem. 2010;1:1789–97. doi: 10.1016/j.bmc.2010.01.056. [DOI] [PubMed] [Google Scholar]
- Magne N, Fischel JL, Dubreuil A, et al. Influence of epidermal growth factor receptor (EGFR), p53 and intrinsic MAP kinase pathway status of tumour cells on the antiproliferative effect of ZD1839 (“Iressa”). Br. J. Cancer. 2002;86:1518–1523. doi: 10.1038/sj.bjc.6600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon FX, Deininger MW, Schultheis B, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- Manley PW, Cowan-Jacob- SW, Fendrich G, Metan J. Molecular interactions between the highly selective pan-Bcr-Abl inhibitor, AMN107 and the kinase domain of Abl. Blood. 2005;106:940a. [Google Scholar]
- Marches R, Uhr JR. Enhancement of the p27KIP1-mediated antiproliferative effect of trastuzumab (Herceptin) on HER2-overexpressing tumor cells. Int. J. Cancer. 2004;112:492–501. doi: 10.1002/ijc.20378. [DOI] [PubMed] [Google Scholar]
- Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Nahta R, Esteva EJ. HER-2-targeted therapy: lessons learned and future directions. Clin. Cancer. Res. 2003;9:5078–5084. [PubMed] [Google Scholar]
- Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27kip1 down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–28. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- Neve RM, Sutterluty H, Pullen N, et al. Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene. 2000;19:1647–1656. doi: 10.1038/sj.onc.1203470. [DOI] [PubMed] [Google Scholar]
- Nowell PC, Hungerford DA. A minute chromosome in human granulocytic leukemia. Science. 1960;132:1497. [Google Scholar]
- O'Hare T, Eide CA, Tyner JW, et al. SGX393 inhibits the CML mutant Bcr-AblT315I and preempts in vitro resistance when combined with nilotinib or dasatinib. Proc. Natl. Acad. Sci. USA. 2008;105:5507–5512. doi: 10.1073/pnas.0800587105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005a;66:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- Ono M, Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeted drugs. Clin. Cancer Res. 2006;12:7242–7251. doi: 10.1158/1078-0432.CCR-06-0646. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA, Kris MG. ‘Targeting’ the epidermal growth factor receptor tyrosine kinase with gefitinib (Iressa®) in non-small cell lung cancer (NSCLC). Sem. Cancer Biol. 2004a;14:33–40. doi: 10.1016/j.semcancer.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PloS Med. 2005a;3:225–235. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005b;2:57–61. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004b;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torres M, Guix M, Gonzalez A, Arteaga CL. Epidermal growth factor receptor (EGFR) antibody down-regulates mutant receptors and inhibits tumors expressing EGFR mutations. J Biol Chem. 2006;281:40183–40192. doi: 10.1074/jbc.M607958200. [DOI] [PubMed] [Google Scholar]
- Petit AM, Rak J, Hung MC, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J. Pharmacol. Exp. Ther. 1999;291:739–748. [PubMed] [Google Scholar]
- Quintás-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat. Rev. Drug Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- Quintás-Cardama A, Cortes J. Therapeutic options against BCR-ABL1 T315I-positive chronic myelogenous leukemia. Clin. Cancer Res. 2008;14:4392–4399. doi: 10.1158/1078-0432.CCR-08-0117. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- Ramirez P, DiPersio JF. Therapy options in imatinib failures. The Oncologist. 2008;13:424–434. doi: 10.1634/theoncologist.2007-0170. [DOI] [PubMed] [Google Scholar]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin. Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- Sawyers CL. Opportunities and challenges in the development of kinase inhibitor therapy for cancer. Genes Dev. 2004;17:2998–3010. doi: 10.1101/gad.1152403. [DOI] [PubMed] [Google Scholar]
- Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B related gene encoding a 185,000-Mr tumor antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Schittenhelm MM, Shiraga S, Schroeder A, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- Shah NP, Sawyers CL. Mechanisms of resistance to STI571 in Philadelphia chromosome-associated leukemias. Oncogene. 2003;22:7389–7395. doi: 10.1038/sj.onc.1206942. [DOI] [PubMed] [Google Scholar]
- Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J. Clin. Invest. 2007;117:2562–2569. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon Dj, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Sliwinski T, Czechowska A, Szemraj J, Morawiec Z, Skorski T, Blasiak J. STI571 reduces NER activity in BCR/ABL-expressing cells. Mutat. Res. 2008;654:162–167. doi: 10.1016/j.mrgentox.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM. Studies with ZD1839 in preclinical models. Semin. Oncol. 2003;30:12–20. doi: 10.1053/sonc.2003.50028. [DOI] [PubMed] [Google Scholar]
- Soverini S, Colarossi S, Gnani A, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Hematologica. 2007;92:404–404. doi: 10.3324/haematol.10822. [DOI] [PubMed] [Google Scholar]
- Soverini S, Martinelli G, Colarossi S, et al. Presence or the emergence of a F317L BCR-ABL mutation may be associated with resistance to dasatinib in Philadelphia chromosome-positive leukemia. J. Clin. Onol. 2006;24:e51–52. doi: 10.1200/JCO.2006.08.9128. [DOI] [PubMed] [Google Scholar]
- Steiner P, Joynes C, Bassi R. Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res. 2007;13:1540–1551. doi: 10.1158/1078-0432.CCR-06-1887. [DOI] [PubMed] [Google Scholar]
- Stoklosa T, Poplawski T, Koptyra M, et al. BCR/ABL inhibits mismatch repair to protect from apoptosis and induce point mutations. Cancer Res. 2008;68:2576–2580. doi: 10.1158/0008-5472.CAN-07-6858. [DOI] [PubMed] [Google Scholar]
- Stover EH, Chen J, Lee BH, et al. The small molecule tyrosine kinase inhibitor AMN107 inhibits TEL-PDGFRbeta and FIP1L1-PDGFRalpha in vitro and in vivo. Blood. 2005;106:3206–13. doi: 10.1182/blood-2005-05-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski JS, Newitt JA, Chang CY, et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- Velu TJ, Beguinot L, Vass WC, et al. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987;238:1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Vennstrom B, Bishop JM. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982;28:135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- Wang Y, Cai D, Brendel C, et al. Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood. 2007;109:2147–2155. doi: 10.1182/blood-2006-08-040022. [DOI] [PubMed] [Google Scholar]
- Ward WH, Cook PN, Slater AM, Davies DH, Holdgate GA, Green LR. Epidermal growth factor receptor tyrosine kinase. Investigation of catalytic mechanism, structure-based searching and discovery of a potent inhibitor. Biochem. Pharmacol. 1994;48:659–666. doi: 10.1016/0006-2952(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Griffin JD. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood. 2000;95:3498–3505. [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, et al. haracterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wendel HG, de Stanchina E, Cepero E, et al. Loss of p53 impedes the antileukemic response to BCR-ABL inhibition. Proc. Natl. Acad. Sci. USA. 2006;103:7444–7449. doi: 10.1073/pnas.0602402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Meng F, Kong LY, et al. Association between imatinib-resistant BCR-ABL mutation-negative leukemia and persistent activation of LYN kinase. J. Natl. Cancer Inst. 2008a;100:926–939. doi: 10.1093/jnci/djn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rivkin A, Pham T. Panitumumab: human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer. Clin Ther. 2008b;30:14–30. doi: 10.1016/j.clinthera.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- Yamamoto T, Hihara H, Nishida T, Kawai S, Toyoshima K. A new avian erythroblastosis virus, AEV-H, carries erbB gene responsible for the induction of both erythroblastosis and sarcomas. Cell. 1983;34:225–232. doi: 10.1016/0092-8674(83)90153-8. [DOI] [PubMed] [Google Scholar]
- Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–738. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. USA. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Adrián FJ, Jahnke W, et al. Targeting Bcr-Abl by combining allosterix with ATP-binding-site inhibitors. Nature. 2010;463:501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]