Abstract
Peripheral T-cell lymphomas (PTCLs) constitute a group of heterogeneous diseases that are uncommon, representing, in Western countries, only approximately 10% of all non-Hodgkin lymphomas. They are typically associated with a poor prognosis compared to their B-cell counterparts and are much less well understood with respect to tumor biology, due to their rarity and biologic heterogeneity, and to the fact that characteristic cytogenetic abnormalities are few compared to B-cell lymphomas. While the outcome for patients with anaplastic large cell lymphoma (ALCL), particularly ALK-positive ALCL, is good, other types of peripheral T-cell lymphomas are associated with a poor prognosis even with aggressive anthracycline-based chemotherapy. In this respect, there is a need for new approaches in these diseases and this review focuses on and explores recent experience with novel therapies in PTCL.
Background
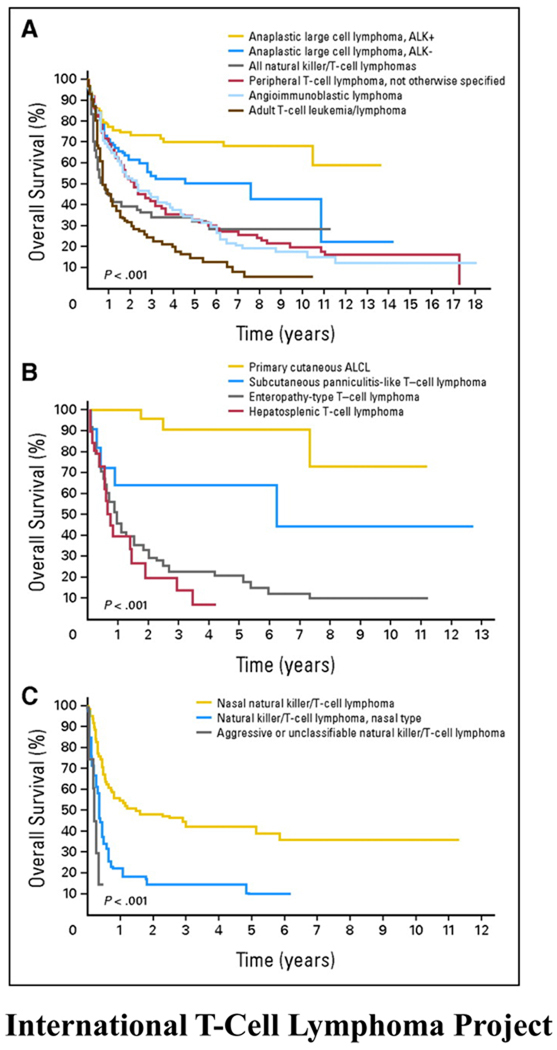
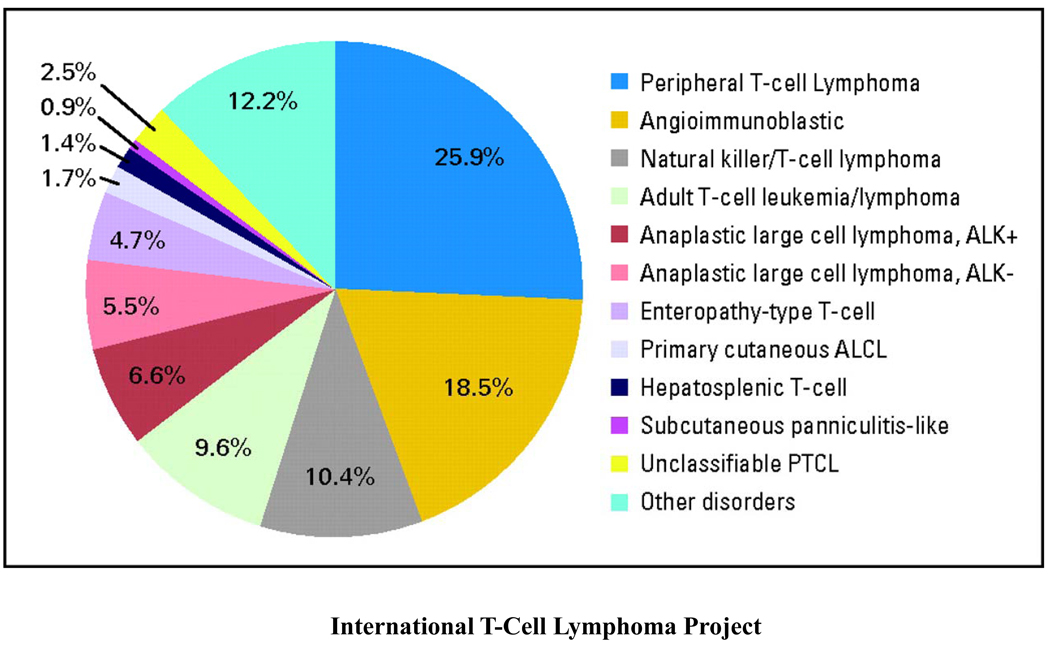
Peripheral T-cell lymphoma represents a heterogeneous group of clinicopathologically defined distinct T-cell lymphomas, the most common of which are peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL) and anaplastic large cell lymphoma (ALCL) (constituting anaplastic lymphoma kinase (ALK) -positive and -negative cases)(figure 1). Rarer subtypes include extranodal natural killer T-cell lymphoma (NKTCL), adult T-cell leukemia/lymphoma (ATLL) and enteropathy associated T-cell lymphoma (EATL). PTCL-NOS are nodal and extranodal mature T-cell lymphomas that do not fit under any of the other specifically defined entities of mature T-cell lymphoma in the current World Health Organization (WHO) classification and will likely be further categorized as gene expression profiling and DNA sequence analysis of these diseases is performed1. The largest evaluation of PTCL to date was performed by the International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study in which a cohort of 1314 patients in 22 centers worldwide was studied (figure 2)2. Though the study was retrospective and therapeutic approaches varied widely, it provided interesting insights into the outcome of these rare lymphomas. While patients with ALCL (particularly ALK-positive) had a good outcome with anthracycline-based therapy (ALK-positive and -negative ALCLs demonstrated 5-year overall survivals (OS) of 70% and 49% respectively), overall survivals for patients with PTCL-NOS and AITL were poor and interestingly, unaffected by the use of anthracyclines in the upfront setting. The 5-year OS for PTCL-NOS, AITL, and all NKTCLs was 32% compared to 14% for ATLL.
