Abstract
Word retrieval deficits are common in Alzheimer’s disease (AD) and are thought to reflect a degradation of semantic memory. Yet, the nature of semantic deterioration in AD and the underlying neural correlates of these semantic memory changes remain largely unknown. We examined the semantic memory impairment in AD by investigating the neural correlates of category knowledge (e.g., living vs. nonliving) and featural processing (global vs. local visual information). During event-related fMRI, 10 adults diagnosed with mild AD and 22 cognitively normal older adults named aloud items from three categories for which processing of specific visual features has previously been dissociated from categorical features. Results showed widespread group differences in the categorical representation of semantic knowledge in several language-related brain areas. For example, the right inferior frontal gyrus showed selective brain response for nonliving items in the CN group but living items in the AD group. Additionally, the AD group showed increased brain response for word retrieval irrespective of category in Broca’s homologue in the right hemisphere and rostral cingulate cortex bilaterally, which suggests greater recruitment of frontally-mediated neural compensatory mechanisms in the face of semantic alteration.
Keywords: dementia, semantic memory, language, fMRI, word retrieval, category-specific deficit
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by neuronal atrophy, synapse loss, and the abnormal accumulation of neuritic plaques and neurofibrillary tangles. In the usual case, AD neuropathology initially involves medial temporal lobe structures (e.g., hippocampus, entorhinal cortex) and then extends to temporal, parietal, and frontal lobe association cortices as the disease progresses (Braak & Braak, 1991; Brewer & Moghekar, 2002). These neuropathological changes cause significant cognitive and behavioral disturbances that characterize the global AD dementia syndrome (Salmon & Bondi, 2009). Although the most prominent feature of this dementia syndrome is a profound amnesia (e.g., episodic memory loss), language dysfunction in the form of word finding difficulties is also an early and ubiquitous aspect of the disease (Salmon, Butters, & Chan, 1999). In fact, some studies suggest that word-finding ability and other manifestations of semantic knowledge deteriorate as much as episodic memory and more than executive function during the prodromal phase of AD (Mickes et al., 2007).
The language dysfunction associated with AD is evident on tests of confrontation naming, verbal fluency, and semantic categorization (Bayles, Tomoeda, & Trosset, 1990; Chan, Salmon, Nordin, Murphy, & Razani, 1998; Hodges & Patterson, 1995; Monsch et al., 1992; Nebes, 1989; Salmon, Heindel, & Lange, 1999). Considerable evidence suggests that patients’ performance on these tests is indicative of a deterioration of semantic knowledge rather than simply an impaired ability to retrieve lexical information from intact semantic stores (Astell & Harley, 1996; Barbarotto, Capitani, Jori, Laiacona, & Molinari, 1998; Bayles, Tomoeda, & Cruz, 1999; Nakamura, Nakanishi, Hamanaka, Nakaaki, & Yoshida, 2000; Paganelli, Vigliocco, Vinson, Siri, & Cappa, 2003). Consistent with a degradation of semantic knowledge, AD patients tend to make highly consistent errors for the same concept (e.g., miss the same items) across test modalities and methods of access (Chertkow & Bub, 1990; Hodges, Salmon, & Butters, 1992; Norton, Bondi, Salmon, & Goodglass, 1997; Salmon, Butters et al., 1999), they are more impaired (relative to healthy control subjects) on semantically-demanding category fluency tasks than on letter fluency tasks (Butters, Granholm, Salmon, Grant, & Wolfe, 1987; Monsch et al., 1992), and they make an abnormally high proportion of semantically-related errors on confrontation naming tests with a propensity to generate the more general superordinate category name (e.g., “an animal”) rather than the specific item name (e.g., “a camel”) (Barbarotto et al., 1998; Hodges, Salmon, & Butters, 1991). These results do not, however, preclude the possibility that AD also impairs the ability to access lexical representations from the semantic store (e.g., Nebes, Martin, & Horn, 1984). Indeed, patients with AD exhibit semantic priming effects under some conditions (Nebes & Brady, 1990) and their semantic memory deficits are more salient when retrieval is difficult (Hodges et al., 1992). Furthermore, AD patients are particularly impaired in producing low-frequency picture names which is consistent with a post-semantic processing deficit since frequency effects are thought to arise during phonological retrieval (Gollan, Salmon, & Paxton, 2006). Thus, the semantic memory impairment exhibited by patients with AD may reflect both a degradation of semantic knowledge and inefficient retrieval.
Although the observation of semantic memory deterioration in AD is well established, its nature is actively debated. A major point of debate is whether the semantic memory deficit in AD reflects loss of specific knowledge of particular concepts, or loss of distributed knowledge of features and attributes (e.g., physical features, function) (Alathari, Trinh Ngo, & Dopkins, 2004; Done & Hajilou, 2005; Harley & Grant, 2004). Support for the claim that specific concepts are lost comes from category-specific effects such as findings that some AD patients perform worse on language tasks that require knowledge of living things versus those that require knowledge of nonliving things (Chan, Salmon, & De La Pena, 2001; Chertkow & Bub, 1990; Garrard, Patterson, Watson, & Hodges, 1998; Silveri, Daniele, Giustolisi, & Gainotti, 1991; Zannino, Perri, Carlesimo, Pasqualetti, & Caltagirone, 2002), whereas other AD patients show the opposite pattern of category-specific deficits, with worse performance on artifacts compared to biological items (Gonnerman et al., 1997). However, the results of several studies, including a recent meta-analysis (Laws, Adlington, Gale, Moreno-Martinez, & Sartori, 2007), suggest that category-specific deficits in AD may be artifactual and actually reflect differences in the degree of featural information (e.g., size, function) needed to identify exemplars in various categories. Evidence of decline in featural knowledge in AD comes from reports that patients show selective difficulties in identifying specific features or properties of objects (Chan, Butters, Salmon, & McGuire, 1993; Sacchett & Humphreys, 1992) and are less consistent than nondemented elderly in their use of features when classifying exemplars into categories (e.g., predation, domesticity, and size for the category “animals”) (Chan, Butters, & Salmon, 1997).
The resolution of this debate is complicated by the ongoing controversy regarding the organization of semantic knowledge in the healthy brain. Briefly, theoretical accounts of category-specific effects differ primarily as to whether they view semantic knowledge as 1) modularly represented in a unitary semantic system (Caramazza & Shelton, 1998; Rogers et al., 2004) or 2) distributed across many attribute-specific subsystems (e.g., visual, sensorimotor, functional) that differ in degree of categorical organization (Coltheart, Inglis, Cupples, Michie, & Budd, 1998; Devlin, Gonnerman, Andersen, & Seidenberg, 1998; Humphreys & Forde, 2001; Moss & Tyler, 2000; Sartori & Lombardi, 2004; Stewart, Parkin, & Hunkin, 1992; Zannino, Perri, Pasqualetti, Caltagirone, & Carlesimo, 2006). While these parallel distributed processing (PDP) models may disagree on whether or not there is a unitary semantic system, they tend to agree that concepts emerge from patterns of activation across sets of distributed features (Aronoff et al., 2006; McRae, de Sa, & Seidenberg, 1997; Shallice, 1988). According to one seminal PDP neural network model of semantic processing put forth by Rogers and colleagues, semantic knowledge arises from the interactive activation of modality-specific representations of objects that are distributed throughout the cortex and converge in a cross-modal ‘hub’. These units are thought to receive input directly from the environment and represent anatomically distinct regions of cortex that subserve a particular function (e.g., visual information) (Lambon Ralph, Lowe, & Rogers, 2007; Rogers et al., 2004). Because concepts within a given category may have more overlapping features than concepts from another category, damage affecting feature knowledge may result in apparent category-specific deficits. In other words, since living things rely more heavily on perceptual features and nonliving things rely more heavily on functional features (Farah & McClelland, 1991; Warrington & McCarthy, 1987), according to computational models, differential category-specific impairments for either living or nonliving items may emerge from widespread damage to distributed features (as in AD) if the features are intercorrelated (e.g., activated simultaneously for many items within a category) or distinguishing (e.g., occurs almost exclusively for one item within a category to differentiate it from related ones) (Gonnerman et al., 1997).
Regardless of whether category-specific deficits are caused by damage to conceptual representations in specialized brain regions or damage to distributed representations within nonspecialized brain areas (Aronoff et al., 2006; Zahn et al., 2006), they appear to emerge from localized changes in neural function that can be detected using fMRI (Thompson-Schill, 2003). Many functional neuroimaging studies in healthy adults suggest that there are localizable regions specialized for processing category and feature knowledge. The fusiform gyrus, for example, is a focal point for the convergence and integration of visual semantic information, and evidence indicates a reliable difference along its medial/lateral dimension for the categorical distinction between nonliving and living things (Chao, Haxby, & Martin, 1999; Ishai, Ungerleider, Martin, Schouten, & Haxby, 1999; Weisberg, van Turennout, & Martin, 2007; Whatmough, Chertkow, Murtha, & Hanratty, 2002; Wierenga et al., 2009). Furthermore, we recently reported a dissociation between category (living vs. nonliving) and attribute (global vs. local form) knowledge in the fusiform gyrus of healthy younger and older adults (Wierenga et al., 2009).
A number of additional studies using fMRI in healthy adults have shown localized function related to various aspects of processing semantic knowledge. For example, studies indicate that the lateral frontal cortex of the language dominant hemisphere (i.e., Broca’s area, cortex along the inferior frontal sulcus, and the possibly pars orbitalis) is involved in selection, retrieval and execution of lexical-semantic responses (Barch, Braver, Sabb, & Noll, 2000; Crosson et al., 1999; Damasio & Anderson, 1993; Gabrieli, Poldrack, & Desmond, 1998; MacDonald, Cohen, Stenger, & Carter, 2000; Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001). Medial frontal lobe cortical regions, especially those at the border of pre-supplementary motor area (SMA) and the rostral cingulate zone, are involved in initiation of language, cognitive control, and monitoring conflict between competing responses (Barch et al., 2000; Carter et al., 2000; Crosson et al., 1999; Damasio & Anderson, 1993). The results of these studies suggest that the left prefrontal cortex as well as bilateral ventral temporal cortex may be involved in a “general-purpose” semantic system for the respective retrieval or storage of semantic knowledge (Thompson-Schill, 2003).
