The possible visual functions of microsaccades have long been debated1–3. Proposed functions range from contributions to the prevention of image fading, the gradual disappearance of the visual percept experienced in the absence of retinal image motion4–6, to participation in the oculomotor strategy by which the eye maintains precise fixation on a cue7,8. An interesting hypothesis is that microsaccades may contribute to visual tasks that require high acuity9. According to this proposal, microsaccades serve the same function as larger saccades: as the visual system uses saccades to explore the scene, microsaccades enable exploration of a narrow region around the point of fixation when necessary.
Experimental data have not supported this proposal. A seminal study, which examined microsaccades while observers aimed and shot a rifle and threaded a sewing needle, found that, in both conditions, microsaccade rates decrease just before the end of a trial, even when the task is successfully performed10. Furthermore, microsaccades are, on average, less frequent during these tasks than during maintained fixation on a small cue10. Similar results were also reported by a second study11. On the basis of these findings, it was concluded that microsaccades are detrimental and, therefore, suppressed during the execution of finely guided visuomotor tasks and/or tasks that require high visual acuity.
While the previous experiments show that some high-acuity judgments can be accomplished without microsaccades, a few observations caution against concluding that microsaccades are not used for exploring small regions within the scene. First, sustained fixation on a marker-the condition used as control reference by previous studies– might not provide an adequate baseline for comparing changes in microsaccade frequency. Many microsaccades performed under this condition might originate from the very requirement of maintaining precise fixation and, thus, serve a different function than the microsaccades that occur when accurate fixation is not demanded7,12,13. Second, a reduction in the rate of microsaccades at the end of an experimental trial may reflect a change in the subject's attention and does not necessarily entail that microsaccades were detrimental for perceptual judgments. These judgments could have benefited from information acquired by means of microsaccades occurring at earlier times during a trial.
In order to reexamine the role of microsaccades in fine spatial vision, we recorded eye movements in human observers while they were threading a needle in a virtual environment. Recent advances in gaze-contingent display technology14,15 now enable accurate localization of the portion of the scene examined with the preferred retinal region, as well as precise analysis of the timing of occurrence of microsaccades relative to adjustments in the thread-needle alignment. Our results show that microsaccades moved the preferred retinal location back and forth between the tip of the thread and the eye of the needle and were generated in order to evaluate the relative alignment of these two objects.
Results
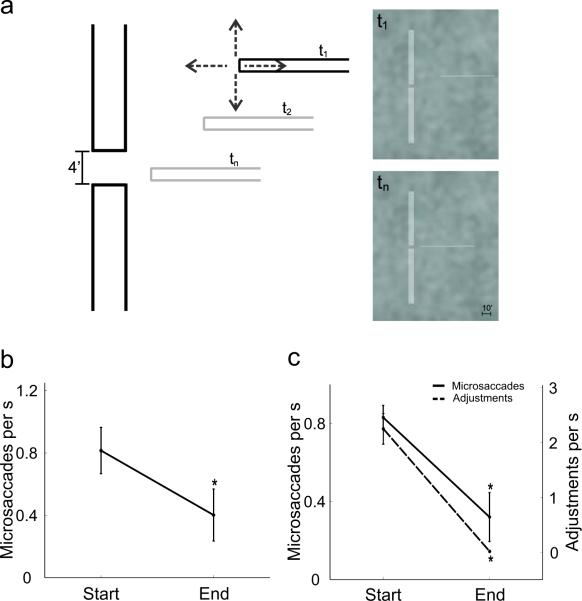
As illustrated in Figure 1a, participants were asked to insert a horizontal bar (the thread) into a small aperture at the center of a stationary vertical bar (the needle). Both bars were displayed on a CRT monitor and embedded in a noisy background. This task enabled replication of the results reported by previous studies. Figure 1b shows data obtained under conditions similar to those of Bridgeman and Palca. As in this previous study, subjects had no control over the thread's position. They were asked to maintain accurate fixation on the eye of the needle, while the thread approached the needle with constant velocity and stopped at a fixed distance. In agreement with previous results, the mean frequency of microsaccades at the end of the trial was significantly lower than at the beginning of the trial. Thus, microsaccades appear to be suppressed at the time of high-acuity judgments.