Figure 1.
These Kaplan-Meier curves show (A) the overall survivals of patients with the most common subtypes of peripheral T-cell lymphoma (PTCL); (B) the overall survivals of patients with the less common subtypes of PTCL; and (C) the overall survivals of patients with natural killer T-cell lymphoma. (Copied with permission from the International T-cell Lymphoma Project: Vose et al. JCO)
Figure 2.
Shown here is the distribution of 1314 cases by consensus diagnosis. NOS, not otherwise specified; ALCL, anaplastic large-cell lymphoma; PTCL, peripheral T-cell lymphoma
Biology of PTCL
The molecular biology of these diseases is poorly understood. This is at least partially due to the rarity of PTCL and has complicated the investigation and development of new targeted therapies3. The notable exceptions to this are ALK (anaplastic lymphoma kinase)-positive anaplastic large cell lymphomas, most commonly characterized by a translocation t(2;5)(p23;q35) between the ALK gene on chromosome 2 and the nucleophosmin (NPM) gene on chromosome 51,4; T-cell prolymphocytic leukemia (PLL) with translocations of the T-cell leukemia 1 (TCL-1) or mature T-cell proliferation 1 (MTCP-1) gene to chromosome 14, the site of the alpha chain of the T-cell receptor; and adult T cell leukemia/lymphoma (ATLL), the first human neoplasm associated with retroviral infection5. While the t(2:5) translocation is found in over 80% of cases of ALK-positive ALCL, variant translocations involving ALK and other partner genes on various chromosomes can also occur6,7. The transforming NPM-ALK fusion protein that results from the t(2:5) translocation encodes a tyrosine kinase receptor, resulting in the constitutive activation of ALK and its downstream pathways8. ALK can be detected by immunohistochemistry and while in the majority of cases with the classical t(2;5)/NPM-ALK translocation, ALK staining is both cytoplasmic and nuclear, it may be membranous or cytoplasmic in variant cases1. PLL can occur in an inherited form associated with ataxia telangiectasia or in a sporadic form. In both cases, the majority of patients have a translocation of the TCL-1 gene into chromosome 14. TCL-1 and MTCP-1 have a similar protein structure and associate with protein kinase B (Akt) to drive proliferation of the cells9. In ATL, cells infected with HTLV1 integrate the viral RNA and express the tax gene, which induces an autocrine growth loop through upregulation of interleukin 2 and its receptor. This immortalizes the infected T cells and presumably, over the course of decades, allows the accumulation of genetic defects that results in overt malignancy. The only other molecular aberration identified in PTCL is isochromosome 7q, which is associated with hepatosplenic T-cell lymphoma. Most other T-cell lymphomas have not been defined molecularly10. ALK negative ALCL is poorly understood and its inferior outcome suggests a derivation distinct from that of ALK-positive cases although gene expression profiling has demonstrated some overlap in expression patterns of kinases and apoptosis inhibitors, suggesting a common pathogenic mechanism. While the pathogenesis of AITL is also poorly elucidated, recent work has demonstrated overexpression of the chemokine CXCL13 by the neoplastic cells suggesting derivation from follicular helper T-cells11–13.
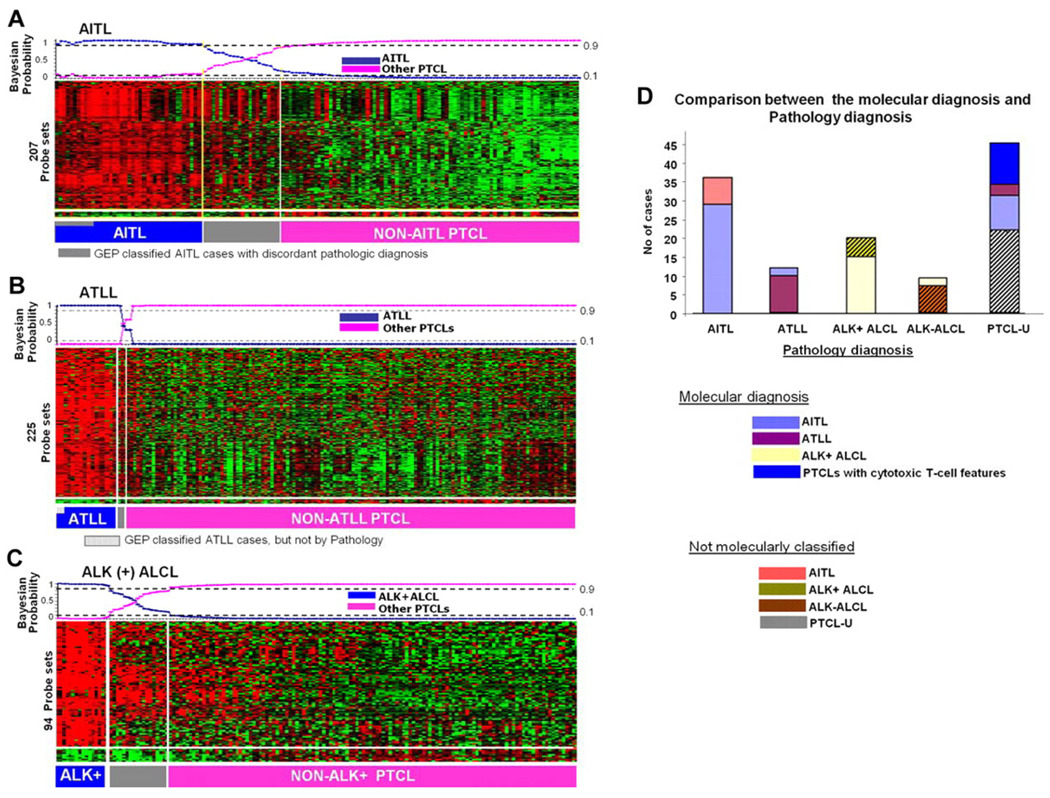
Though gene expression profiling studies in PTCL lag behind those in B-cell lymphomas, some recent studies, albeit with small numbers of patients due to the infrequency of these diseases, have suggested that the histopathologically defined PTCL subtypes have distinct molecular profiles3,14–16. Molecular classifiers have been constructed for AITL, ALK-positive ALCL and ATLL (figure 3). In a recent study where gene expression profiling was performed on 144 cases of PTCL, the identification of a molecular subgroup, with features of cytotoxic T lymphocytes and a poor survival compared to the remaining PTCL-NOS cases, suggests that PTCL-NOS is a molecularly heterogeneous entity3. This heterogeneity of lymphomagenesis makes it challenging to identify selective targets for drug development.