Relatively few studies have examined the effects of AD on the neural substrates of semantic memory. Zahn et al. (2006) reported that left posterior fusiform gyrus hypometabolism was correlated with impaired knowledge of visual properties of living objects in patients with AD, whereas hypometabolism in the left anterior temporal region was correlated with impaired knowledge of visual and functional properties of nonliving objects. We found that adults genetically at risk for AD by virtue of the APOE ε4 allele exhibited a greater object naming-related brain response in the left inferior temporal lobe (including fusiform gyrus), right perisylvian region (i.e., insula, inferior frontal gyrus, superior temporal gyrus, inferior parietal lobe), and medial prefrontal cortex (i.e., anterior cingulate, rostral cingulate zone, pre-SMA, SMA) than those with only the APOE ε3 allele (perhaps reflecting a compensatory response); however, category-specific effects that differed by group were restricted to left frontal (e.g, superior and middle frontal gyri, anterior cingulate) and right posterior cingulate regions (Wierenga et al., 2010). Other functional neuroimaging studies in patients with AD have reported increased diffuse cortical activity in the lateral temporal lobe during a category judgment task (Grossman et al., 2003), and in the left inferior frontal gyrus and the right prefrontal cortex during semantic decision-making (Saykin et al., 1999).
In light of these previous findings, the aim of the current study was to investigate the effects of AD on the neural correlates of semantic knowledge measured by an object naming task that allowed evaluation of categorical (living vs. non-living) and featural (global form vs local detail) knowledge effects. This task contrasted naming animals, vehicles, and tools because the categories represent living and non-living objects and previous research showed that they differ in the amount of global or local features needed for identification of exemplars (animals most global, tools most local, and vehicles intermediate). We predicted that 1) AD patients and normal control subjects would differ in the pattern of functional BOLD responses they produced to category distinctions in language-related regions of interest such as the inferior frontal gyrus, medial prefrontal cortex, and fusiform gyrus, and 2) AD patients would exhibit a compensatory increase (relative to normal controls) in functional BOLD response in frontal cortices during word retrieval in the object naming tasks. We have previously shown that processing of specific visual features (global form vs. local detail) is dissociable from processing of semantic category (Wierenga et al., 2009). Thus, we anticipated that AD patients’ ability to make such fine-grained distinctions in semantic processing would be informative regarding the nature of semantic memory impairment in AD. For instance, if semantic impairments occur for category knowledge, then we would expect an altered distinction between living and nonliving items in AD. In contrast, if changes in processing visual attributes contribute to semantic impairments, then we would expect decreased lateralization for processing global and local visual attributes in AD.
Methods
Participants
Eleven adults diagnosed with probable AD and 24 neurologically and cognitively normal (CN) elderly adults enrolled in a longitudinal study of healthy aging participated. CN participants were recruited through newspaper advertisements and community lectures (i.e., no clinic-based or medical referral sources). All CN participants were considered normal based on extensive medical, neurologic, laboratory, and neuropsychologic evaluations. AD participants were selected for this study from a larger cohort of research volunteers at the UCSD Alzheimer’s Disease Research Center (ADRC). AD patients received a diagnosis of probable AD by two senior staff neurologists according to the criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association. All participants were native English speakers and were strongly right-handed (Oldfield, 1971). Potential participants were excluded if they had causes of dementia other than AD (e.g., stroke, hypothyroidism, vitamin B12 deficiency, electrolyte imbalance), a history of severe head injury, uncontrolled hypertension, the apolipoprotein E (APOE) ε2 isoform, or a Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Axis 1 diagnosis of learning disability, attention deficit disorder, or substance abuse. Persons with significant cerebrovascular disease were excluded. In addition, participants were excluded if they had metal in their body other than dental fillings, or if they were taking prescription psychoactive medications. No participant reported a significant level of depressive symptoms on the Geriatric Depression Scale (i.e., GDS ≥ 10) (Yesavage et al., 1983). Of the 35 adults scanned, two CN individuals and one AD adult were excluded from further analysis due to excessive movement (i.e., met a priori criterion of excessive motion as determined by the number of outliers in the time series exceeding 1.2 standard deviations above the group mean as determined by AFNI’s 3dToutcount program). Data from the CN group were previously reported in a comparison of APOE ε4 vs. APOE ε3 isoforms (Wierenga et al., 2010). Of the participants who completed the neuroimaging procedure, no CN participants were taking cognitive enhancing medications at the time of scanning or neuropsychological testing. However, 6 of the 10 AD participants were taking Aricept, Namenda, and/or Razadyne (dosages ranged from 5–20 mg/day) at the time of the study.
As shown in Table 1, the AD group and the CN group did not differ significantly in age, level of education, sex distribution, APOE genotype, or family history of Alzheimer’s disease (defined as having at least one first degree relative with AD). As expected, on formal neuropsychological testing, the AD group performed significantly worse than the CN group on a test of global cognitive functioning, the Mattis Dementia Rating Scale-II (DRS)(Mattis, 1988), and on tests of confrontation naming, verbal fluency, aspects of executive function, and memory (i.e., the Wechsler Memory Scale-Revised (WMS-R)(Wechsler, 1997) and the California Verbal Learning Test (CVLT)(Delis, Kramer, Kaplan, & Ober, 2000). The AD group had significantly less total brain volume, gray matter volume, and right and left hippocampal volumes than the CN group, but the groups did not differ on volume of white matter or white matter hyperintensities. The AD group also had decreased cortical thickness in the right fusiform gyrus, right pars triangularis, right pars opercularis, and left and right posterior cingulate compared to the CN group (see Table 1).
Table 1.
Demographics, neuropsychological raw scores, and brain structural characteristics of the cognitively normal (CN) and Alzheimer’s disease (AD) groups
| Diagnosis | ||||||
|---|---|---|---|---|---|---|
| Variables | CN, n = 22 | AD, n = 10 |
t or Χ2, value |
p-value (two-tailed) |
||
| Mean | SD | Mean | SD | |||
| Demographics | ||||||
| Age, y | 78.09 | (6.47) | 77.70 | (9.71) | 0.14 | .89 |
| Education, y | 16.05 | (2.28) | 15.40 | (2.37) | 0.73 | .47 |
| Women/men | 11 | 11 | 5 | 5 | 0.00 | 1.00 |
| +FH AD/−FH AD | 13 | 9 | 7 | 3 | 0.35 | .70 |
| APOE ε4/APOE ε3 | 11 | 11 | 7 | 3 | 1.12 | .45 |
| Global cognition | ||||||
| DRS total (144 points total) | 140.64 | (3.66) | 126.80 | (7.16) | 7.29 | .00a |
| MMSE | -- | -- | 24.30 | (3.56) | -- | -- |
| CDR | -- | -- | 0.75 | (0.49) | -- | -- |
| Language | ||||||
| Boston Naming Test | 57.41 | (3.45) | 45.90 | (10.75) | 4.60 | .00a |
| Letter Fluency (FAS Total) | 46.73 | (12.24) | 36.50 | (8.50) | 2.38 | .02a |
| Category Fluency (animals) | 21.68 | (6.45) | 12.20 | (4.89) | 4.12 | .00a |
| Learning and memory | ||||||
| WMS-R LM immediate recall | 27.09 | (7.95) | 10.30 | (4.76) | 6.17 | .00a |
| WMS-R LM delayed recall | 23.77 | (9.17) | 4.30 | (3.65) | 6.44 | .00a |
| CVLT List 1–5 total recall | 48.00b | (12.21) | 24.90 | (9.12) | 5.30 | .00a |
| CVLT long delay free recall | 9.57b | (3.63) | 1.50 | (1.65) | 6.69 | .00a |
| Executive function | ||||||
| Trails B, seconds | 85.29b | (45.96) | 165.33c | (55.29) | −4.12 | .00a |
| WCST-48, categories | 5.48b | (1.03) | 3.40 | (1.90) | 3.97 | .00a |
| WCST-48, total errors | 6.71b | (7.01) | 21.70 | (10.75) | −4.67 | .00a |
| Emotional function | ||||||
| GDS | 2.76b | (3.08) | 2.50 | (1.84) | 0.25 | .81 |
| bBrain volume (Z-scores)* | ||||||
| Total brain volume | 0.40 | (0.77) | −0.79 | (0.77) | 4.00 | .00a |
| White matter | 0.19 | (1.04) | −0.37 | (0.61) | 1.57 | .13 |
| Gray matter | 0.42 | (0.82) | −0.84 | (0.55) | 4.35 | .00a |
| Hypointensities | −0.06 | (0.93) | 0.13 | (1.02) | −0.52 | .61 |
| Left hippocampus | 0.45 | (0.71) | −0.99 | (0.68) | 5.39 | .00a |
| Right hippocampus | 0.33 | (0.72) | −0.72 | (1.08) | 3.26 | .00a |
| cCortical thickness (Z-scores)* | ||||||
| Left fusiform gyrus | 0.19 | (0.92) | −0.38 | (.99) | 1.57 | .13 |
| Right fusiform gyrus | 0.33 | (0.82) | −0.67 | (0.92) | 3.08 | .00a |
| Left pars orbitalis | 0.09 | (1.09) | −0.19 | (0.65) | 0.75 | .46 |
| Right pars orbitalis | 0.13 | (0.93) | −0.26 | (1.04) | 1.03 | .31 |
| Left pars triangularis | −0.01 | (1.03) | 0.02 | (0.87) | −0.10 | .92 |
| Right pars triangularis | 0.25 | (0.96) | −0.49 | (0.80) | 2.10 | .05a |
| Left pars opercularis | 0.16 | (0.91) | −0.32 | (1.03) | 1.32 | .20 |
| Right pars opercularis | 0.36 | (0.82) | −0.72 | (0.85) | 3.36 | .00a |
| Left rostral anterior cingulate | 0.20 | (0.83) | −0.41 | (1.13) | 1.69 | .10 |
| Right rostral anterior cingulate | 0.15 | (0.95) | −0.31 | (0.96) | 1.26 | .22 |
| Left caudal anterior cingulate | −0.09 | (0.96) | 0.18 | (1.00) | −0.73 | .47 |
| Right caudal anterior cingulate | 0.14 | (0.96) | −0.29 | (0.95) | 1.12 | .27 |
| Left posterior cingulate | 0.35 | (0.80) | −0.70 | (0.92) | 3.25 | .00a |
| Right posterior cingulate | 0.33 | (0.91) | −0.66 | (0.74) | 2.97 | .01a |
statistically significant pairwise comparison: AD<CN adults.