Figure 1.
Threading a virtual needle. (a) The arena in which all the threading experiments of this study were conducted. Subjects used a joypad to align a horizontal bar (the thread) with the gap in a vertical bar (the needle). The gray bars represent the positions of the thread at various times during the course of the trial. The actual stimulus on the display is shown in the right panels for two different times, t1 and tn. (Bottom Row) Results from two experiments with conditions similar to those of previous studies: (b) Bridgeman and Palca (1980), and (c) Winterson and Collewijn (1976). The two intervals refer to the initial 4 s (Start) and the last 0.5 s (End) in each trial. In (c), both the mean microsaccade rate and the frequency of adjustments in the thread's vertical position are shown. Significant differences between the initial and final periods are marked by * (p < 0.01; one-tailed t-test). In this and all the following figures, error-bars represent s.e.m..
Figure 1c shows data obtained under conditions similar to those of Winterson and Collewijn. In this experiment, subjects were free to move their eyes normally and fully controlled the position of the thread. Each trial ended when the thread successfully passed through the eye of the needle. Again, the rate of microsaccades dropped significantly at the end of the trial, as previously reported10. Thus, microsaccades also appear to be suppressed during finely-guided visuomotor tasks.
Findings of microsaccade suppression similar to those shown in Figure 1b and c suggest that microsaccades are detrimental in tasks that require high visual acuity. However, a reduction in microsaccade rate before perceptual reports (Fig. 1b) and at the completion of the visuomotor task (Fig. 1c) does not imply that microsaccades were not helpful at earlier times during the course of the trial, when perceptual judgments were formed. In the conditions of Figure 1b, the requirement for sustained fixation could have influenced microsaccade production. In the conditions of Figure 1c, the probability of correcting the vertical position of the thread also decreased together with the microsaccade frequency at the end of the trial. That is, microsaccade rates reached their minimum at a time at which observers no longer adjusted the position of the thread.
To further investigate the possible contributions of microsaccades in high-acuity vision, we simulated threading under conditions intermediate to those of the experiments in Figure 1b and c. Subjects were allowed to freely move their eyes and control the thread's position, but the trial ended when the thread and the needle were at a distance at which evaluation of their correct alignment was still difficult. In order to ensure that modifications in the thread position were always the result of perceived misalignment between the thread and the needle, we restricted control of the thread to the vertical axis only. On the horizontal axis, the thread approached the needle at a constant velocity (1.4'/s).
Figure 2 compares the saccades measured in this experiment to those recorded from the same observers during prolonged fixation on a stationary marker, and during free viewing of images of natural scenes. As shown by these data, both the frequency and amplitude of saccades varied greatly with the task. Amplitude distributions were very similar during threading and sustained fixation. In both cases, the median was around 20'. In contrast, the amplitudes of saccades were spread more uniformly in free viewing, a condition in which saccades were more frequent than in the threading task (saccade rate during free viewing: 2.49 ± 0.46; threading: 1.56 ± 0.25; p=0.002, paired t-test). In agreement with previous reports, the rate of microsaccades was on average lower during threading than during sustained fixation, even though this difference fell short of statistical significance (p=0.074; one-tailed paired t-test). This rate was, however, five times higher than the rate measured during free viewing of natural images. Thus, the condition chosen as comparison plays a critical role in evaluating microsaccade frequency.
Figure 2.
Comparison of saccade characteristics in three different tasks: threading, sustained fixation on a marker, and free viewing of natural images. (a–c) Distributions of saccade amplitudes. The triangles mark the medians of the distributions. The insert panel in (c) displays the range of small amplitudes. (d) Mean amplitudes of microsaccades, defined as saccades smaller than 20' (ANOVA with Scheffe post-hoc comparisons: (*) p = 0.009; (**) p = 0.004). Movies of individual trials in the threading task can be found as Supplementary Videos 1 and 2.