Figure 3.
Shown here are gene-expression-based molecular predictors of the major subgroups of PTCL from the International Peripheral T-Cell Lymphoma Project. (A) AITL, (B) ATLL, and (C) ALK+ ALCL. (D) illustrates the correlation between the molecular and the pathology-based diagnoses.
The biological basis for the poor treatment outcome for patients with PTCL, in contrast to patients with aggressive B-cell lymphomas, is not well understood. In the future, studies that incorporate technology such as microarray (that can interrogate the expression of thousands of genes) or deep DNA sequencing (that can identify all genetic changes) will likely help elucidate which distinct pathways are activated and transformed and provide insights into the role of epigenetic changes in these diseases.
Treatment of PTCL
With the exception of ALK-positive ALCL, which is highly curable with CHOP-like therapy and has a 5 year overall survival that approaches 80%, the outcome for patients with a new diagnosis of PTCL who receive anthracycline-based therapy is poor and most patients relapse soon after initial treatment and die from their disease2. While historically, many studies in PTCL have included patients with ALCL, studies confined to PTCL-NOS using CHOP or CHOP-like regimens show disappointing outcomes with long-term survivals in the range of 20%17. In one study of 36 newly diagnosed patients with PTCL-NOS who received CHOP-based chemotherapy, one and two year overall survivals were 61% and 25%, respectively. In the International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study, while over 85% of patients received an anthracycline-containing regimen, the 5 year overall survival for patients with PTCL-NOS, AITL and NKTCLs was merely 32%. These results confirm the poor efficacy of current standard therapies and argue for the evaluation of new agents in these diseases. Over the past few years, a number of novel therapies with efficacy in the relapsed setting have emerged for PTCL. The suboptimal outcome for newly diagnosed patients with current standard therapeutic approaches suggests that novel agents should be investigated in clinical trials in the upfront setting in a window of opportunity approach to define their efficacy and their molecular mechanisms. Autologous bone marrow transplantation following second line therapy with regimens like ICE and DHAP, can be curative for patients with relapsed disease, particularly for patients with ALK-positive ALCL18. The experience of allogeneic transplantation in relapsed PTCL is limited to a small number of studies and its role continues to be investigated19,20. Further studies to define the optimal conditioning regimens and the group of patients who would benefit most from allogeneic transplantation are necessary.
On the Horizon
Pralatrexate
Pralatrexate was the first drug approved specifically for use in PTCL by the Food and Drug Administration (FDA) based on its single agent activity in relapsed/refractory T-cell lymphoma21. It is a targeted anti-folate and specifically, a novel 10-deazaaminopterin, structurally similar to methotrexate, but rationally designed to have greater affinity for the reduced folate carrier (SLC19A1 or RFC-1) (a fetal oncoprotein highly expressed on fetal and malignant tissue and the principal transporter through which folates and anti-folates enter the cell), enabling the drug to be selectively accumulated in tumor cells22.
Based on preclinical modeling in both T and B cell lymphoma cell lines, which demonstrated better cytotoxicity for pralatrexate compared to methotrexate (in lymphoma cytotoxicity assays, pralatrexate had at least a 1-log lower 50% inhibitory concentration than methotrexate), a phase I/II study was undertaken in patients with both T and B-cell lymphomas23,24. While an every other week dose of 135mg/m2 was associated with problematic stomatitis, a weekly schedule of 30mg/m2 for 6 of 7 weeks, with folate and vitamin B12 supplementation, abrogated significant stomatitis that had been seen with the every other week schedule and reduced other toxicities25. Though minimally effective in B-cell lymphoma, the activity in T-cell lymphoma was significant with complete and partial response rates of 28% and 24% respectively in 25 patients with relapsed/refractory T-cell lymphoma who received the once weekly schedule. Responses were seen across various types of PTCL including many patients with PTCL-NOS. In responders, over 50% achieved a durable remission of at least 6 months. Following on from the results of this study, an international phase II study in PTCL (PROPEL) was carried out22, and was the basis for the accelerated approval granted by the FDA in 2009. In 109 evaluable patients, the overall response rate was 28% with 9% of patients achieving a complete response – the median duration of response was 9.4 months. In this multi-center study, the principal toxicities were thrombocytopenia, mucositis, neutropenia and anemia. Shifting to a weekly schedule and treatment with folate and vitamin B12 has mitigated the risk of mucositis. The full clinical potential of pralatrexate in combination with other agents has yet to be determined. Recent studies have demonstrated synergy with agents such as gemcitabine, paving the way for combination studies26.
Histone deacetylase inhibitors
Acetylation of proteins is a post-translational modification that occurs on the e-amino side chain of lysine amino acids; deacetylases are a family of enzymes that remove these acetyl groups. To date, histones have been the most characterized proteins for the post-translational modification of acetylation. As a result, agents that block the activity of deacetylases are commonly referred to as histone deacetylase inhibitors (HDIs). Furthermore, the most commonly accepted hypothesis for their mechanism of antitumor activity is thought to be the ability of these agents to regulate gene expression. They have been shown to alter gene expression in a wide variety of tumor types and specifically have been shown to mediate increased expression of cell cycle regulators, cell type-specific differentiation genes, tumor antigens, and genes encoding pro-apoptotic proteins27–30. However, with the discovery that multiple other proteins undergo post-translational modification by acetylation, including numerous nuclear and cellular proteins, mechanisms of anti-tumor activity independent of regulation of gene expression through histones are potentially as important31.