CN n=21
AD n=9
standardized residual z-scores after controlling for age, gender and intracranial volume for volume comparisons and controlling for age and gender for cortical thickness comparisons.
+FH AD = positive family history of Alzheimer’s disease; DRS = Dementia Rating Scale; MMSE = Mini Mental State Exam; CDR = Clinical Dementia Rating; WMS-R = Wechsler Memory Scale-Revised, LM = Logical Memory subtest; CVLT = California Verbal Learning Test; WCST-48 = Wisconsin Card Sorting Test-48 card version; GDS = Geriatric Depression Scale
This research was approved by the Ethics Committee and Institutional Review Board at the University of California at San Diego and VA San Diego Healthcare System. Written informed consent was obtained from all participants and/or their caregivers according to guidelines established by the Declaration of Helsinki.
Experimental Design and Procedure
FMRI naming task
Participants alternated between an overt event-related picture naming task and a passive viewing task during four functional imaging runs (Wierenga et al., 2009; Wierenga et al., 2010). During the picture naming task, 20 grayscale photographs of animals, 20 grayscale photographs of tools or implements, and 20 grayscale photographs of vehicles were presented for a total of 60 naming trials during the scanning session. Photographs were chosen based on a previous study in nondemented younger and older adults that assessed the amount of high spatial frequency content needed for object identification and showed that adults rely primarily on global form for identifying animals, local details for identifying tools, and both for identifying vehicles (Wierenga et al., 2009). This study also showed that processing specific visual features (global form vs. local detail) could be dissociated from processing semantic category for these three categories (animals, tools, and vehicles): living vs. nonliving category membership drove a respective lateral vs. medial fusiform distinction in processing whereas global form vs. local detail drove a respective right vs. left fusiform distinction in processing.
Photographs were equated for size and resolution. Stimuli in each category did not differ according to frequency of occurrence in English [F(2, 55) = .18, p = .84] or familiarity [F(2,55) = 1.48, p = .24] (CELEX; (Baayen, Piepenbrock, & Run, 1993). Although normative data on name agreement do not exist for these stimuli, the number of acceptable responses for each item was similar across categories [F(2,58) = 0, p = 1]. Mean accuracy rates for animals (89.1% correct, SD = 8.3), tools (92.1% correct, SD = 6.5), and vehicles (91.3% correct, SD = 6.6) did not differ across younger and older participants [F (2,76) = 2.74, p = .071] in a previous study (Wierenga et al., 2009); however, participants responded more quickly to tools than vehicles [t (38) = 3.49, p = .001] or animals [t (38) = 5.07, p < .001] and responded more quickly to vehicles than animals [t (38) = 2.86, p = .007].
Pictures were presented one at a time for 3400 ms each, in an event-related format, and participants named each picture aloud. An event-related design was chosen to allow for overt responding so that performance accuracy and response latency could be assessed. Between trials, participants were instructed to rest quietly, and to look at abstract patterns that were derived by pixelating photographs from the naming task using Adobe PhotoShop 7.0. This process served to randomize the pixels in clusters of a predetermined size while maintaining image luminance. The interstimulus interval equaled a variable intertrial interval plus 3400 ms for trial. Intertrial intervals were pseudorandomly varied between 13600 ms (8 images), 15300 ms (9 images), 17000 ms (10 images) and 18700 ms (11 images) to mitigate effects of periodic or quasi-periodic physiological noise, and to allow the hemodynamic response to return to baseline before the participant spoke again to prevent contamination of the latter part of the hemodynamic response by movement during the subsequent response. Experimental runs began and ended with a rest interval. There were 15 trials in each of the 4 experimental runs. Each 15-trial run was 323s in length and acquired 190 functional images for each slice. Stimuli were presented using E-Prime Version 1.1 software via an LCD projector that was projected onto a screen at the participant’s feet. Overt verbal responses were monitored using a bidirectional dual microphone (Resonance Technology, Inc.). Microphone output was run through the penetration panel into the scanner control room and connected to a Dell Inspiron Laptop Computer with Adobe Audition 1.5 software that recorded verbal responses from each scanning run. These responses were scored off-line for accuracy and reaction time. A training phase that used a different set of photographs preceded the experimental phase.
Image acquisition
All data were acquired on a GE Signa Excite 3-T whole body system with a body transmit coil and an 8-channel receive-only head coil. Functional images were obtained with a 1-shot gradient echo EPI scan: 24 cm FOV, 64 × 64 matrix, 3.75 mm × 3.75 mm in-plane resolution, TR=1700 msec, TE= 30 msec, flip angle=70°. Twenty-eight 5 mm thick sagittal slices covering the whole brain were acquired. Two field maps were collected to correct for distortions in EPI images due to susceptibility artifact: 24 cm FOV, 64 × 64 matrix, 3.75 mm × 3.75 mm in-plane resolution, TR=1,000 msec, TE=minimum full (1st field map) or 5.5 (2nd field map), flip angle=60°, 28 5 mm thick sagittal slices covering the whole brain. A high resolution T1-weighted 3D MP-RAGE scan was obtained to provide anatomic reference: 26 cm FOV, 256 × 256 matrix, TR=7 msec, TE=min full, flip angle=8°, inversion recovery prepared: inversion time 900 msec, bandwidth=31.25 kHz, 170 1.2 mm sagittal slices. Head motion was minimized using foam padding.
Data analysis
Behavioral data
Accuracy and response latency measures from the overt naming task during fMRI were submitted to group (AD vs. CN) × category (animals, tools, vehicles) ANOVAs with paired t-tests used for post-hoc pair-wise comparisons (Bonferrroni-corrected for multiple comparisons).
Neuroimaging data
fMRI data were analyzed and overlaid onto structural images with the Analysis of Functional Neuroimaging (AFNI) software package from the National Institutes of Health (Cox, 1996). For each participant, T1-weighted anatomical data and EPI BOLD data were spatially coregistered utilizing the AFNI program 3dAllineate with the local Pearson correlation cost function (Saad et al., 2009). The goodness of the alignment was then assessed visually and manually edited as necessary. To minimize the effects of head motion, each individual’s functional time series were corrected for motion by alignment to that base image which necessitated the least interpolation using a three-dimensional iterated, linearized, weighted least-squares method with Fourier interpolation (Seidenberg et al., 2009). Following automated motion correction, the time series was examined for uncorrected motion outliers, and time-points with more than 10 times the mean number of outliers within a run were excluded from statistical analysis (via censor file). Slice timing correction was applied to the four imaging runs for the naming task and runs were detrended of low frequency signal drifts (Birn, Saad, & Bandettini, 2001) and concatenated into a single time series. Standard signal normalization procedures were performed on each individual voxel to have a timecourse mean of 100 and percent signal change from the mean was calculated. The association between measured BOLD signal and the object naming task was calculated with multiple regression using the program 3dDeconvolve. The following predictors were included in the model: a constant, a linear trend, three parameters indicating the degree of motion correction performed in three rotational angles, and stimulus functions indicating the initiation of the 3400 ms presentation of pictures of animals, tools, and vehicles to model the hemodynamic response for each category. The 3dDeconvolve command was repeated with a stimulus function indicating the initiation of the 3400 ms presentation of all pictures to model the hemodynamic response for object naming collapsed across category for within-group comparisons. For each voxel, the observed fMRI intensity time-series was modeled as the convolution of the experimental stimulus vector (comprised of 60 picture stimuli) and the estimated best-fit 11-lag impulse response using tent functions, allowing the hemodynamic response to return to baseline. Area under the curve (AUC) of the deconvolved hemodynamic response (HDR) was the dependent variable for group analyses. AUC was calculated by adding the deconvolved image intensity at each deconvolved time point of the impulse response. The first image following stimulus presentation, during which the participant responded overtly, was excluded to eliminate stimulus-correlated signal artifact (Carter et al., 2000) since the vast majority of responses occurred within the first 1.7 seconds following stimulus presentation. Functional images were spatially smoothed with a Gaussian kernel of 4 mm full-width at half-maximum. The T1-weighted anatomic images and the AUC functional activation maps were warped to the coordinates of the co-planar stereotactic atlas of Talairach & Tournoux (Talairach J. & Tournoux, 1988) and resampled at a 4 mm3 resolution.
Region-of-interest (ROI) analysis
Based on previous studies showing the importance of the fusiform gyrus in resolving visual semantic information (Chao et al., 1999; Ishai et al., 1999; Weisberg et al., 2007; Wierenga et al., 2009), group differences were examined in a bilateral fusiform gyrus (FG) ROI. We manually outlined each participant’s fusiform gyrus in order to increase the specificity in this small but important brain region. Left and right fusiform gyrus ROIs were drawn on each participant’s high-resolution MP-RAGE brain image in coronal view rotated into alignment with the anterior commissure-posterior commissure (AC-PC) plane. Following the guidelines of Lee et al. (2002) and Behrmann et al. (2007), the outlining began one slice posterior to the appearance of the mammillary body and continued posteriorly to a slice midway between the posterior commissure and the posterior end of the occipital lobe at the AC-PC level. The collateral and occipitotemporal sulci were used to determine the medial and lateral fusiform gyrus borders, respectively. In cases in which the sulci were interrupted or duplicated, the more laterally located sulcus was used as the border. Interrater reliability was computed for the FG ROI by 2 independent raters (C.W., S.D.) who were blind to group membership. Ten cases were selected randomly for interrater reliability. An intraclass correlation coefficient used to compute interrater reliability for the 2 raters was 0.86 for the FG ROI. A voxel was classified as falling within the fusiform gyrus if it was located in the fusiform gyrus in 6 out of 10 AD patients and 12 out of 22 CN adults. These thresholds were used because the resulting region represented >50% of the participants in each group and resulted in a cluster of relatively equal volume across groups; the conjunction of these two masks was then transformed into standard atlas space and used as the final mask. The blurred and standard-space transformed AUC images for each participant were masked with the resampled 4×4×4 mm bilateral FG ROI (volume = 20,352 mm3). Significant clusters resulting from within-group t-tests comparing object naming-related activity vs. baseline in both AD and CN adults were retained. Additionally, significant clusters for the main effect of group, the main effect of category, and the group × category interaction resulting from a random effects 2 group × 3 category ANOVA were retained. Clusters in the FG ROI were considered significant if each voxel was significant at p<0.05 (AD: t>2.3, df=9, CN: t>2.1, df=21 for within group comparisons; t>4.2, df=1,30 for main effect of group, t>3.2, df=2,60 for main effect of category and group × category interaction) and had a volume of at least 448 mm3. This threshold/volume combination was determined by Monte Carlo simulation (AlphaSim program) to protect ROI-wise probability of false positives of p<0.05. Effect sizes were calculated according to the following equation: eta-squared = (t2/(t2+df)) where t = t-value and df = degrees of freedom.
