Abstract
Objective
To determine the cost effectiveness of a strategy of near patient Helicobacter pylori testing and endoscopy for managing dyspepsia.
Design
Randomised controlled trial.
Setting
31 UK primary care centres.
Participants
478 patients under 50 years old presenting with dyspepsia of longer than four weeks duration.
Interventions
Near patient testing for H pylori and open access endoscopy for patients with positive results. Control patients received acid suppressing drugs or specialist referral at general practitioner's discretion.
Main outcome measures
Cost effectiveness based on improvement in symptoms and use of resources at 12 months; quality of life.
Results
40% of the study group tested positive for H pylori. 45% of study patients had endoscopy compared with 25% of controls. More peptic ulcers were diagnosed in the study group (7.4% v 2.1%, P=0.011). Paired comparison of symptom scores and quality of life showed that all patients improved over time with no difference between study and control groups. No significant differences were observed in rates of prescribing, consultation, or referral. Costs were higher in the study group (£367.85 v £253.16 per patient).
Conclusions
The test and endoscopy strategy increases endoscopy rates over usual practice in primary care. The additional cost is not offset by benefits in symptom relief or quality of life.
What is already known on this topic
Patients younger than 50 without H pylori infection are unlikely to have treatable disease detected at endoscopy
Such patients can be managed by acid suppression and reassurance alone
Test and endoscopy (referral of patients testing positive for H pylori in primary care) has been recommended as a way to reduce endoscopic workload
What this paper adds
Applying a test and endoscopy strategy increased the endoscopy referral rate from 25% to 40%
The strategy produced no significant differences in symptoms or quality of life compared with usual management
The increased costs of this strategy cannot be justified
Introduction
The NHS spent £1.1bn on managing dyspepsia in 1998,1 and 450 000 patients had endoscopy. If endoscopy is reserved for patients who test positive for Helicobacter pylori, it should maximise the yield of peptic ulceration (for which eradication therapy is effective2,3) and reduce overall endoscopy workload. Patients negative for H pylori can be given empirical acid suppression treatment.
Two non-randomised studies in secondary care have examined this “test and endoscopy” strategy. A retrospective cohort study found that positive H pylori test results were highly predictive of peptic ulcer and suggested that screening out negative patients could have reduced endoscopy workload by 23%.4 A controlled before and after study found that test and endoscopy was as effective in reducing dyspeptic symptoms as the previous practice of endoscopy in all patients referred.5 However, the study did not follow up the whole screened cohort, and the control group consisted only of patients negative for H pylori who had had endoscopy. The groups are not therefore representative. Furthermore, all the patients examined in these two studies had been referred for endoscopy by their general practitioner. The test and endoscopy strategy has not been investigated in a randomised controlled trial, and there are no studies based in primary care.
Near patient testing allows general practitioners to base their initial management on the results of tests.6 However, there are few outcome studies of near patient tests in clinical decision making.7 A recent systematic review of H pylori tests in primary care showed that the Helisal rapid blood test has variable performance in primary care, with a sensitivity of 77-92% and a specificity of 56-69%.8 However, when the test was evaluated in the population local to our trial, the sensitivity was 89% and specificity 84%.9 Analysis with a Markov model suggested that the test would be cost effective.10 We therefore used the test in this study to determine the cost effectiveness of the test and endoscopy strategy in primary care.
Participants and methods
Participants
All patients aged 18-49 years who consulted their general practitioner with dyspepsia of more than four weeks duration were eligible for the trial. We excluded patients who had had endoscopy or a positive barium meal examination in the past three years, who were unable to give informed consent, or who were unfit for endoscopy. Dyspepsia was defined as epigastric pain or heartburn with or without nausea and bloating.11
Randomisation and concealment of allocation
We randomised patients individually using sealed, opaque, sequentially numbered envelopes. The randomisation schedule was done on a 60:40 basis (study: control) and used a computerised random number sequence without blocking or stratification. We kept a log of numbers issued to practices.
Interventions
Patients were randomised to “test and endoscopy” or to usual management. The Helisal test (Cortecs Diagnostics, Deeside) was done by the general practitioner or practice nurse. Endoscopies on patients with positive results were carried out according to usual practice at open access services at six local hospitals. Patients with negative results were not referred for endoscopy but received empirical acid suppressing drugs chosen by their general practitioner.