Romidepin (FK228 or depsipeptide) was the first HDAC inhibitor to demonstrate efficacy in patients with PTCL or cutaneous T-cell lymphoma (CTCL). In a report of 4 patients treated on a phase 1 study, 1 patient with PTCL-NOS had a CR and 3 patients with cutaneous T-cell lymphoma (CTCL) a PR, prompting a phase II trial to assess its efficacy in patients with CTCL or PTCL29,32. Romidepsin is administered at a dose of 14 mg/m2 given on days 1,8 and 15 of an every 28-day cycle. An overall response rate of 34% and a CR rate of 6% (with a median duration of response of 13.7 months) in 71 patients with CTCL was reported from a multi-institutional study and in a separate registration trial of 96 patients, a similar overall response rate of 34% and CR rate of 6% was observed33,34 The pooled data from these two trials were the basis of the FDA approval of this agent for patients with CTCL. Romidepsin was also studied in patients with PTCL in a multi-center study; of 46 evaluable patients, the overall response rate was 33% with a complete response rate of 11% - the median duration of response was 9 months35. Based on these results, a confirmatory international study of romidepsin in PTCL is ongoing. The principal drug-related toxicities were fatigue, nausea, and thrombocytopenia. EKG changes consisting of T wave flattening are noted in a majority of patients but have not been associated with cardiac damage36. Electrolyte replacement to maintain high-normal potassium and magnesium and concurrent administration of antiemetics, integral to ongoing studies, have made romidepsin well-tolerated in both the CTCL and PTCL patient populations33. Future clinical trials that will combine romidepsin with other agents in PTCL are important to improve efficacy and outcome.
Belinostat (PXD101) is another pan-HDAC inhibitor that has demonstrated activity in both CTCL and PTCL. Recently, its activity in PTCL was reported37. Patients received a dose of 1000 mg/m2 IV on days 1 to 5 of a 3-week cycle and in 20 patients, the overall response rate was 25% with a CR rate of 10%.
At this point in time, there is no data available on the efficacy of the other HDIs in patients with PTCL, but it is very possible that these will also show efficacy given the apparent activity of the entire class of agents in T cell lymphomas. These include vorinostat, already FDA approved for the treatment of refractory CTCL, panobinostat, and entinostat38,39.
Denileukin Diftitox
Denileukin diftitox (Ontak), a recombinant fusion protein, consists of interleukin-2 (IL-2) genetically fused with diptheria toxin. It binds to the IL-2 receptor (IL-2R) and the fusion toxin is endocytosed and cleaved, resulting in the release of the active diptheria toxin. This leads to ADP ribosylation of the eukaryotic translation factor - elongation factor 2 - with subsequent inhibition of protein synthesis and cell death. The limited expression of the IL-2R on activated T cells and T regulatory cells and its high level of expression on many hematologic malignancies make it an attractive agent for clinical evaluation.
Denileukin diftitox was approved by the FDA for patients with refractory or relapsed CD25-positive CTCL based on its activity in this disease40,41. It has also shown some efficacy in PTCL42 and is currently being tested in combination with CHOP chemotherapy for patients with minimally treated PTCL. A phase II trial that evaluated a dose of 18µg/kg daily for 5 days every 3 weeks preliminarily reported an overall response rate of 48% and a complete response rate of 22% with minimal toxicity42. A higher response rate was observed in patients with CD25+ tumors (61.5% versus 45.4%) and the median progression-free survival was 6 months. At present, denileukin difitoxin administration in the setting of autologous transplantation is being investigated in PTCL.
Novel Monoclonal Antibodies
Alemtuzumab
Alemtuzumab (Campath) is a humanized anti-CD52 monoclonal antibody that is approved by the FDA for relapsed and untreated B-cell chronic lymphocytic leukemia (B-CLL) but also has efficacy in T-cell lymphomas, particularly T cell prolymphocytic leukemia (PLL). Alemtuzumab was evaluated in 39 patients with PLL and produced responses in 76% (60% CR and 16% PR)43. The median survival of patients was 10 months, but reached 16 months for patients who achieved a CR. In a pilot study, it was investigated in 14 patients with heavily pretreated relapsed or refractory PTCL44. Patients received a rapidly escalating dose during the first week followed by 30 mgs, 3 times per week, for a maximum of 12 weeks. Though associated with significant hematological toxicity and infectious complications (these included military tuberculosis, herpes zoster and pulmonary aspergillosis), the overall response rate was 36% with 21% of patients achieving a complete response. This study demonstrated that alemtuzumab has antitumor activity in PTCL but the toxicity suggests that lower doses/different schedules should be explored. Sustained remissions with alemtuzumab have been reported in patients with AITL45. Though its spectrum of activity and mechanism of action have not yet been elucidated, it likely works through a combination of different pathways that include complement-dependent cytolysis, antibody-dependent cellular cytotoxicity (ADCC) and apoptosis. Alemtuzumab has been combined with doxorubicin-based chemotherapy and although associated with a high response rate, treatment related toxicity has been problematic over several studies 46. Intravenously administered alemtuzumab appears to be associated with a higher mortality rate than subcutaneous administration, again cautioning that dose and schedule need to be optimized 47–49. A phase III trial of CHOP-14 with or without subcutaneous alemtuzumab is currently ongoing.
SGN-30
CD30 is a cell membrane protein of the tumor necrosis factor receptor family and a regulator of cell growth and apoptosis50. CD 30 ligation with monoclonal antibodies or CD30 ligand produced apoptosis of ALCL cell lines in vitro and in murine xenograft models51–54. Based on activity in animal models, anti-CD30 monoclonal antibodies were tested in CD30-positive hematologic malignancies. SGN-30 (anti-CD30 mAB) is a chimeric anti-CD30 monoclonal antibody that demonstrated potent preclinical antitumor activity in both Hodgkin lymphoma and ALCL50. In a phase I study where the drug was administered once weekly, there was modest activity in ALCL and HL55. Adverse events were mild and the maximum tolerated dose (MTD) was not reached. A subsequent phase II study (where again the drug was administered according to a weekly schedule) treated 41 patients with ALCL. The objective response rate in this group was 17.1% and interestingly all the responses were in patients with ALK-negative disease, who typically have a worse prognosis than ALK-positive cases56. Currently, there are ongoing clinical trials combining SGN-30 with combination chemotherapy.