Whole brain analysis
As an exploratory analysis, we examined voxel-wise task-related whole brain response using within group t-tests and a 2 group × 3 category ANOVA with subjects as a random factor and AUC of the HDR as the dependent variable. Regions were considered activated if each voxel was significant at p<0.05 (AD: t>2.3, df=9, CN: t>2.1, df=21 for within group comparisons; t>4.2, df=1,30 for main effect of group, t>3.2, df=2,60 for main effect of category and group × category interaction) and the cluster had a volume of at least 1536 mm3. This threshold/volume combination protected a whole-brain probability of false positives of p<0.05.
Given that characteristics of the BOLD signal may differ between individuals due to potential changes in cerebrovascular dynamics, we examined the temporal characteristics of the BOLD HDRs in AD and CN adults during word retrieval. We generated a mask of the significant clusters that showed differences in activity between groups at previously reported threshold/volume combinations protecting a whole-brain probability of false positives of p<0.05 and derived the HDR (based on percent signal change from baseline) for each participant and averaged across voxels. The averaged HDRs for each subject and cluster were entered into a 2 (group) × 10 (image number) repeated measures ANOVA to investigate differences in the time course of the HDR in those clusters that showed a significant difference in activation between groups or categories. The first image was excluded from the group × time repeated measures ANOVA to remain consistent with the independent samples t-test and 2 group × 3 category ANOVAs for AUC that eliminated the first image to reduce motion artifact from overt speaking.
Correlations with performance
To aid interpretation of observed clusters of activity during object naming, we calculated the Pearson’s correlation between naming accuracy or response time and mean brain response (e.g., AUC) in clusters of significant activity.
Anatomical analysis
T1-weighted 3D MP-RAGE image files in DICOM format were transferred to a Linux workstation for morphometric analysis. Images were reviewed for quality, and automatically corrected for spatial distortion due to gradient nonlinearity (Jovicich et al., 2006) and B1 field inhomogeneity, and were then rigid body registered to a probabilistic brain atlas. Volumetric segmentation (Fischl et al., 2002; Fischl et al., 2004) and cortical surface reconstruction (A. Dale & Sereno, 1993; A. M. Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999; Fischl et al., 2004) methods based on FreeSurfer software were used. To measure thickness, the cortical surface was reconstructed (A. Dale & Sereno, 1993; A. M. Dale et al., 1999) and parcellated into distinct ROIs (Desikan et al., 2006; Fischl et al., 2004). These tools have been well-validated for use in aging and dementia research and have been shown to successfully segment and normalize atrophic brains without loss of anatomical specificity (Desikan et al., 2009; Desikan et al., 2010; Dickerson, Bakkour et al., 2009; Dickerson, Feczko et al., 2009; Gold et al., 2005). ANCOVAs were then conducted to assess group differences in brain volume or cortical thickness. Volumetric data were corrected for individual differences by regressing the estimated total intracranial volume (eTIV), age, and gender (Buckner et al., 2004) and then group comparisons were performed on the resultant standardized residual z-scores using independent samples t-tests. Participant age and gender were included in the model as covariates for group comparisons of cortical thickness.
Manual outlining of hippocampal ROIs was completed by experienced operators (A.J., D.N.) blind to participant identity and group using AFNI software. High levels of intra- and inter-rater reliability for the procedure were established on a separate set of images not among those studied presently (intraclass correlation coefficients >.90). Hippocampal ROIs were delineated using a stereotactic approach using methods published previously (Jak, Houston, Nagel, Corey-Bloom, & Bondi, 2007). Briefly, the anterior boundary of the hippocampus was traced on the coronal slice through the fullest portion of the mammillary bodies, and the posterior boundary was traced on the last coronal slice on which the superior colliculi could be fully visualized. Whole-brain volume was used in normalizing hippocampal volumes (Bigler & Tate, 2001).
Results
Behavioral results
Naming performance during the fMRI task is presented in Table 2. There was a significant interaction between group and category for naming accuracy [F(2,56) = 16.23, p = .004]. CN adults and AD patients had similar naming accuracy rates for animals [t(28) = 1.33, p = 0.194], but AD patients had poorer accuracy than CN adults for tools [t(28) = 4.49, p= .000] and vehicles [t(28) = 2.39, p = .024]. Overall, there was a main effect of group [F(1,28) = 11.28, p = .002], with AD patients worse than CN adults collapsed across category, but no main effect of category [F(2,56) = 2.86, p = .066]. There was no significant interaction between group and category for naming response time (RT) [F(2,56) = 1.36, p = .265]. However, the main effect of group was significant [F(1,28) = 8.65, p = .006] with AD patients having longer RT than CN adults. Response times for correctly identified objects differed significantly between categories when performance was collapsed across subjects [F(2,56) = 9.60, p = .000]. As in a previous study (Wierenga et al., 2010), participants responded more quickly to tools than vehicles [t = −5.16, p = .000] or animals [t = 3.45, p = .002], but responded similarly to vehicles and animals [t = −.55, p = .585]. Although RT differences were not small (e.g., up to 430 ms), given the poor temporal resolution of fMRI (e.g., one image acquired every 1700 ms) this difference in RT is unlikely to have much bearing on the fMRI category comparisons. Implications of differences in performance on fMRI comparisons are discussed later. No outliers were identified for these comparisons, so all subjects and items were included in these analyses.
Table 2.
Performance on the fMRI naming task for the 22 cognitively normal (CN) and 10 Alzheimer’s disease (AD) adults.
| Total | Animals | Tools | Vehicles | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Accuracy (%correct) |
CN | 92.8a | (5.8) | 92.6 | (7.7) | 94.5a | (5.8) | 91.2a | (8.2) |
| AD | 84.2a | (7.9) | 88.5 | (8.2) | 80.0a | (12.0) | 84.0a | (6.6) | |
| Response Time (ms) |
CN | 1696b | (299) | 1780 | (354) | 1553c | (311) | 1754 | (340) |
| AD | 2058b | (355) | 2045 | (400) | 1944c | (334) | 2185 | (430) | |
AD<CN adults; main effect of group and group × category interaction, p<.05.
AD>CN adults; main effect of group, p<.05.
tools<animals, vehicles; main effect of category, p<.05.
FMRI results
Fusiform gyrus ROI
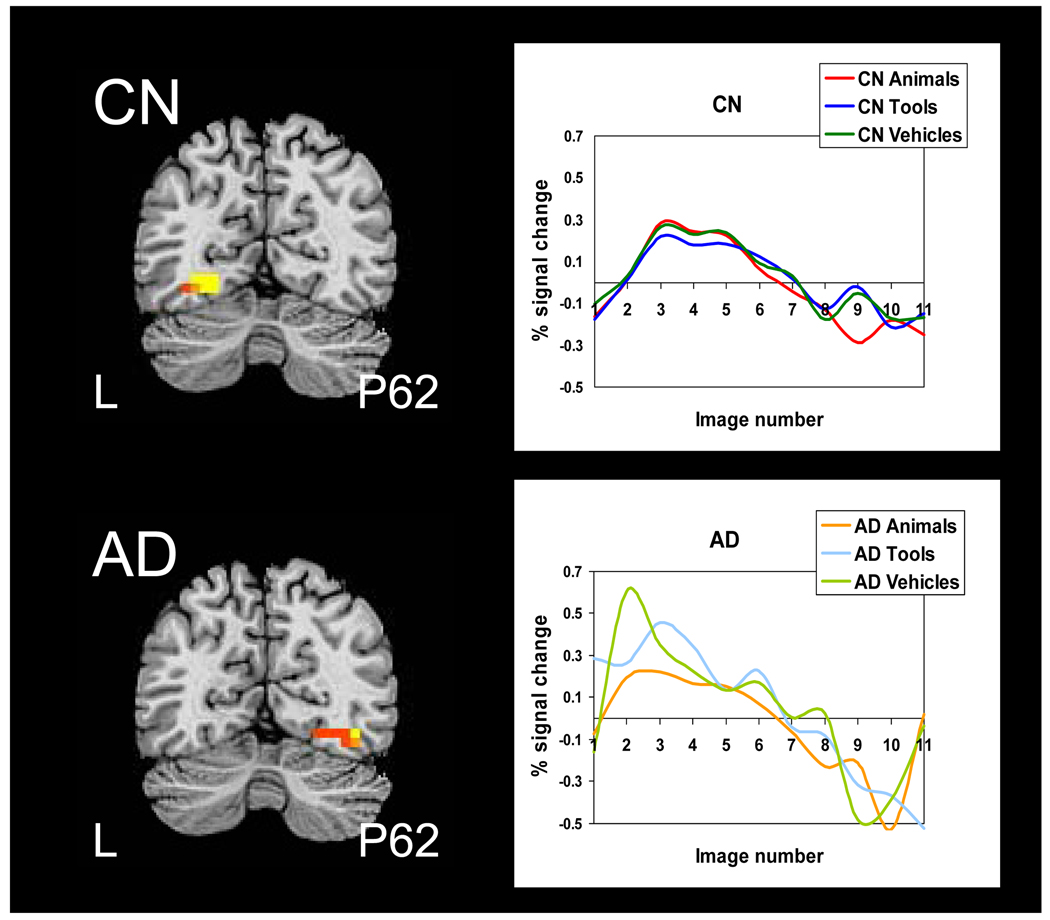
Within-group t-tests comparing naming objects vs. viewing pixelated images showed significant clusters of naming-related brain response within the FG ROI in both the CN adults and AD patients (Table 3; Figure 1). The two groups exhibited comparably-sized clusters of activity that encompassed the medial and lateral aspects of the FG with peak intensity located in the same posterior slice. However, the CN group activated the left FG and the AD group activated the right FG. Mean brain response in the left FG (r=−0.45, p=.05) was significantly negatively related to naming accuracy in the CN group. No main effects of group or category, or a group × category interaction, were found for degree of activation in the FG ROI. Subjective analysis of the temporal dynamics of the HDR revealed that the CN group had a tightly coupled response for each of the three semantic categories, with greater peak amplitude for animals and vehicles than for tools. The AD group showed greater variability in HDR across categories with greater peak amplitude for vehicles than tools, and greater peak amplitude for tools than animals.