Patients randomised to the control arm of the trial were managed according to the practitioner's usual management strategy. This allowed outpatient referral to a specialist gastroenterologist but excluded initial referral to open access endoscopy. Patients in whom initial management failed could be referred for endoscopy after six weeks.
Outcomes
The main outcomes were effectiveness (assessed by symptoms) and costs of managing dyspepsia. We measured symptoms at recruitment and 15-18 months using the Birmingham dyspepsia symptom score, a postal measure previously validated in the local population.12 We calculated the costs of dyspepsia from a health service perspective. We assessed use of resources in primary and secondary care for 12 months after randomisation by abstracting data from primary care case records. All data were double entered, and we verified inconsistencies by referring to the original case records.
We used a questionnaire derived from a validated measure for patients with peptic ulcer disease to measure quality of life in terms of pain, emotion, and social function.13 Patient satisfaction was assessed by a validated measure of satisfaction with the primary care consultation14 supplemented with additional questions relating to secondary care and endoscopy.
Analysis
We analysed data by intention to treat. A sample of 430 patients would detect differences of 2 units (SD=4) in the dyspepsia score, 9 units (SD=22) in the pain dimension of the quality of life, and 8 units (SD=20) in the emotion and social dimensions, and would detect a reduction in general practice consultation rates from 3 to 1 a year (SD=3). These estimates were based on a power of 90% at the 5% significance level and assumed 25% loss to follow up. Ethical approval was obtained from all local research ethics committees.
We recorded numbers of endoscopies, barium meal examinations, and primary care consultations from each patient's notes. Drugs prescribed, including those for eradication of H pylori, were recorded as defined daily doses of drug per patient. Table 1 shows the unit costs for management, which we obtained from national reference sources for 1998.
Table 1.
Unit costs of care for dyspeptic patients used in economic analysis
| Procedure | Mean cost (£) |
|---|---|
| Attendance at accident and emergency15 | 98 |
| Barium meal examination16 | 246 |
| Test for campylobacter-like organisms* | 15 |
| Dilation of oesophagus16 | 323 |
| Endoscopy16 | 246 |
| Helico G ELISA test* | 2 |
| Helisal rapid blood test† | 17 |
| Histology for H pylori* | 8 |
| Outpatient appointment15 | 63 |
| Primary care consultation15 | 17 |
| Prescribing costs17 | Individual defined daily doses |
Public Health Laboratory Service. †Cortecs Diagnositics.
Secondary analyses included comparison of procedure, rates of diagnosis, and use of services in the first year after randomisation; changes in quality of life; and patient satisfaction. We compared use of resources per patient using t tests18 and changes in symptom and quality of life scores from baseline to 18 months using two sample t tests. We identified variables relating to response rates using multiple logistic regression analysis and included them in analysis of covariance models to assess their effect on symptom and quality of life scores. Data were analysed with SAS (version 6.12).
Results
The 31 participating practices had a registered population of 195 700. Four hundred and seventy eight patients entered the trial; 285 were randomised to “test and endoscopy” and 193 to usual management. The patients were recruited over three years from May 1995 at a mean rate of 2 per 1000 registered practice population per month. Full details of ascertainment and recruitment by practice have been reported.19
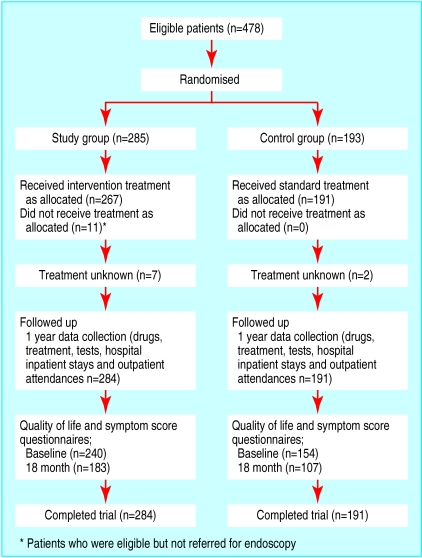
Figure 1 shows the trial profile. Full data on use of resources were collected for 475 patients (99%). Records for three patients could not be traced. We obtained evaluable symptom scores and quality of life scores from 290 (61%) patients. Two hundred and seventy three (57%) patients returned satisfaction questionnaires. The baseline characteristics of the patients entered into the analysis were similar in the two randomised groups (table 2).
Figure 1.
Trial profile
Table 2.