MDX-060
MDX-060 is a fully human anti-CD30 immunoglobulin (Ig) G1 κ monoclonal antibody that binds to the CD30 ligand with nanomolar affinity57. It has been shown to inhibit growth of CD30 expressing tumor cells in preclinical models58. It inhibits cellular proliferation through modulation of signaling and induces ADCC58. In a phase I/II study, in patients with recurrent HL and ALCL, the drug was administered on a once weekly schedule, up to a dose of 15mg/kg - the maximum tolerated dose was not reached57. Although this study only enrolled 7 patients with ALCL, 2 (28%) of these had a complete response. Interestingly, these were patients who had predominantly skin involvement by their lymphoma.
SGN-35
SGN-35 (brentuximab vedotin) is an antibody-drug conjugate (ADC) in which anti CD30-antibody is attached by an enzyme cleavable linker to a potent, synthetic drug payload: monomethyl auristatin E, which inhibits microtubule polymerization. Preliminary results from a phase 1 weekly dosing study of SGN-35 suggested good efficacy in systemic ALCL59.
Zanolimumab
Zanolimumab is a fully human monoclonal antibody that targets the CD4 antigen present on T-helper lymphocytes60. The antibody prevents interaction between the CD4 receptor and the major histocompatibility complex class II molecules and in this way interferes with T-cell activation. It is very effective in refractory CTCL and preliminary results in a study in patients with PTCL demonstrated encouraging activity with an overall response rate of 23% - there were 2 complete responses, one in a patient with PTCL-NOS and another in AITL61. The development of zanolimumab was recently discontinued by Genmab due to slow patient recruitment into its pivotal study for zanolimumab in CTCL.
Siplizumab
Siplizumab is a humanized monoclonal antibody that targets the CD2 antigen present on most T and NK cells. Siplizumab demonstrated activity in an animal model of ATLL with 50% of tumor-bearing animals cured with 4 weekly administrations of the antibody and complete elimination of disease with a six-month course of treatment. Two separate phase I trials of siplizumab demonstrated promising activity in T cell malignancies with partial and complete responses observed in patients with PTCL, ATLL and large granular lymphocyte leukemia but the development of Epstein-Barr virus-related lymphoproliferative disease (EBV-LPD) in five of 51 patients prompted closure of the single agent trials62. Siplizumab is under evaluation in combination with rituximab in an attempt to prevent EBV-LPD. Down modulation of the CD2 receptor in response to antibody administration represents another potential problem in the use of this antibody.
KW-0761
KW-0761 is a defucosylated humanized anti-CCR4 antibody that has been evaluated in a phase I trial in patients with ATL or PTCL. The antibody is unique in that defucosylation enhances ADCC and permits a lower dose of antibody to be effective. Responses were observed in patients treated at doses ranging from .01– 1 mg/kg with the latter dose being evaluated in phase II trials63. Interestingly, this antibody also depletes T regulatory cells and may exert some of its antitumor effect through this mechanism.
Daclizumab
Daclizumab is a humanized monoclonal antibody that inhibits IL-2 binding to its receptor IL-2R and is approved by the FDA for the prevention of renal transplant rejection64. Basiliximab, a chimeric antibody that binds to the same receptor would be anticipated to have similar activity. Neither antibody depletes CD25-expressing T cells, but daclizumab has activity in patients with smoldering and chronic ATLL, where it interferes with the autocrine growth loop stimulated by tax64.
A major difficulty in the use of most monoclonal antibodies directed at T-cell antigens to treat lymphoma resides in the necessary depletion of normal T-cells that accompanies their use. T-cell depletion predisposes to the development of opportunistic infections and secondary malignancies and these combinations appear to be greater with the combination of monoclonal antibodies and chemotherapy. The addition of rituximab may prevent the development of EBV-LPD and the use of antiviral and antifungal prophylaxis may help reduce the incidence of opportunistic infections.
Immunosuppressants and Immunomodulatory agents
Cyclosporine A (CsA) is an antifungal metabolite with immunosuppressive effects. It has a suppressive effect on T-cells at the early stages of activation and a direct cytotoxic effect on lymphocytes. This rationale led investigators to investigate its role in AITL, a disease that is characterized by complex immune dysregulation. Cyclosporine was administered twice daily for 6–8 weeks, with gradual tapering over several weeks and responding patients received maintenance therapy65. Considering the poor outcome with standard therapy in this disease, the results with this drug were noteworthy. Eight of 12 (67%) patients with AITL experienced a response with 2 patients achieving a complete response; the median duration of response was 13 months65. The investigators proposed that cyclosporine may act by inhibiting deregulated T-cell activation through the calcineurin-nuclear factor of activated T-cell (NF-AT) signaling pathway.
Lenalidomide, a derivative of thalidomide, has shown interesting efficacy across a broad range of lymphoid diseases66–68. Its mechanism of action is poorly understood - in vitro it has direct anti-tumor effects, inhibits angiogenesis and results in increased NK cells in tumor tissue. Recently, it was tested in patients with relapsed or refractory PTCL and administered at a dose of 25mgs once daily on days 1 to 21 of a 28-day cycle69. In a preliminary report, the overall response rate was 30% in 23 evaluable patients, suggesting that further investigation of this drug in PTCL is warranted69. Thalidomide, in a case report, demonstrated efficacy in AITL70.
Other agents in PTCL
Gemcitabine is a novel nucleoside analog that competes with the natural nucleotide deoxycytidine, arresting tumor growth and causing apoptosis. It also causes cell apoptosis through inhibition of ribonucleotide reductase. It has demonstrated activity in relapsed T-cell lymphomas71,72. One study investigated its activity in patients with relapsed PTCL; gemcitabine was administered at a dose of 1200mg/m2 on days 1,8 and 15 of a 28-day cycle for a total of 3 to 6 cycles71,73. Albeit a small study, 55% of patients with PTCL had a response, with 30% achieving a complete response. Response duration ranged from 15 to 60 months71,73.
Pentostatin was tested at a dose of 3.75 or 5 mg/m2 by intravenous bolus administration daily for three days on an every three week schedule in patients with relapsed T cell lymphoma74. There were forty-two patients evaluable for response with an overall response rate of 54.8% observed. The median duration of remission was short at 4.3 months but some responses were prolonged to more than five years. The major toxicities included nausea, neutropenia and CD4 T cell depletion.