Table 3.
Clusters of significant brain response within the fusiform gyrus search region for object naming vs. passive viewing
| Direction of response | Hemisphere | Subregion (Brodmann’s area) |
Volume (mm3) |
Coordinates of maximum intensity voxel |
t for maximum intensity voxel |
η2(Mean ±SEM) |
|---|---|---|---|---|---|---|
| NC adults | ||||||
| Name > View | L | ąąC1. Lateral and medial fusiform gyrus (37) | 1,280 | 22L, 57P, 4I | 6.2 | .32±.03 |
| AD patients | ||||||
| Name > View | R | C1. Lateral and medial fusiform gyrus (37) | 1,216 | 38R, 57P, 12I | 5.4 | .30±.02 |
Clusters shown survived our cluster threshold alpha-protection procedure (p<.05, volume > 448 mm3; see text for details). Note: L = left, R = right, A = anterior, P = posterior, S = superior, I = inferior, C = cluster.
brain response negatively correlated with naming accuracy performance (p<.05)
Figure 1.
Brain response to object naming vs. viewing pixelated images in the fusiform gyrus ROI. Thresholded and clustered results (protecting an ROI-wise p≤.05) for a single-sample t-test are presented in the top panel for the CN participants and the bottom panel for the AD participants with the corresponding hemodynamic response function for each category by group. Warm colors represent areas more active during object naming than passive viewing (red: p≤.05, orange: p≤.01, yellow: p≤.005). Results are overlaid onto coronal slices of a high-resolution anatomical image (L: left, P: posterior).
Whole brain analysis
Within-group analysis
Whole brain voxel-wise within-group t-test analysis revealed multiple areas of task-related brain response in both groups (Table 4). Specifically, CN adults showed task-related activation when naming objects compared to viewing pixelated images in a large region that included the lateral and medial frontal cortex bilaterally extending to the left and right inferior temporal cortex, and in several smaller posterior regions that included the right precentral gyrus, superior and middle temporal gyri, the left inferior parietal lobe, the right middle and inferior occipital gyrus extending to the lingual gyrus, the right postcentral gyrus and precuneus, the right posterior middle temporal gyrus and angular gyrus, the left postcentral gyrus, and the right cerebellum. In contrast, they showed greater activity for viewing pixelated images vs. object naming in regions of the right temporal pole and anterior superior temporal gyrus, the right caudate, and the right and left cingulate gyrus. The AD patients showed a widespread task-related brain response when naming objects vs. viewing pixelated images that encompassed large regions of the left frontal and temporal cortex extending to the right medial frontal cortex and thalamus bilaterally, and smaller clusters of activity localized to the right perisylvian cortex, right cuneus, right superior temporal gyrus, right culmen and left lingual gyrus, right precentral gyrus, and right middle temporal gyrus extending to the angular and supramarginal gyri. No clusters of activity for viewing pixelated images vs. naming objects were observed within the AD group.
Table 4.
Clusters of significant brain response across the whole brain for within subject contrast of picture naming vs. passive viewing and 2 group × 3 category ANOVA.
| Direction of response | Hemisphere | Subregion (Brodmann’s area) |
Volume (mm3) |
Coordinates of maximum intensity voxel |
t or F-score for maximum intensity voxel |
η2(Mean ±SEM) |
|---|---|---|---|---|---|---|
| NC adults | ||||||
| Name > View | R & L | R & L lateral frontal cortex, R & L medial frontal cortex, R & L inferior temporal cortex | 179,072 | 46L, 13P, 36S | 7.6 | .34±.02 |
| R | Precentral gyrus, superior and middle temporal gyrus | 10,752 | 42R, 13P, 32S | 6.1 | .31±.05 | |
| L | Inferior parietal lobe | 9,280 | 26L, 53P, 40S | 4.7 | .30±.05 | |
| R | Middle and inferior occipital gyrus, lingual gyrus | 4,352 | 38R, 69P, 8I | 4.3 | .28±.06 | |
| R | Postcentral gyrus, precuneus | 2,560 | 10R, 37P, 64S | 5.2 | .29±.09 | |
| R | Middle temporal gyrus, angular gyrus | 2,240 | 30R, 61P, 28S | 4.2 | .28±.08 | |
| L | Postcentral gyrus | 2,048 | 22L, 37P, 60S | 5.2 | .31±.01 | |
| R | Cerebellum | 1,984 | 2R, 57P, 28I | 4.4 | .30±.01 | |
| View > Name | ||||||
| R | Temporal pole, superior temporal gyrus | 3,456 | 38R, 23A, 20I | −3.6 | .27±.04 | |
| R | Caudate | 3,328 | 22R, 33P, 24S | −4.7 | .27±.08 | |
| R | Cingulate gyrus | 1,920 | 10R, 17P, 28S | −6.3 | .34±.02 | |
| L | Cingulate gyrus | 1,728 | 6L, 17P, 24S | −3.9 | .28±.09 | |
| AD patients | ||||||
| Name > View | R & L | L lateral frontal cortex, L superior temporal lobe, L superior parietal lobe, L & R medial frontal cortex, L & R inferior temporal cortex, L & R thalamus | 185,088 | 38L, 3A, 12S | 10.3 | .32±.02 |
| R | Perisylvian cortex (inferior frontal gyrus, insula, superior temporal gyrus) | 12,992 | 38R, 7A, 20S | 7.3 | .31±.06 | |
| R | Precuneus | 10,752 | 14R, 29P, 60S | 5.6 | .31±.05 | |
| R | Superior temporal gyrus | 8,192 | 50R, 29P, 8S | 6.6 | .31±.07 | |
| R & L | R culmen, L lingual gyrus | 3,968 | 6R, 65P, 8I | 5.9 | .30±.09 | |
| R | Precentral gyrus | 2,560 | 58R, 9P, 24S | 3.8 | .29±.07 | |
| R | Middle temporal gyrus, angular gyrus, supramarginal gyrus | 1,920 | 42R, 69P, 28S | 7.0 | .31±.02 | |
| Main Effect of Group | ||||||
| AD > NC | ||||||
| R & L | C1. Anterior cingulate (32, 24) | 3,392 | 10R, 35A, 12S | 16.6 | .67±.03 | |
| AD > NC | R | ąąC2. Inferior frontal gyrus, pars triangularis (45), pars orbitalis (47), pars opercularis (44), insula (13) | 3,328 | 46R, 27A, 4S | 11.2 | .59±.02 |
| NC > AD | R | C3. Middle frontal gyrus (10, 9) | 1,728 | 30R, 43A, 12S | 16.0 | .71±.05 |
| Main Effect of Category | ||||||
| Veh > Ani > Tool | R & L | *C1. Anterior cingulate, cingulate gyrus, posterior cingulate (24, 31, 32) | 94,400 | 10L, 43A, 4I | 61.5 | ,88±.02 |
| Veh > Ani = Tool | R | C2. Superior temporal gyrus, insula (22, 41, 42, 13) | 2,432 | 50R, 5P, 4I | 8.8 | .46±.02 |
| Ani = Veh > Tool | R | C3. Rostral cingulate zone (32) | 2,432 | 14R, 31A, 20S | 9.2 | .49±.03 |
| Ani > Tool = Veh | L | C4. Inferior frontal gyrus, pars triangularis (45) | 1,600 | 34L, 19A, 12S | 8.2 | .45±.03 |
| Interaction of Group × Category | ||||||
| R | *C1. Superior temporal gyrus (39) | 6,208 | 38R, 45A, 0 | 13.0 | .49±.02 | |
| L | *C2. Pre-SMA (6, 24) | 3,200 | 10L, 15P, 44S | 8.0 | .45±.02 | |
| R | *C3. Inferior frontal gyrus, pars triangularis (45), pars opercularis (44), pars orbitalis (47), insula (13) | 2,432 | 42R, 11P, 8S | 7.1 | .49±.02 | |
| L | *C4. Inferior parietal lobe, supramarginal gyrus (40, 39) | 2,432 | 54L, 37P, 24I | 6.7 | .45±.02 | |
| L | *C5. Inferior parietal lobe (40) | 2,048 | 30L, 33P, 40I | 6.3 | .43±.02 | |
| R | C6. Middle frontal gyrus (9, 10) | 1,920 | 38R, 43P, 8I | 10.9 | .52±.04 | |
| R | *C7. Inferior parietal lobe, supramarginal gyrus (40) | 1,728 | 34R, 25P, 16I | 7.6 | .47±.02 | |
| L | *C8. Anterior cingulate (24, 6) | 1,664 | 18L, 13P, 44I | 9.2 | .53±.04 |
Clusters shown survived our cluster threshold alpha-protection procedure (p<.05, volume>1536 mm3, whole brain p<.05; see text for details). Note: L = left, R = right, A = anterior, P = posterior, S = superior, I = inferior, C = cluster.