Baseline characteristics of participants. Values are numbers (percentages) of participants unless stated otherwise
| Study (n=284) | Control (n=191) | |
|---|---|---|
| Mean (SD) age (years) | 36.9 (8.4) | 37.1 (7.2) |
| Men | 165 (58) | 106 (55) |
| Smokers | 126/276 (46) | 75/182 (41) |
| Taking non-steroidal anti-inflammatories at study entry | 7/284 (2) | 6 (3) |
| Previous confirmed diagnosis of ulcer | 13/280 (5) | 13 (7) |
| Previous barium meal examination | 50/281 (18) | 26 (14) |
| Previous endoscopy | 38/280 (14) | 28 (15) |
| Endoscopy in past 3 years | 6/280 (2) | 2 (1) |
| Mean (SD) quality of life scores: | ||
| Pain | 57.1 (20.8) | 54.2 (20.6) |
| Emotion | 57.4 (18.2) | 56.7 (18.1) |
| Social | 69.0 (19.5) | 67.2 (20.1) |
| Total | 60.3 (15.2) | 58.8 (16.2) |
| Mean (SD) symptoms score | 10.1 (3.8) | 10.6 (3.9) |
| Epigastric pain more than once a month | 202/238 (85) | 130/152 (86) |
| Heartburn more than once a month | 161/238 (68) | 103/152 (68) |
| Epigastric pain and heartburn more than once a month | 149/238 (63) | 92/152 (61) |
Patients for whom accurate historical data could not be attained are excluded from comparisons of previous investigations.
Interventions and diagnostic findings
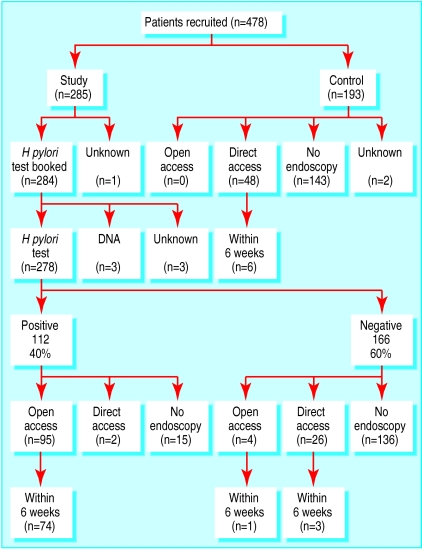
The Helisal test gave positive results in 40% (112/278) of patients (fig 2 ). The expected prevalence of H pylori in this population was 30%.12 We used the test performance and the observed numbers of positive and negative results to calculate underlying prevalence and predictive values. With a sensitivity of 89% and a specificity of 84%, a positive rate of 40% reflected an underlying prevalence of 33%.9 On this basis, 27% of positive results and 6% of negative results would be false.
Figure 2.
Numbers of participants having open and direct access endoscopy in study and control groups
Overall, 127 (45%) of the study group had endoscopy compared with 48 (25%) of the control group. Figure 2 shows the numbers of patients who had open access endoscopy, consultant booked endoscopy, and no endoscopy. Fifteen patients who were positive for H pylori did not have endoscopy, of whom one refused, nine did not attend, and five were not referred. Of the 166 patients who were negative for H pylori in the test group, 30 (18%) had endoscopy during the 12 month follow up. Only one of these patients had open access endoscopy within six weeks. No control patients were inappropriately managed by test and endoscopy.
Among the patients who had endoscopy, significantly more peptic ulcers were detected by the test and endoscopy strategy than by standard management (21 (7%) v 4 (2.1%), χ2= 6.4, df=1, P=0.011). Compared with the control patients, fewer patients in the study group had oesophagitis (17% v 31%, χ2= 4.1, df=1, P=0.04) and more had duodenitis (19% v 6%, χ2= 4.3, df=1, P=0.04; table 3 ).
Table 3.
Diagnostic findings in patients who had endoscopy
| Diagnosis at endoscopy | No (%) in study group (n=127) | No (%) in control group(n=48) | Difference in % (95% CI) | χ2 (df=1) | P value |
|---|---|---|---|---|---|
| Normal | 48 (38) | 17 (35) | 3 (−4 to 18) | 0.1 | 0.7 |
| Duodenal ulcer | 17 (13) | 3 (6) | 7 (−2 to 16) | 1.8 | 0.2 |
| Gastric ulcer | 4 (3) | 1 (2) | 1 (−4 to 6) | 0.99† | |
| Oesophagitis | 22* (17) | 15 (31) | −14 (−29 to 1) | 4.1 | 0.04 |
| Duodenitis | 24 (19) | 3 (6) | 13 (3 to 22) | 4.3 | 0.04 |
| Gastritis | 26 (20) | 11 (23) | −3 (−16 to11) | 0.1 | 0.7 |
| Gastric cancer | 0 | 0 | — | — | — |
Includes one patient with diagnosis of Barrett's oesophagus.