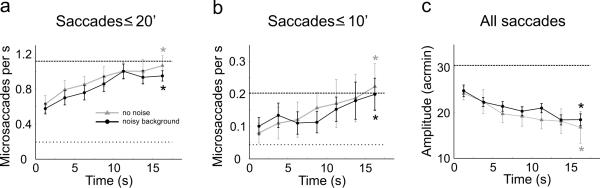
Unlike during fixation on a stationary marker and free viewing of static pictures, the stimulus displayed during threading changed dynamically according to the subject's commands. We examined whether these changes in the stimulus modulated the characteristics of saccades. As shown in Figure 3, the frequency of microsaccades increased during the course of a trial. Both saccades smaller than 20' and 10' were significantly more frequent during the final 2.5 s of a trial than during the initial 2.5 s. This increment in microsaccade rate coincided with a decrement in the mean amplitude across all saccades (see Fig. 3c). That is, saccades became progressively smaller during the course of a trial, a change which reflected the narrowing of the separation between the thread and the needle. This effect was not a consequence of background noise, which could have forced subjects to look for points in which the thread and the needle were more visible. Indeed, highly similar results were obtained in the absence of background noise, when the thread and the needle were clearly visible.
Figure 3.
Modulation of saccade characteristics. (a, b) Mean instantaneous frequency of microsaccades, defined as: (a) saccades smaller than 20'; and (b) saccades smaller than 10'. (c) Mean instantaneous saccade amplitude. The two curves in each panel represent data obtained in the presence and absence of background noise. In this latter condition, the background was at a constant grey level and the stimulus was displayed at maximum contrast. Horizontal lines in each panel indicate mean values during sustained fixation (dashed line) and free viewing (dotted line). * marks conditions in which measured values were significantly higher during the last 2.5 s of a trial than during the initial 2.5 s (p < 0.04; one-tailed t-test in a, b and Wilcoxon signed-rank test in c).
The data in Figure 3 suggest that the visual system calibrated saccades on the basis of the distance between the thread and the needle: the closer the tip of the thread to the needle, the smaller was the saccade. This dependence might originate from an oculomotor strategy in which microsaccades relocate the line of sight back and forth between the two objects. To determine whether this was indeed the case, we examined the spatial distribution of fixations during a trial.
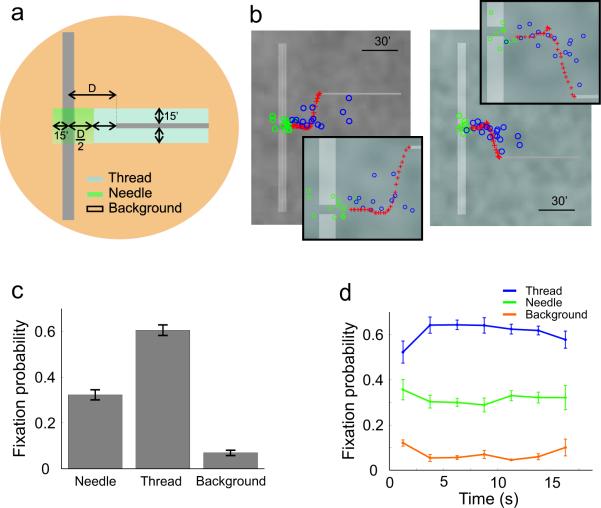
In the periods in between successive saccades, slow movements keep the eye continually in motion. With the low velocity of the thread used in this study, no sign of pursuit was present in the recorded data. Ocular drift was highly similar to that measured during sustained fixation on a marker and kept the eye within a region with mean radius 6' ± 3'. Because of this motion, we classified each intersaccadic interval as a fixation on the thread, on the needle, or on the background according to the location of the mean position of the eye's trajectory during the considered interval (Fig. 4a).