Cluster survived cluster threshold alpha-protection procedure in post-hoc comparison of a subset of participants matched for whole brain volume
brain response negatively correlated with naming accuracy performance (p<.05) for CN group
Between group analysis
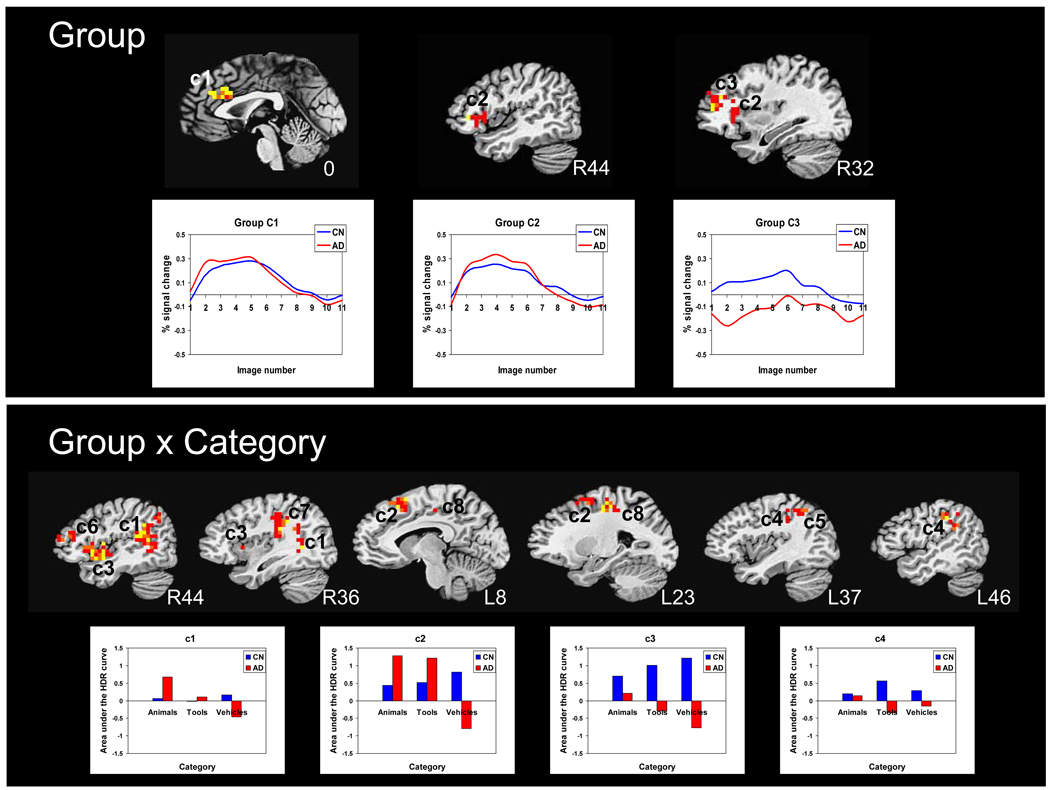
Results of the whole-brain voxel-wise group × category ANOVA revealed main effects of group in 1) the anterior cingulate (BA 32, 24) bilaterally, 2) the right inferior frontal gyrus involving pars triangularis, pars orbitalis, pars opercularis, and the insula, and 3) the right middle frontal gyrus (BA 10, 9) (Table 4). The AD patients showed greater activity than the CN adults in the anterior cingulate and right inferior frontal gyrus, whereas the CN group showed greater activity than the AD group in the right middle frontal gyrus. Analysis of the temporal dynamics of the HDR revealed that the group × image number interaction was not significant for the anterior cingulate gyrus (C1) [F(9, 270) = 1.05, p = .40] or the right inferior frontal gyrus (C2) [F(9, 270) = 1.44, p = .17] clusters, but was significant for the right middle frontal gyrus (C3) [F(9, 270) = 2.00, p = .04] cluster. In the CN group, task-related brain response in the right inferior frontal gyrus (C2) was significantly negatively correlated with naming accuracy (r=−0.45, p=.05).
A main effect of category was found in a large cluster extending the length of the cingulate gyrus (C1) (vehicles greater than animals and animals greater than tools), in the right superior temporal gyrus extending to the insula (C2) (vehicles greater than animals and tools), the right rostral cingulate zone (C3) (animals and vehicles greater than tools), and the left inferior frontal gyrus involving pars triangularis (C4) (animals greater than tools and vehicles). Analysis of the temporal dynamics of the HDR revealed that the category × image number interaction was significant for the anterior cingulate (C1) [F(18, 837) = 1.64, p = .04] (the HDR for vehicles showed a delayed return to baseline). The category × image number interaction was not significant for the other three active clusters [right superior temporal gyrus (C2): F(18, 837) = 1.11, p = .34; right rostral cingulate zone (C3): F(18, 837) = 0.72, p = .79; and left inferior frontal gyrus (C4) F(18, 837) = 1.47, p = .09].
A group × category interaction was found in several regions, including: 1) the right superior temporal gyrus, 2) left pre-supplementary motor area (pre-SMA), 3) right inferior frontal gyrus and insula, 4) left lateral inferior parietal lobe, 5) left medial inferior parietal lobe, 6) right middle frontal gyrus, 7) right inferior parietal lobe, and 8) left anterior cingulate. In the largest cluster (e.g., the right superior temporal gyrus), the CN group showed little categorical differentiation in HDR, whereas the AD group showed preferential response for animals. In the left pre-SMA (C2), both groups showed increased brain response to animals and tools (AD adults to a greater degree), but the CN adults showed greatest response to vehicles whereas the AD adults showed a negative response to vehicles. In the right inferior frontal gyrus (C3), the CN group showed a graded brain response for vehicles greater than tools and tools greater than animals. The left inferior parietal lobe (C4) showed selective brain response for tools in the CN group. In contrast, the AD group showed a weak selective brain response for animals in both these regions. Analysis of the temporal dynamics of the HDR revealed that the category × image number interaction effect was significant only for the cluster in the left inferior parietal lobe (C4) and restricted to the AD group [F(18, 243) = 2.42, p = .000]. The HDR for vehicles showed greater fluctuation in amplitude over the time domain than did other categories. No correlations were found between naming performance and brain response in clusters that showed significant activation in category main effects or group × category interaction effects.
Anatomical results
Anatomical image data from one CN participant did not pass quality control evaluation for brain volume analyses, and anatomical image data from one AD participant did not pass quality control evaluation for cortical thickness analyses. These participants were excluded from further anatomical analysis. After controlling for total intracranial volume, age, and gender, group differences were found for total brain volume, cerebral gray matter volume, and left and right hippocampal volumes (AD less than CN for all regions). The CN and AD groups did not differ in volume of cerebral white matter or white matter lesions (Table 1). After controlling for age and gender, the AD group had reduced cortical thickness in regions of interest that included the right fusiform gyrus, right pars triangularis, right pars opercularis and left and right posterior cingulate (Table 1).
Since the AD group had significantly lower whole brain volume than the CN group, the 2 group × 3 category repeated measures ANOVA was conducted on a subset of the participants with similar total brain volume (AD n=7, CN n=14). Whole brain results on this subset of participants were relatively consistent with the results including the entire sample, suggesting that differences in brain volume did not largely contribute to differences in functional brain response between groups (see Table 4). Specifically, previously observed group by category interaction effects survived statistical threshold procedures in all clusters except the right middle frontal gyrus (C6). Only the large cingulate cluster survived statistical threshold procedures for the main effect of category, and none of the original clusters survived statistical threshold procedures for the main effect of group.
Discussion
Our results indicate that the neural correlates of semantic knowledge related to object naming are altered in AD. Patients with AD demonstrated increased response in a few language-related frontal brain regions during picture naming (collapsed across semantic categories) compared to healthy control participants. In addition, group by category interaction effects on brain activity were observed in language areas that included the right inferior frontal gyrus extending to the anterior insular cortex (e.g., CN showed a graded response of vehicles > tools > animals that AD did not), the right superior temporal gyrus (e.g., CN showed equal activation across categories while AD showed greater response to animals than to tools or vehicles), the pre-supplementary motor area bilaterally (e.g., CN showed response to all categories while AD showed response to animals and tools but a negative response to vehicles), left and right supramarginal gyrus, the left inferior parietal lobe (e.g., CN showed a selective response to tools but AD showed a weak selective response to animals), and the middle frontal gyrus. When considered with previous work showing no group by category interaction effects on brain activation in older and younger adults, and such effects in only two brain areas in those elderly at genetic risk for AD (Wierenga et al., 2010), the present results suggest a continuum of semantic impairment across the spectrum of dementia risk that culminates in widespread changes in AD.
Another aim of the present study was to examine whether the semantic memory impairment in AD differentially involves the neural correlates of category or feature processing. To address this question we compared brain responses across three categories (animals, tools, and vehicles) for which processing of specific visual features (global form vs. local detail) could be dissociated from processing of semantic category (e.g., living vs. nonliving). In regions showing a group × category interaction effect, CN individuals tended to show tightly coupled HDRs with expectedly graded selectivity for category, while patients with AD showed atypical or exaggerated categorical distinctions in their HDRs. It should be noted, however, that a consistent pattern of response corresponding to living vs. nonliving categories or global vs. local attributes was not clearly evident. For example, the brain response of AD patients in the right superior temporal gyrus was selective for animals, but in the left pre-supplementary motor area their brain response for animals and tools was similar with a largely negative response for vehicles. Both the AD and CN groups exhibited a categorical differentiation in HDR in the right inferior frontal region of interest, but in the opposite direction: AD patients showed greater brain response for animals than for tools or vehicles, while CN individuals showed greater brain response for vehicles than for tools, and greater for tools than for animals. Group differences in the fusiform gyrus did not survive statistical thresholding. However, the AD group showed a cluster of brain activity in the right fusiform gyrus for naming vs. baseline that had greater amplitude for vehicles than for tools, and for tools than for animals. In contrast, the CN group showed brain activity in the left fusiform gyrus for naming vs. baseline with activity for animals and vehicles having slightly greater amplitude than for tools.