Fisher's exact test.
Outcomes and costs
Symptoms and quality of life scores in the test and control groups significantly improved by 18 months. There was no evidence of a difference in the size of improvement between the groups (table 4). Non-respondents were more likely to smoke and were younger than respondents (smoking odds ratio=1.63, 95% confidence interval 1 to 2.65; age 0.96, 0.93 to 0.99), but no difference in sex or baseline symptoms was observed. Analysis of covariance found that age and smoking had no significant effect on symptoms or quality of life. No significant differences were observed in the satisfaction questionnaire.
Table 4.
Improvement in symptom and quality of life scores from baseline at 18 months
| Score | Mean (SD) change from baseline at 18 months
|
Mean (95% CI) difference in change | P value* | |
|---|---|---|---|---|
| Study | Control | |||
| Symptom | 3.8 (4.8) | 3.5 (4.5) | 0.3 (−0.9 to 1.5) | 0.61 |
| Quality of life: | ||||
| Pain | 16.9 (25.3) | 14.3 (21.5) | 2.5 (−3.5 to 8.6) | 0.41 |
| Social | 9.6 (18.4) | 10.3 (17.3) | 0.7 (−3.9 to 5.2) | 0.78 |
| Emotion | 5.4 (18.6) | 7.2 (18.0) | 1.8 (−2.8 to 6.4) | 0.44 |
t test.
There were no significant differences in the use of drugs for dyspepsia between the groups (table 5). The numbers of outpatient attendances, general practice consultations, or regimens for eradication of H pylori did not differ significantly between the groups (table 6). Mean total costs were £367.85 for test and endoscopy and £253.16 for usual management. This increased cost of £114.69 per patient was not associated with any significant difference in effects. The test and endoscopy strategy was thus less cost effective than usual management.
Table 5.
Mean (SD) defined daily doses of drugs for dyspepsia in study and control groups
| Type of drug | Study | Control | Difference (95% CI) | P value* |
|---|---|---|---|---|
| Antacid | 8.0 (19.9) | 6.2 (12.9) | 0.3 (−0.9 to 1.5) | 0.26 |
| H2 receptor antagonist | 33.5 (66.6) | 35.7 (82.4) | 2.5 (−3.5 to 8.6) | 0.75 |
| Proton pump inhibitor | 39.4 (80.0) | 45.5 (100.5) | −0.7 (−5.2 to 3.9) | 0.48 |
| Prokinetic | 3.5 (14.4) | 4.6 (15.3) | −1.8 (−6.4 to 2.8) | 0.43 |
| Total | 84.3 (112.8) | 92.1 (127.0) | 0.61 (0.47 to 0.75) | 0.49 |
t test.
Table 6.
Mean (SD) use of resources per patient
| Study | Control | Difference (95% CI) | P value* | |
|---|---|---|---|---|
| Endoscopy | 0.59 (0.76) | 0.28 (0.49) | 0.31 (0.19 to 0.43) | <0.0001 |
| Barium meal examination | 0.06 (0.23) | 0.05 (0.25) | 0.004 (−0.04 to 0.05) | 0.86 |
| Outpatient appointment | 0.23 (0.82) | 0.21 (0.63) | 0.01 (−0.13 to 0.15) | 0.87 |
| Inpatient episode | 0.02 (0.26) | 0.01 (0.1) | 0.01 (−0.02 to 0.05) | 0.40 |
| H pylori test | 1.35 (0.78) | 0.74 (0.73) | 0.61 (0.47 to 0.75) | <0.0001 |
| GP consultation | 3.26 (2.73) | 3.30 (2.67) | 0.03 (−0.46 to 0.53) | 0.89 |
| H pylori eradication | 0.20 (0.45) | 0.28 (0.52) | −0.07 (−0.16 to 0.01) | 0.11 |
t test.
Discussion
In contrast to non-randomised studies in secondary care,4,5 our study shows that the test and endoscopy strategy increased endoscopy referral rates by almost twofold over usual practice. Some of the increase in referral was due to the choice of non-invasive test. However, even if a carbon-13 urea breath test had been used, at least 92 patients would have been referred (33% of the total would test H pylori positive) compared with the 69 (25%) expected from the rate in the control group.