Forodesine is a potent purine nucleoside phosphorylase (PNP) inhibitor, that leads to T-cell selective intracellular dGTP accumulation, causing apoptosis. It has in-vitro activity against a wide range of B and T-cell diseases and has demonstrated moderate efficacy in patients with CTCL in a preliminary analysis75. Clofarabine is a deoxyadenosine analog that has increased stability compared to cladribine or fludarabine and is under investigation in PTCL.
Bortezomib, a proteasome inhibitor, was initially approved for use in relapsed/refractory multiple myeloma and subsequently was also found to be very effective in mantle-cell lymphoma76–78. It has also demonstrated activity in T-cell lymphoma, mostly in CTCL but also in PTCL-NOS with skin involvement79. In previously untreated patients with PTCL and NK-T-cell lymphoma, it has been combined with CHOP and was well-tolerated and active80.
A recently published study that evaluated the first clinically available spleen tyrosine kinase (Syk) inhibitor in recurrent B-cell lymphoma demonstrated high objective response rates, particularly in patients with CLL and SLL81. Over expression of Syk has been demonstrated in PTCL and inhibition of SyK induces apoptosis and blocks proliferation in T-cell NHL cell lines82,83. Therefore, SyK inhibition is an interesting therapeutic strategy and fostamatinib disodium is being investigated in PTCL.
Bone marrow transplantation
While there is an established role for autologous transplantation in relapsed ALK-positive ALCL, as discussed earlier, the role of this strategy in the setting of other relapsed PTCLs or as frontline consolidation in PTCL has not been well established84,85. Allogeneic transplantation is a potentially effective therapy for some patients with relapsed T-cell lymphoma and is under investigation in prospective studies. 86. For histologies like enteropathy associated T-cell lymphoma and hepatosplenic γ-δ T-cell lymphoma, where outcomes with conventional approaches are extremely poor and survivals short, consolidation allogeneic transplantation following induction therapy should be investigated in clinical trials.
Conclusions
With the exception of ALK-positive ALCL, the outcome for most patients with PTCL is poor and significantly inferior to that of patients with aggressive B-cell lymphomas. Therapeutic advancement in T-cell lymphoma has been curtailed at least in part by the rarity of these tumors, which has made instituting large-scale clinical trials challenging. Additionally, there is a lack of reagents to perform pre-clinical studies. There are limited cell lines available from patients with T-cell neoplasms and efforts to develop these lines has the potential to increase our understanding of these diseases. Whereas the biology of many B-cell lymphomas has been well elucidated, the pathogenesis and pivotal pathways that characterize distinct T-cell lymphomas remain poorly understood at present. Recent gene expression profiling studies, albeit in small numbers of patients, have demonstrated that certain key signatures are associated with PTCL subtypes and suggest that more extensive and expansive molecular analyses are critical to advancement of the field. It is likely that defining the molecular abnormalities in T-cell lymphomas will provide an opportunity to develop specifically targeted agents.
In the preceding paragraphs, we have discussed a long list of agents with potential utility in PTCL: novel agents that are under study prior to registration and previously FDA-approved agents in which PTCL would represent an expanded indication. These agents include small molecules, monoclonal antibodies, immunomodulators and conjugates and represent a pipeline that is one of the most extensive in oncology. The fundamental question is how to move these compounds forward. At the present time, only one of these (pralatrexate) is FDA approved for PTCL. The approval was accelerated, indicating that further studies are required to confirm clinical benefit. Trials with histone deacetylase inhibitors are completed and submission to the FDA expected. For these therapeutic advances to be translated into long-term clinical benefit for patients with PTCL, it is imperative that a systematic clinical trial effort is instituted in this disease. Given its rarity, every patient with a PTCL subtype other than ALK-positive ALCL should be considered for enrollment in clinical trials. In the context of these trials, it is critical to further develop international consortiums, and attempt to pair molecular characterization of tumors with drug development and investigation. Novel agents with activity demonstrated in the relapsed setting need to be incorporated into upfront clinical trials, given the aggressiveness and frequent poor outcome of PTCL subtypes other than ALK-positive ALCL. Novel clinical trial designs, including adaptive designs, also need to be considered to increase efficiency. Window of opportunity trials offer the opportunity to test agents in newly diagnosed patients. The goal is to move agents from the long list of potential agents either off the list, or into their appropriate places in the armamentarium, and to bring the outcome for patients with PTCL up to that expected for patients with more curable types of lymphoma.
References
- 1.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon. 2008 [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 115:1026–1036. doi: 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamant L, Meggetto F, al Saati T, et al. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin's disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996;87:284–291. [PubMed] [Google Scholar]
- 5.Russo G, Isobe M, Gatti R, et al. Molecular analysis of a t(14;14) translocation in leukemic T-cells of an ataxia telangiectasia patient. Proc Natl Acad Sci U S A. 1989;86:602–606. doi: 10.1073/pnas.86.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falini B, Pileri S, Zinzani PL, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999;93:2697–2706. [PubMed] [Google Scholar]
- 7.Falini B, Pulford K, Pucciarini A, et al. Lymphomas expressing ALK fusion protein(s) other than NPM-ALK. Blood. 1999;94:3509–3515. [PubMed] [Google Scholar]