The inconsistent pattern of categorical differentiation in the brain activity of patients with AD during the object naming task does not provide evidence for preferential damage to either living or nonliving items but rather suggests a graded semantic deficit. Additionally, the AD brain does not clearly respect the distinction in global vs. local feature processing, suggesting possible deterioration of perceptual featural processing (Chan et al., 1998; Chan et al., 1997; Chan et al., 1993; Chan et al., 2001). Our findings are most consistent with contemporary PDP models of semantic memory that posit semantic knowledge arises from the convergence of interactive activation of representations of objects (e.g., either modality-specific or feature-driven) that are distributed throughout the cortex. Regions of significant BOLD group differences may be reflective of the convergence of this information in the brain (e.g., Roger’s “cross-modal hub”). Aronoff et al.’s (2006) assertion that the connections among distributed sets of features are randomly damaged in AD, resulting in semantic deficits, may also explain the variability in our findings (e.g., lack of clear evidence for neural instantiation of a category-specific deficit). While Aronoff’s view suggests that loss of featural knowledge drives the semantic memory deficit in AD, it acknowledges that the entire network underlying a word’s meaning is not necessarily damaged. Thus, AD distorts the “semantic space” but may leave semantic knowledge partially intact in the early stages of disease, with more comprehensive losses expected as the disease progresses (Aronoff et al., 2006).
Consistent with our prediction and some previous results (Wierenga et al., 2009), patients with AD exhibited a larger word retrieval-related brain response (collapsed across category) than CN individuals in the right inferior frontal gyrus and the left and right anterior cingulate (including the rostral cingulate zone). This increased activity in task-related brain regions may reflect neural compensatory mechanisms invoked to support cognitive processing in the face of cortical compromise. Furthermore, the observed increase in brain response in the right inferior frontal gyrus in the AD group is also seen in normal aging (Wierenga et al., 2008) and can be interpreted in light of Cabeza’s (2002) hemispheric asymmetry reduction in old adults (HAROLD) model. According to this model, the activity observed in contralateral areas in the right hemisphere reflects increased neurocognitive effort to maintain an equivalent level of performance (Hernandez, 2009). These compensatory mechanisms may not be uniformly invoked throughout the brain, however, since the CN group showed greater brain response than the AD group in the right middle frontal gyrus.
In contrast to previous reports that AD patients show a delayed BOLD response, we observed no differences in the time course of the HDR in the AD and CN groups. However, we may have reduced our ability to detect changes in the temporal dynamics of the HDR by excluding individuals with cerebrovascular disease in an attempt to decrease the possibility of confounding compromised vascular responses with changes in cognitive processing. Because the AD and CN groups did not differ on quantitative measures of cerebrovascular integrity (e.g., white matter hyperintensities), it is unlikely that group differences in vascular response contributed to observed BOLD effects. Future studies should include direct measures of vascular responsiveness (e.g., hypercapnia) to adequately assess changes in the physiological basis of group differences in the BOLD response.
Several factors that could influence the observed differences in the brain responses associated with semantic processing in the AD patients and CN individuals should be considered. First, patients with AD performed less accurately than CN individuals when naming tools and vehicles, and responded more slowly when naming in all categories, raising the possibility that the present results reflect differences in difficulty and retrieval effort rather than differences in the processing of stimulus category or global/local information. It should be noted, however, that activation in some brain areas showed a double dissociation across groups and categories that makes the influence of task difficultly unlikely. For example, in the right inferior frontal area CN individuals showed greater brain response when processing vehicles and tools than when processing animals, whereas AD patients showed greater brain response when processing animals than when processing tools or vehicles. Furthermore, the level of brain response in many other activated regions did not consistently correspond to level of task performance. For example, AD patients showed elevated BOLD response in some regions when naming tools, but decreased BOLD in others, even though they were less accurate in naming tools than animals, and faster at naming tools than vehicles. The lack of correlation between brain response and naming performance would not necessarily be expected if level of brain activity were driven by task difficulty. It may be the case that group differences in brain areas (e.g., medial prefrontal cortex and right inferior frontal gyrus) that were activated when categories were collapsed might reflect the influence of task difficulty, but differences in brain responses across categories most likely reflects group differences in semantic processing of the objects that were named. The behavioral findings also suggest that AD results in category-specific semantic deficits given that there was a group × category interaction effect in naming accuracy even though stimuli were carefully selected so that categories did not differ in difficulty for younger and older cognitively intact adults, and there were no category differences in naming accuracy in the CN group.
Second, group differences in brain response could be influenced by demographic or genetic factors. However, the AD and CN groups did not differ significantly with regard to age, level of education, family history of AD, sex, or APOE genotype, thereby reducing the likelihood that these factors contributed to the present findings. Third, the AD group had reduced (compared to the CN group) total brain volume, cerebral gray matter volume, left and right hippocampal volumes, and reduced cortical thickness in regions of interest that included the right fusiform gyrus, right pars triangularis, right pars opercularis and left and right posterior cingulate. This raises the possibility that functional changes were related to morphological differences between the groups. However, whole brain results on a subset of participants matched for total whole brain volume were consistent with the results for the entire sample, suggesting that differences in brain volume or thickness did not largely contribute to differences in functional brain response between groups. Importantly, within group analyses that compared task (object naming) to baseline (viewing pixelated images) provided essential confirmation that activity associated with naming occurred in expected regions (including frontal and inferior temporal cortices).
In summary, the widespread group differences we observed in the neural correlates of categorical representation of semantic knowledge provide support for the notion that patients with AD suffer semantic network disruption that does not clearly follow living vs. nonliving categorical distinctions or global vs. local featural distinctions. Both category and feature knowledge may be impacted by AD consistent with the theory that category distinctions emerge from attributes shared by category members. The neuropathology of AD may damage the connections among features that results in observable category-specific differences. Group differences in word retrieval (irrespective of semantic category) were restricted to frontal cortical regions. Patients with AD showed greater than normal brain response in the right inferior frontal gyrus and rostral cingulate zone bilaterally, presumably due to the application of more effortful semantic retrieval. This provides further support for frontal lobe-mediated compensatory mechanisms, and generally concurs with the notion that executive functions mediated by the frontal lobes may be better preserved than temporal lobe-mediated episodic or semantic memory functions during the early period of AD (Mickes et al., 2007).
Figure 2.
Whole brain response to object naming vs. passive viewing overlaid onto sagittal slices of a high-resolution anatomical scan. Thresholded and clustered results (protecting a whole brain p≤.05; red: p ≤.05, orange: p ≤.01, yellow: p≤.005) for the 2 group × 3 category repeated measures ANOVA are presented in the top panel for the main effect of group (with corresponding hemodynamic response functions for each group) and in the bottom panel for the interaction of group × category, with corresponding AUC for each category per group for the four largest clusters (R: right, L: left).
Acknowledgments
This work was supported by National Institute on Aging grants R01 AG012674 (M.W.B.), K24 AG026431 (M.W.B.) and P50 AG05131 (D.P.S.), Alzheimer’s Association grants IIRG 07-59343 (M.W.B.), NIRG-07-59143 (A.J.J.), and NIRG 09-131856 (C.E.W.), VA Career Development Awards (A.J.J. and C.E.W.), National Institutes of Health Ruth L. Kirschstein National Research Service Award MH18399-20 (C.E.W.) and NINDS F31 NS059193 (K.J.B.). The authors gratefully acknowledge the assistance of Dr. Lisa Eyler and Sheena Dev, as well as the staff, patients, and volunteers of the UCSD Alzheimer’s Disease Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alathari L, Trinh Ngo C, Dopkins S. Loss of distinctive features and a broader pattern of priming in Alzheimer's disease. Neuropsychology. 2004;18(4):603–612. doi: 10.1037/0894-4105.18.4.603. [DOI] [PubMed] [Google Scholar]
- Aronoff JM, Gonnerman LM, Almor A, Arunachalam S, Kempler D, Andersen ES. Information content versus relational knowledge: semantic deficits in patients with Alzheimer's disease. Neuropsychologia. 2006;44(1):21–35. doi: 10.1016/j.neuropsychologia.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Astell AJ, Harley TA. Tip-of-the-tongue states and lexical access in dementia. Brain Lang. 1996;54(2):196–215. doi: 10.1006/brln.1996.0071. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Run HV. The CELEX Lexical Database. Philadelphia: Linguistic Data Consortium; 1993. [Google Scholar]
- Barbarotto R, Capitani E, Jori T, Laiacona M, Molinari S. Picture naming and progression of Alzheimer's disease: an analysis of error types. Neuropsychologia. 1998;36(5):397–405. doi: 10.1016/s0028-3932(97)00124-3. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12(2):298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK, Cruz RF. Performance of Alzheimer's disease patients in judging word relatedness. J Int Neuropsychol Soc. 1999;5(7):668–675. doi: 10.1017/s1355617799577096. [DOI] [PubMed] [Google Scholar]
- Bayles KA, Tomoeda CK, Trosset MW. Naming and categorical knowledge in Alzheimer's disease: the process of semantic memory deterioration. Brain Lang. 1990;39(4):498–510. doi: 10.1016/0093-934x(90)90158-d. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex. 2007;17(10):2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36(9):539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. Spatial heterogeneity of the nonlinear dynamics in the FMRI BOLD response. Neuroimage. 2001;14(4):817–826. doi: 10.1006/nimg.2001.0873. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Moghekar A. Imaging the medial temporal lobe: exploring new dimensions. Trends Cogn Sci. 2002;6(5):217–223. doi: 10.1016/s1364-6613(02)01881-8. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. 1987;9(5):479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain the animate-inanimate distinction. J Cogn Neurosci. 1998;10(1):1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Salmon D, Nordin S, Murphy C, Razani J. Abnormality of semantic network in patients with Alzheimer's disease. Evidence from verbal, perceptual, and olfactory domains. Ann N Y Acad Sci. 1998;855:681–685. doi: 10.1111/j.1749-6632.1998.tb10645.x. [DOI] [PubMed] [Google Scholar]