We found that test and endoscopy did not improve dyspeptic symptoms or quality of life compared with usual management. The number of questionnaires returned was lower than expected, but the numbers returned were still large enough to detect the predefined differences with adequate power (80%). As the trial was subject to 39% attrition on the symptom and quality of life scores, the possibility of bias needs to be considered. Logistic regression analysis for the effect of differential follow up by age and smoking status had no significant effect on the result.
Investigation of dyspeptic patients by test and endoscopy increased the use of resources without producing benefit. Contrary to expectation, there was no fall in primary care consultations for dyspepsia or outpatient attendance in the test and endoscopy group. Most patients investigated had non-ulcer dyspepsia, and the number of peptic ulcers was too small to detect an effect of H pylori eradication. The low prevalence of treatable disease in patients under 50 means that relatively expensive methods of case finding such as endoscopy are not cost effective. Empirical prescribing is therefore the best treatment. In older patients, however, who have a greater frequency of treatable disease, a primary care based randomised controlled trial has shown that initial endoscopy may be cost effective compared with empirical management.20
Although acid suppression is effective for undiagnosed dyspepsia, especially reflux symptoms,21 treatment with these drugs misses the opportunity to cure an important minority of patients with recurrent peptic ulcer disease due to H pylori. Eradication of H pylori may also have a small but important effect in non-ulcer dyspepsia, possibly by preventing the development of ulcers in susceptible patients.22 It is unclear whether a strategy to test for H pylori and then eradicate is cost effective as an initial management strategy in primary care. Future trials should evaluate the cost effectiveness of this strategy compared with empirical prescribing. Until then, near patient testing for H pylori is probably unwarranted in patients under 50.
Acknowledgments
We thank Dr A Briggs, Health Economics Research Centre, Oxford University, for advice on the economic analysis and Dr R P Walt, Heartlands Hospital, Birmingham, Dr B Cooper, City Hospital Birmingham, and Mr M Hallissey, Queen Elizabeth Hospital, Birmingham, for providing open access endoscopy for study patients. We thank the following practices for enrolling patients: Bellevue Medical Centre, Riverbrook Medical Centre, Laurie Pike Health Centre, Frankley Health Centre, Dr M Fernell and partners, Cofton Medical Centre, Hill Top Surgery, Dr J Crosland and partners, Northfield Health Centre, Swanswell Medical Centre, Dr D Taylor and partners, Ley Hill Surgery, The Reabrook Surgery, University Medical Centre, Ash Tree Medical Centre, Dr J Parle and partners, Dr B Dicker and partner, Green Ridge Surgery, Harborne Medical Practice, Dr Hayes, Dr P Machin and partners, Fernley Medical Centre, Dr E Pennington and partners, Severn House Surgery, Dr N Gaballa, Moor Green Medical Centre, Dr P Beyer, Northgate Medical Centre, Yardley Wood Health Centre, Grange Hill Surgery, West Heath Surgery, Stockland Green Health Centre, Kendrick Surgery, James Preston Health Centre, Ashfurlong Health Centre, Medical Centre, Selly Oak Health Centre, Dr B Pattni, Kingsmount Surgery, Church Lane Medical Centre, Hollyoaks Medical Centre, Mirfield Surgery, Castle Practice.
Footnotes
Funding: The study was funded by the NHS research and development primary secondary care interface programme, grant no PSI 37-01 and the NHS Executive, West Midlands. The Astra Foundation supplied the Helisal tests. BCD holds a NHS research and development national primary care career scientist award. LR holds a NHS Executive, West Midlands new blood fellowship.
Competing interests: None declared.