- 8.Bischof D, Pulford K, Mason DY, Morris SW. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol. 1997;17:2312–2325. doi: 10.1128/mcb.17.4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laine J, Kunstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell. 2000;6:395–407. doi: 10.1016/s1097-2765(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 10.Alonsozana EL, Stamberg J, Kumar D, et al. Isochromosome 7q: the primary cytogenetic abnormality in hepatosplenic gammadelta T cell lymphoma. Leukemia. 1997;11:1367–1372. doi: 10.1038/sj.leu.2400742. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30:490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Dunleavy K, Wilson WH, Jaffe ES. Angioimmunoblastic T cell lymphoma: pathobiological insights and clinical implications. Curr Opin Hematol. 2007;14:348–353. doi: 10.1097/MOH.0b013e328186ffbf. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Delgado B, Cuadros M, Honrado E, et al. Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia. 2005;19:2254–2263. doi: 10.1038/sj.leu.2403960. [DOI] [PubMed] [Google Scholar]
- 15.de Leval L, Rickman DS, Thielen C, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 16.Piccaluga PP, Agostinelli C, Califano A, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117:823–834. doi: 10.1172/JCI26833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima H, Hasegawa Y, Suzukawa K, et al. Clinicopathological features and prognostic factors of Japanese patients with "peripheral T-cell lymphoma, unspecified" diagnosed according to the WHO classification. Leuk Res. 2004;28:1287–1292. doi: 10.1016/j.leukres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez J, Munsell M, Yazji S, et al. Impact of high-dose chemotherapy on peripheral T-cell lymphomas. J Clin Oncol. 2001;19:3766–3770. doi: 10.1200/JCO.2001.19.17.3766. [DOI] [PubMed] [Google Scholar]
- 19.Jagasia M, Morgan D, Goodman S, et al. Histology impacts the outcome of peripheral T-cell lymphomas after high dose chemotherapy and stem cell transplant. Leuk Lymphoma. 2004;45:2261–2267. doi: 10.1080/10428190412331272749. [DOI] [PubMed] [Google Scholar]
- 20.Le Gouill S, Milpied N, Buzyn A, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 21.Thompson CA. FDA approves pralatrexate for treatment of rare lymphoma. Am J Health Syst Pharm. 2009;66:1890. doi: 10.2146/news090080. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor O, Pro B, Pinter-Brown L, et al. PROPEL: Results of the pivotal, multicenter, phase II study of pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) J Clin Oncol (ASCO Annual meeting abstracts) 2009;27(15s) doi: 10.1200/JCO.2010.29.9024. (suppl; abstr 8561) 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toner LE, Vrhovac R, Smith EA, et al. The schedule-dependent effects of the novel antifolate pralatrexate and gemcitabine are superior to methotrexate and cytarabine in models of human non-Hodgkin's lymphoma. Clin Cancer Res. 2006;12:924–932. doi: 10.1158/1078-0432.CCR-05-0331. [DOI] [PubMed] [Google Scholar]
- 24.Wang ES, O'Connor O, She Y, Zelenetz AD, Sirotnak FM, Moore MA. Activity of a novel anti-folate (PDX, 10-propargyl 10-deazaaminopterin) against human lymphoma is superior to methotrexate and correlates with tumor RFC-1 gene expression. Leuk Lymphoma. 2003;44:1027–1035. doi: 10.1080/1042819031000077124. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor OA, Horwitz S, Hamlin P, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol. 2009;27:4357–4364. doi: 10.1200/JCO.2008.20.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz SM, Vose J, Advani R, et al. Pralatrexate and Gemcitabine in Patients with Relapsed or Refractory Lymphoproliferative Malignancies: Phase 1 Results. Blood (ASH Annual Meeting Abstracts) 2009 Nov;114:1674. [Google Scholar]
- 27.Archer SY, Meng S, Shei A, Hodin RA. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci U S A. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates SE, Zhan Z, Steadman K, et al. Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br J Haematol. 148:256–267. doi: 10.1111/j.1365-2141.2009.07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandor V, Bakke S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide ( FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 30.Sandor V, Senderowicz A, Mertins S, et al. P21-dependent g(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer. 2000;83:817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009;15:3918–3926. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piekarz RL, Robey R, Sandor V, et al. Inhibitor of histone deacetylation, depsipeptide ( FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 33.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Whittaker S, Demierre MF, et al. Clinically significant responses achieved with romidepsin in treatment-refractory cutaneous T-cell lymphoma: Final results from a phase 2B, international, multicenter, registration study. Blood (ASH Annual Meeting Abstracts) 2008;112:263. [Google Scholar]
- 35.Piekarz R, Wright J, Frye R, et al. Final Results of a Phase 2 NCI Multicenter Study of Romidepsin in Patients with Relapsed Peripheral T-Cell Lymphoma (PTCL) Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 1657. [Google Scholar]
- 36.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 37.Pohlman B, Advani R, Duvic M, et al. Final Results of a Phase II Trial of Belinostat (PXD101) in Patients with Recurrent or Refractory Peripheral or Cutaneous T-Cell Lymphoma. Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 920. [Google Scholar]
- 38.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 39.Ellis L, Pan Y, Smyth GK, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 40.LeMaistre CF, Saleh MN, Kuzel TM, et al. Phase I trial of a ligand fusion-protein (DAB389IL-2) in lymphomas expressing the receptor for interleukin-2. Blood. 1998;91:399–405. [PubMed] [Google Scholar]
- 41.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 42.Dang NH, Pro B, Hagemeister FB, et al. Phase II trial of denileukin diftitox for relapsed/refractory T-cell non-Hodgkin lymphoma. Br J Haematol. 2007;136:439–447. doi: 10.1111/j.1365-2141.2006.06457.x. [DOI] [PubMed] [Google Scholar]
- 43.Dearden CE, Matutes E, Catovsky D. Alemtuzumab in T-cell malignancies. Med Oncol. 2002;19 Suppl:S27–S32. doi: 10.1385/mo:19:2s:s27. [DOI] [PubMed] [Google Scholar]
- 44.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 45.Halene S, Zieske A, Berliner N. Sustained remission from angioimmunoblastic T-cell lymphoma induced by alemtuzumab. Nat Clin Pract Oncol. 2006;3:165–168. doi: 10.1038/ncponc0430. quiz 169. [DOI] [PubMed] [Google Scholar]
- 46.Janik J, Dunleavy K, Pittaluga S, et al. A Pilot Trial of Campath-1H and Dose-Adjusted EPOCH in CD52-Expressing Aggressive T-Cell Malignancies. Blood. 2005 November 16;Volume 106(issue 11) Abstract #3348. [Google Scholar]
- 47.Kim JG, Sohn SK, Chae YS, et al. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother Pharmacol. 2007;60:129–134. doi: 10.1007/s00280-007-0469-9. [DOI] [PubMed] [Google Scholar]