Figure 4.
Analysis of fixation locations. (a) Each intersaccadic period was classified as a fixation on the eye of the needle, the thread, or the background according to the location of its centroid. The distance D between the needle and the thread varied during the course of the trial. (b) Two examples of spatial distributions of fixations. Each panel corresponds to a different experimental trial. Blue and green circles represent fixations on the thread and on the eye of the needle, respectively. Orange circles indicate fixations on the background. The red crosses mark the trajectory followed by the thread. The insert panels zoom in on the center of the display. (c) Mean probabilities of fixation locations. Differences across all conditions are significant (ANOVA with Scheffe post-hoc comparisons, p < 0.002). (d) Fixation probabilities at successive intervals during the course of the trial.
Fixations were clustered around the eye of the needle and the tip of the thread (see examples in Fig. 4b). Very few fixations fell far from these two regions. Notably, the distributions of fixations covered the path followed by the thread during the course of the trial, suggesting that subjects precisely calibrated microsaccades in order to fixate on the moving thread. As shown in Figure 4c, subjects fixated more often on the thread than on any other part in the image; more than 50% of fixations were on the thread. This preference is understandable, given that the thread was the only component of the stimulus that changed position during a trial. Subjects also fixated often on the eye of the needle, with approximately one every three fixations in this area of the image. This distribution of fixation locations remained constant during the course of the trial (Fig. 4d).
We then examined how microsaccades contributed to oculomotor strategies. Microsaccades were often used to relocate the line of sight across objects (Fig. 5a). While fixating on the needle, microsaccades most often moved the line of sight to the thread. While fixating on the thread, however, microsaccades had a higher probability of maintaining fixation on the thread. This difference occurred because subjects made multiple consecutive fixations on the thread and spent longer time looking at this object before moving their gaze, via a microsaccade, to another region of the image (median interval consecutively spent on the thread: 876 ms; on the needle: 578 ms). It should be observed that these probability distributions might underestimate the number of relocations of gaze between the thread and the needle, particularly toward the end of the trial, when the two objects were very close to each other. As shown in Figure 5b, during the last 2.5 s in each trial, the horizontal direction of a microsaccade was very likely to be opposite to that of the previous microsaccade.
Figure 5.
Analysis of microsaccades. (a) Probabilities of various types of microsaccades during fixation on the needle and on the thread. Microsaccades are subdivided according to where they landed. Data refer to saccades smaller than 20'. (b) Influence of microsaccade direction on the direction of the following microsaccade. During the last 2.5 s in each trial, consecutive microsaccades possessed opposite directions on the horizontal axis. In both graphs, all differences within each group are statistically significant (paired z-test with Bonferroni corrections, p < 0.001).
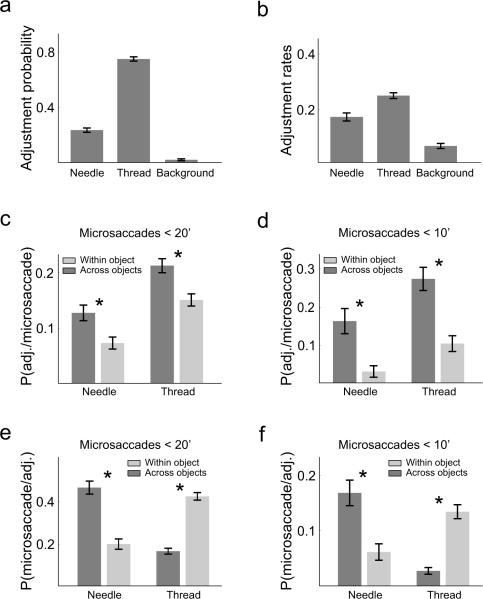
We wondered whether microsaccades contributed to the estimation of the alignment between the thread and the needle. To this end, we first determined during which fixations subjects were more likely to adjust the position of the thread. Figure 6a shows the probability of realigning the thread during fixation on different regions of the scene. Not surprisingly, adjustments were more likely to occur while subjects fixated on the thread than other parts of the image. This result was, however, a consequence of the uneven distributions of fixations within the scene, since most fixations were also dedicated to the thread (see Fig. 4c). Indeed, the mean rates of adjustments, i.e., the mean numbers of corrections per fixation, were actually quite similar during fixations on the thread and the needle (Fig. 6b).