References
- 1.Asante M, Lord J, Mendall M, Northfield T. Endoscopy for Helicobacter pylori seronegative young dyspeptic patients: an economic evaluation based on a randomized trial. Eur J Gastroenterol Hepatol. 1999;11(8):851–856. doi: 10.1097/00042737-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Axon ATR, Bell GD, Jones RH, Quine MA, McCloy RF. Guidelines on appropriate indications for upper gastrointestinal endoscopy. BMJ. 1995;310:853–856. doi: 10.1136/bmj.310.6983.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiba N, Lahaie R, Fedorak RN, Bailey R, Veldhuyzen vZS, Bernucci B. Helicobacter pylori and peptic ulcer disease. Current evidence for management strategies. Can Fam Physician. 1998;44:1481–1488. [PMC free article] [PubMed] [Google Scholar]
- 4.Sobala GM, Crabtree JE, Pentith JA, Rathbone BJ, Shallcross TM, Wyatt JI, et al. Screening dyspepsia by serology to Helicobacter pylori. Lancet. 1991;338:94–96. doi: 10.1016/0140-6736(91)90085-4. [DOI] [PubMed] [Google Scholar]
- 5.Patel P, Khulusi S, Mendall MA, Lloyd R, Jazrawi R, Maxwell JD, et al. Prospective screening of dyspeptic patients by Helicobacter pylori serology. Lancet. 1995;346:1315–1318. doi: 10.1016/s0140-6736(95)92340-3. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs R. Near patient testing in primary care. BMJ. 1996;312:263–264. doi: 10.1136/bmj.312.7026.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney BC, Hyde CJ, McManus RJ, Wilson S, Fitzmaurice DA, Jowett S, et al. Systematic review of near patient test evaluations in primary care. BMJ. 1999;319:824–827. doi: 10.1136/bmj.319.7213.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts AP, Childs SM, Rubin G, de Wit NJ. Tests for Helicobacter pylori: a critical appraisal from primary care. Fam Pract. 2000;17:S12–S20. doi: 10.1093/fampra/17.suppl_2.s12. [DOI] [PubMed] [Google Scholar]
- 9.Delaney BC, Holder RL, Allan TF, Kenkre J, Hobbs FDR, et al. Performance of a whole blood point of care test for Helicobacter pylori in primary care: a Bayesian analysis [Abstract] Gastroenterology. 1999;116(suppl 4):G0228. [Google Scholar]
- 10.Delaney B, Hobbs FD. Near patient tests for Helicobacter pylori in primary care: how accurate do they need to be? Eur J Gen Pract. 1998;4:149–154. [Google Scholar]
- 11.Management of dyspepsia: report of a working party. Lancet. 1988;i:576–579. [PubMed] [Google Scholar]
- 12.Hobbs FDR, Delaney BC, Rowsby M, Kenkre JE. Effect of Helicobacter pylori eradication therapy on dyspeptic symptoms in primary care. Fam Pract. 1996;13:225–228. doi: 10.1093/fampra/13.3.225. [DOI] [PubMed] [Google Scholar]
- 13.Korman MG. Quality of life in duodenal ulcer disease. Scand J Gastroenterol. 1993;suppl 199:28–31. doi: 10.3109/00365529309098352. [DOI] [PubMed] [Google Scholar]
- 14.Baker R. Characteristics of practices, general practitioners and patients related to levels of patients' satisfaction with consultations. Br J Gen Pract. 1996;46:601–605. [PMC free article] [PubMed] [Google Scholar]
- 15.Netten A, Dennett J, Knight J. Unit costs of health and social care. Canterbury: University of Kent; 1998. [Google Scholar]
- 16.The new NHS—reference costs. London: NHS Executive; 1998. [Google Scholar]
- 17.British Medical Association; Royal Pharmaceutical Society of Great Britain. British national formulary. London: BMA, RPS; 1997. [Google Scholar]
- 18.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320:1197–1200. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson S, Delaney BC, Roalfe A, Roberts L, Redman V, Wearn A, et al. Randomised controlled trials in primary care: case study. BMJ. 2000;321:24–27. doi: 10.1136/bmj.321.7252.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaney BC, Wilson S, Roalfe A, Roberts L, Redman V, Wearn AM, et al. Cost effectiveness of initial endoscopy for dyspepsia in patients over the age of 50 years: a randomised controlled trial in primary care. Lancet. 2000;356:1965–1969. doi: 10.1016/s0140-6736(00)03308-0. [DOI] [PubMed] [Google Scholar]
- 21.Delaney BC, Innes MA, Deeks J, Wilson S, Oakes R, Moayyedi P, et al. Initial management strategies for dyspepsia Cochrane Database Syst Rev 2000;(2):CD001961. [DOI] [PubMed]
- 22.Moayyedi P, Soo S, Deeks J, Innes MA, Forman D, Delaney BC. A systematic review and economic analysis of the cost effectiveness of H pylori eradication therapy in non-ulcer dyspepsia. BMJ. 2000;321:659–664. doi: 10.1136/bmj.321.7262.659. [DOI] [PMC free article] [PubMed] [Google Scholar]