- 48.Weidmann E, Hess G, Chow KU, et al. A phase II study of alemtuzumab, fludarabine, cyclophosphamide, and doxorubicin (Campath-FCD) in peripheral T-cell lymphomas. Leuk Lymphoma. 51:447–455. doi: 10.3109/10428190903580402. [DOI] [PubMed] [Google Scholar]
- 49.Gallamini A, Zaja F, Patti C, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–2323. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- 50.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 51.Tian ZG, Longo DL, Funakoshi S, et al. In vivo antitumor effects of unconjugated CD30 monoclonal antibodies on human anaplastic large-cell lymphoma xenografts. Cancer Res. 1995;55:5335–5341. [PubMed] [Google Scholar]
- 52.Zhang M, Yao Z, Zhang Z, et al. Effective therapy for a murine model of human anaplastic large-cell lymphoma with the anti-CD30 monoclonal antibody, HeFi-1, does not require activating Fc receptors. Blood. 2006;108:705–710. doi: 10.1182/blood-2005-11-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willers J, Dummer R, Kempf W, Kundig T, Burg G, Kadin ME. Proliferation of CD30+ T-helper 2 lymphoma cells can be inhibited by CD30 receptor cross-linking with recombinant CD30 ligand. Clin Cancer Res. 2003;9:2744–2754. [PubMed] [Google Scholar]
- 54.Mir SS, Richter BW, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood. 2000;96:4307–4312. [PubMed] [Google Scholar]
- 55.Bartlett NL, Younes A, Carabasi MH, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111:1848–1854. doi: 10.1182/blood-2008-01-127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forero-Torres A, Leonard JP, Younes A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 57.Ansell SM, Horwitz SM, Engert A, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25:2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 58.Borchmann P, Treml JF, Hansen H, et al. The human anti-CD30 antibody 5F11 shows in vitro and in vivo activity against malignant lymphoma. Blood. 2003;102:3737–3742. doi: 10.1182/blood-2003-02-0515. [DOI] [PubMed] [Google Scholar]
- 59.Fanale M, Bartlett N, Forero-Torres A, et al. The Antibody-Drug Conjugate Brentuximab Vedotin (SGN-35) Induced Multiple Objective Responses in Patients with Relapsed or Refractory CD30-Positive Lymphomas in a Phase 1 Weekly Dosing Study. Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract 2731. [Google Scholar]
- 60.Kim YH, Duvic M, Obitz E, et al. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109:4655–4662. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- 61.d’Amore F, Radford J, Jerkeman M, et al. Zanolimumab (HuMax-CD4TM), a Fully Human Monoclonal Antibody: Efficacy and Safety in Patients with Relapsed or Treatment-Refractory Non-Cutaneous CD4+ T-Cell Lymphoma. Blood (ASH Annual Meeting Abstracts) 2007;110 Abstract 3409. [Google Scholar]
- 62.O'Mahony D, Morris JC, Stetler-Stevenson M, et al. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clin Cancer Res. 2009;15:2514–2522. doi: 10.1158/1078-0432.CCR-08-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 28:1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 64.Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26:3699–3703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- 65.Advani R, Horwitz S, Zelenetz A, Horning SJ. Angioimmunoblastic T cell lymphoma: Treatment experience with cyclosporine. Leuk Lymphoma. 2007;48:521–525. doi: 10.1080/10428190601137658. [DOI] [PubMed] [Google Scholar]
- 66.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 67.Verhelle D, Corral LG, Wong K, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 68.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 69.Dueck G, Chua N, Prasad A, et al. Activity of lenalidomide in a phase II trial for T-cell lymphoma: Report on the first 24 cases. J Clin Oncol. 2009;27(15s) (suppl; abstr 8524) [Google Scholar]
- 70.Gottardi GottardiM, Danesin C, Canal F, et al. Complete remission induced by thalidomide in a case of angioimmunoblastic T-cell lymphoma refractory to autologous stem cell transplantation. Leuk Lymphoma. 2008;49:1836–1838. doi: 10.1080/10428190802233165. [DOI] [PubMed] [Google Scholar]
- 71.Zinzani PL, Magagnoli M, Bendandi M, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol. 1998;9:1351–1353. doi: 10.1023/a:1008409601731. [DOI] [PubMed] [Google Scholar]
- 72.Sallah S, Wan JY, Nguyen NP. Treatment of refractory T-cell malignancies using gemcitabine. Br J Haematol. 2001;113:185–187. doi: 10.1046/j.1365-2141.2001.02743.x. [DOI] [PubMed] [Google Scholar]
- 73.Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2009 doi: 10.1093/annonc/mdp508. [DOI] [PubMed] [Google Scholar]
- 74.Tsimberidou AM, Giles F, Duvic M, Fayad L, Kurzrock R. Phase II study of pentostatin in advanced T-cell lymphoid malignancies: update of an M.D. Anderson Cancer Center series. Cancer. 2004;100:342–349. doi: 10.1002/cncr.11899. [DOI] [PubMed] [Google Scholar]
- 75.Duvic M, Forero-Torres A, Foss F, Olsen E, Pinter-Brown L, Kim Y. Long-term treatment of CTCL with the oral PNP inhibitor, forodesine. ASCO Meeting Abstracts. 2009;27:8552. [Google Scholar]
- 76.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 77.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 78.O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 79.Zinzani PL, Musuraca G, Tani M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:4293–4297. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
- 80.Lee J, Suh C, Kang HJ, et al. Phase I study of proteasome inhibitor bortezomib plus CHOP in patients with advanced, aggressive T-cell or NK/T-cell lymphoma. Ann Oncol. 2008;19:2079–2083. doi: 10.1093/annonc/mdn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non Hodgkin's lymphoma and chronic lymphocytic leukemia. Blood. 2009 doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feldman AL, Sun DX, Law ME, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22:1139–1143. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilcox RA, Sun DX, Novak A, Dogan A, Ansell SM, Feldman AL. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin's lymphoma cell lines. Leukemia. 24:229–232. doi: 10.1038/leu.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez J, Conde E, Gutierrez A, et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: the Spanish Lymphoma and Autologous Transplantation Group experience. Ann Oncol. 2007;18:652–657. doi: 10.1093/annonc/mdl466. [DOI] [PubMed] [Google Scholar]
- 85.Chen AI, McMillan A, Negrin RS, Horning SJ, Laport GG. Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant. 2008;14:741–747. doi: 10.1016/j.bbmt.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutierrez A, Caballero MD, Perez-Manga G, Rodriguez J. Hematopoietic SCT for peripheral T-cell lymphoma. Bone Marrow Transplant. 2008;42:773–781. doi: 10.1038/bmt.2008.332. [DOI] [PubMed] [Google Scholar]