Figure 6.
Interplay between microsaccades and corrections in the thread-needle alignment. (a) Probability distributions of adjustments to the thread's position as a function of the location of fixation during which they occurred. (b) Rates of adjustments. Data points represent the average numbers of changes in the thread's position per fixation. In both a and b, all differences are statistically significant (paired z-test with Bonferroni corrections, p < 0.01). (c–d) Conditional probabilities of adjustments following different types of microsaccades. The fixation in which the adjustment occurred (x-axis) is the target destination of the microsaccade. (e–f) Conditional probabilities of performing different types of microsaccades following an adjustment. The fixation in which the adjustment occurred (x-axis) is the origin of the microsaccade. Data are shown for both microsaccades smaller than 20' (c,e) and 10' (d,f). In c–f, microsaccades are arranged according to whether they maintained fixation on the same object (thread or needle) or moved the line of sight from one to the other. * marks significant differences (paired z-test with Bonferroni corrections, p < 0.05).
The two bottom rows of Figure 6 show the oculomotor strategies preceding and following adjustments in the thread's position. The data in Figure 6c and d represent the probability of changing the position of the thread immediately after different types of microsaccades. Subjects were more likely to correct the thread's position after executing a microsaccade which moved the preferred retinal location from one object (the thread or the needle) to the other, than after prolonged fixation on the same object. This effect occurred for microsaccades of all amplitudes, but was particularly pronounced after microsaccades smaller than 10' (Fig. 6d). In contrast, after performing an adjustment, microsaccades were likely to move the preferred retinal location toward the thread (Fig. 6e and f). That is, the first microsaccade following an adjustment had a high probability of (a) moving the line of sight onto the thread when the subject performed the adjustment while fixating on the needle, and (b) keeping the center of gaze on the thread when the correction was performed during fixation on the thread. Thus, microsaccades were used to either look back or gain a new view of the moving thread after realigning its position.
These data, together with the different patterns of eye movements observed in successful and unsuccessful trials (see Supplementary Results and Supplementary Fig. 1), show that microsaccades were part of the oculomotor strategy by which subjects acquired information about the alignment between the thread and the eye of the needle.
Discussion
Although microsaccades have attracted considerable interest since their first quantitative measurements16,17, their visual functions have remained subject of controversy2,3. In our experiments, saccades smaller than 20' precisely relocated the line of sight according to the ongoing demands of the task. These results contradict the widespread assumption that microsaccades are suppressed during high-acuity judgments and support the proposal that microsaccades are exploratory movements like larger saccades9,12.
The proposal that microsaccades differ from saccades in amplitude but not in function is consistent with multiple experimental observations. It is known that the area of preferred target location in the retina is small, with a standard deviation of only 3.4'18,19. Thus, targets displaced by more than a few minutes of arc are likely to fall outside this region, and the line of sight may need to be reoriented in order to ensure optimal vision. For example, under the assumption that the probability of target location is uniformly distributed within the preferred retinal region, a stimulus located only 5' away from the current fixation would fall outside of the preferred retinal region in more than 50% of the cases. Furthermore, microsaccades and saccades exhibit similar motor characteristics and share a common neural substrate20. In fact, there is no clear distinction between the two types of movements, and their amplitudes form a continuum (see Fig. 2c). It is also known that saccades with amplitudes within the range of microsaccades can be voluntarily executed both to track small displacements of a fixated target21 and to look away from a stimulus22. Thus, our findings are consistent with the observations that (a) relocations of the line of sight of a few minutes of arc are sometimes necessary in order to ensure optimal vision, and (b) the oculomotor system is capable of performing such relocations by means of microsaccades.