- Chan AS, Butters N, Salmon DP. The deterioration of semantic networks in patients with Alzheimer's disease: a cross-sectional study. Neuropsychologia. 1997;35(3):241–248. doi: 10.1016/s0028-3932(96)00067-x. [DOI] [PubMed] [Google Scholar]
- Chan AS, Butters N, Salmon DP, McGuire KA. Dimensionality and clustering in the semantic network of patients with Alzheimer's disease. Psychol Aging. 1993;8(3):411–419. doi: 10.1037//0882-7974.8.3.411. [DOI] [PubMed] [Google Scholar]
- Chan AS, Salmon DP, De La Pena J. Abnormal semantic network for "animals" but not "tools" in patients with Alzheimer's disease. Cortex. 2001;37(2):197–217. doi: 10.1016/s0010-9452(08)70568-9. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2(10):913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer's type. What do various measures measure? Brain. 1990;113(Pt 2):397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Inglis L, Cupples L, Michie P, Budd W. A semantic subsystem of visual attributes. Neurocase. 1998;4:353–370. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Bobholz JA, Gokcay D, Mohr CM, Leonard CM, et al. Activity in the paracingulate and cingulate sulci during word generation: an fMRI study of functional anatomy. Cereb Cortex. 1999;9(4):307–316. doi: 10.1093/cercor/9.4.307. [DOI] [PubMed] [Google Scholar]
- Dale A, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction--A linear-approach. Journal of Cognitive Neuroscience. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Anderson SW. The frontal lobes. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 1993. pp. 409–460. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test-II. New York City: The Psychological Corporation; 2000. [Google Scholar]
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, et al. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132(Pt 8):2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, et al. Selective disruption of the cerebral neocortex in Alzheimer's disease. PLoS One. 2010;5(9):e12853. doi: 10.1371/journal.pone.0012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, et al. Selective disruption of the cerebral neocortex in Alzheimer's disease. PLoS One. 5(9):e12853. doi: 10.1371/journal.pone.0012853. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Gonnerman LM, Andersen ES, Seidenberg MS. Category-specific semantic deficits in focal and widespread brain damage: a computational account. J Cogn Neurosci. 1998;10(1):77–94. doi: 10.1162/089892998563798. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, et al. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30(3):432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done DJ, Hajilou BB. Loss of high-level perceptual knowledge of object structure in DAT. Neuropsychologia. 2005;43(1):60–68. doi: 10.1016/j.neuropsychologia.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Farah MJ, McClelland J. A computational model of semantic memory impairment: Modality specificity and emergent category specificity. Journal of Experimental Psychology: General. 1991;120:339–357. [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard P, Patterson K, Watson PC, Hodges JR. Category specific semantic loss in dementia of Alzheimer's type. Functional-anatomical correlations from cross-sectional analyses. Brain. 1998;121(Pt 4):633–646. doi: 10.1093/brain/121.4.633. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Cortese MJ, Sergent-Marshall SD, Snyder AZ, Salat DH, et al. Differing neuropsychological and neuroanatomical correlates of abnormal reading in early-stage semantic dementia and dementia of the Alzheimer type. Neuropsychologia. 2005;43(6):833–846. doi: 10.1016/j.neuropsychologia.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gollan TH, Salmon DP, Paxton JL. Word association in early Alzheimer's disease. Brain Lang. 2006;99(3):289–303. doi: 10.1016/j.bandl.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, Kempler D, Seidenberg MS. Double dissociation of semantic categories in Alzheimer's disease. Brain Lang. 1997;57(2):254–279. doi: 10.1006/brln.1997.1752. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, et al. Neural basis for semantic memory difficulty in Alzheimer's disease: an fMRI study. Brain. 2003;126(Pt 2):292–311. doi: 10.1093/brain/awg027. [DOI] [PubMed] [Google Scholar]
- Harley TA, Grant F. The role of functional and perceptual attributes: evidence from picture naming in dementia. Brain Lang. 2004;91(2):223–234. doi: 10.1016/j.bandl.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Hernandez AE. Language switching in the bilingual brain: what's next? Brain Lang. 2009;109(2–3):133–140. doi: 10.1016/j.bandl.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33(4):441–459. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. The nature of the naming deficit in Alzheimer's and Huntington's disease. Brain. 1991;114(Pt 4):1547–1558. doi: 10.1093/brain/114.4.1547. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer's disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30(4):301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EM. Hierarchies, similarity, and interactivity in object recognition: "category-specific" neuropsychological deficits. Behav Brain Sci. 2001;24(3):453–476. discussion 476–509. [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A. 1999;96(16):9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23(6):382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph A, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: Evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130:1127–1137. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Laws KR, Adlington RL, Gale TM, Moreno-Martinez FJ, Sartori G. A meta-analytic review of category naming in Alzheimer's disease. Neuropsychologia. 2007;45(12):2674–2682. doi: 10.1016/j.neuropsychologia.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59(9):775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale: professional manual. Odessa, Florida: 1988. [Google Scholar]
- McRae K, de Sa VR, Seidenberg MS. On the nature and scope of featural representations of word meaning. J Exp Psychol Gen. 1997;126(2):99–130. doi: 10.1037//0096-3445.126.2.99. [DOI] [PubMed] [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, Thal LJ, et al. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer's disease. Neuropsychology. 2007;21(6):696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Moss HE, Tyler LK. A progressive category-specific semantic deficit for non-living things. Neuropsychologia. 2000;38(1):60–82. doi: 10.1016/s0028-3932(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakanishi M, Hamanaka T, Nakaaki S, Yoshida S. Semantic priming in patients with Alzheimer and semantic dementia. Cortex. 2000;36(2):151–162. doi: 10.1016/s0010-9452(08)70521-5. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Semantic memory in Alzheimer's disease. Psychol Bull. 1989;106(3):377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Preserved organization of semantic attributes in Alzheimer's disease. Psychol Aging. 1990;5(4):574–579. doi: 10.1037//0882-7974.5.4.574. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Martin DC, Horn LC. Sparing of semantic memory in Alzheimer's disease. J Abnorm Psychol. 1984;93(3):321–330. doi: 10.1037//0021-843x.93.3.321. [DOI] [PubMed] [Google Scholar]
- Norton LE, Bondi MW, Salmon DP, Goodglass H. Deterioration of generic knowledge in patients with Alzheimer's disease: evidence from the Number Information Test. J Clin Exp Neuropsychol. 1997;19(6):857–866. doi: 10.1080/01688639708403766. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paganelli F, Vigliocco G, Vinson D, Siri S, Cappa S. An investigation of semantic errors in unimpaired and Alzheimer's speakers of Italian. Cortex. 2003;39(3):419–439. doi: 10.1016/s0010-9452(08)70257-0. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland J, Hodges JR, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychological Review. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44(3):839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchett C, Humphreys GW. Calling a squirrel a squirrel, but a canoe a wigwam: a category-specific deficit for artefactual objects and body parts. Cognitive Neuropsychology. 1992;9:73–86. [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annu Rev Psychol. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Butters N, Chan AS. The deterioration of semantic memory in Alzheimer's disease. Can J Exp Psychol. 1999;53(1):108–117. doi: 10.1037/h0087303. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer's disease: implications for the integrity of semantic memory. J Int Neuropsychol Soc. 1999;5(7):692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]
- Sartori G, Lombardi L. Semantic relevance and semantic disorders. J Cogn Neurosci. 2004;16(3):439–452. doi: 10.1162/089892904322926773. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Flashman LA, Frutiger SA, Johnson SC, Mamourian AC, Moritz CH, et al. Neuroanatomic substrates of semantic memory impairment in Alzheimer's disease: patterns of functional MRI activation. J Int Neuropsychol Soc. 1999;5(5):377–392. doi: 10.1017/s135561779955501x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, et al. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73(8):612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. From Neuropsychology to Mental Structure. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Silveri MC, Daniele A, Giustolisi L, Gainotti G. Dissociation between knowledge of living and nonliving things in dementia of the Alzheimer type. Neurology. 1991;41(4):545–546. doi: 10.1212/wnl.41.4.545. [DOI] [PubMed] [Google Scholar]
- Stewart F, Parkin AJ, Hunkin NM. Naming impairments following recovery from herpes simplex encephalitis: category-specific? Q J Exp Psychol A. 1992;44(2):261–284. doi: 10.1080/02724989243000037. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. New York: Thiem Medical Publishers; 1988. [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring "how" from "where". Neuropsychologia. 2003;41(3):280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy R. Categories of knowledge: Further fractionations and an attempted integration. Brain. 1987;110:1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Weisberg J, van Turennout M, Martin A. A neural system for learning about object function. Cereb Cortex. 2007;17(3):513–521. doi: 10.1093/cercor/bhj176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40(2):174–186. doi: 10.1016/s0028-3932(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, et al. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008;29(3):436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Perlstein WM, Benjamin M, Leonard CM, Rothi LG, Conway T, et al. Neural substrates of object identification: Functional magnetic resonance imaging evidence that category and visual attribute contribute to semantic knowledge. J Int Neuropsychol Soc. 2009;15(2):169–181. doi: 10.1017/S1355617709090468. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Stricker NH, McCauley A, Simmons A, Jak AJ, Chang YL, et al. Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer's disease. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, T.L B, T.L R, O L, V H, M A, et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zahn R, Garrard P, Talazko J, Gondan M, Bubrowski P, Juengling F, et al. Patterns of regional brain hypometabolism associated with knowledge of semantic features and categories in Alzheimer's disease. J Cogn Neurosci. 2006;18(12):2138–2151. doi: 10.1162/jocn.2006.18.12.2138. [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Carlesimo GA, Pasqualetti P, Caltagirone C. Category-specific impairment in patients with Alzheimer's disease as a function of disease severity: a cross-sectional investigation. Neuropsychologia. 2002;40(13):2268–2279. doi: 10.1016/s0028-3932(02)00110-0. [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Pasqualetti P, Caltagirone C, Carlesimo GA. (Category-specific) semantic deficit in Alzheimer's patients: the role of semantic distance. Neuropsychologia. 2006;44(1):52–61. doi: 10.1016/j.neuropsychologia.2005.04.008. [DOI] [PubMed] [Google Scholar]