The conclusions of the present study conflict with prior reports; the results of the prior reports were confirmed (Fig. 1b,c) but now subject to a very different interpretation. The observation that microsaccades are rare immediately before shooting a rifle or threading a needle10, two tasks in which shifts of attention between small details would be expected to be crucial, has been taken to imply that microsaccades are detrimental in tasks that require high visual acuity. However, a similar reduction in microsaccade frequency is also to be expected if microsaccades contribute information about the stimulus. Microsaccades might no longer be needed at the end of the trial, when the positions of the thread and needle practically overlap on the retina and their alignment can be assessed comfortably without the need for relocating the line of sight. In fact, a decrement in microsaccade rate also occurred in our experiments when the thread was allowed to reach and go through the needle (see Fig. 1c). However, the frequency of microsaccades only dropped when the distance between the tip of the thread and the needle was smaller than 5'. In contrast, microsaccades occurred frequently at earlier times, when the thread was farther from the needle, and subjects still adjusted the thread/needle alignment.
It is also known that the mean rates of microsaccades during threading and shooting are lower than during sustained fixation on a marker10. Across the five naive subjects who participated in this previous study, the mean rate decreased by approximately 30%, a value similar to that measured in our experiments (26% for saccades smaller than 20'). Again, this reduction does not imply that microsaccades are not helpful in high-acuity tasks. In our experiments, the mean rate of microsaccades measured during threading was lower than that observed during fixation, but also substantially higher than that measured during free viewing of natural images. This task-dependence of microsaccades emphasizes the difficulty of choosing a control condition to use as baseline.
The results of this study stress the need for distinguishing between different types of microsaccades23,24. As previously reported in the literature11 and confirmed in our experiments (see Fig. 1b), a reduction in microsaccade rate occurs when subjects are asked to maintain fixation on the eye of the needle and passively observe the motion of the thread. This effect is probably caused by a change in the accuracy of fixation control during the course of the experiment. Most (but not all) observers perform frequent microsaccades while attempting to maintain steady fixation on a marker, even if no stimulus other than the fixation cue is displayed. These fixational saccades appear to serve a different function from the exploratory microsaccades observed when subjects are free to move their eyes. Fixational saccades can be voluntarily suppressed12 and their frequency depends on the precision of intended fixation13. In the experiment of Figure 1b, a suppression of fixational saccades is to be expected at the time of perceptual judgments, as these saccades are not pertinent to the task and may impair performance. Exploratory microsaccades and fixational saccades can hardly be distinguished in experiments in which stimuli are observed while maintaining accurate fixation on a marker. Confusion between these two types of eye movements has probably contributed to the long-standing controversy over the visual functions of microsaccades.
In our experiments, the probability of correcting the thread's position was significantly higher after a microsaccade shifted the line of sight from one object to the other than during prolonged fixation, a finding reminiscent of the way saccades precede hand movements in manipulation tasks25–28. The precision by which microsaccades relocated the line of sight between the thread and the needle suggests that these movements contributed to visual acuity. Such a contribution would also provide an explanation for previously reported improvements in fine spatial discrimination measured in the presence of microsaccades29. Our findings, however, do not exclude other hypotheses, including possible enhancements in contrast sensitivity following microsaccades. Further studies are needed to distinguish among hypotheses and investigate the physiological mechanisms by which microsaccades enhance the perception of fine detail.
Methods
Participants
Ten subjects with normal vision participated in the experiments of this study. Six subjects participated in the main experiment (Figs. 2–6), with three of them also taking part in the control experiments of Figure 1. Five subjects participated in the experiment of Figure 7. With the exception of one experienced observer, all subjects were naive about the purposes of the experiments and were paid to participate. Informed consent was obtained from all participants following the procedures approved by the Boston University Charles River Campus Institutional Review Board.
Apparatus
Stimuli were displayed on a fast phosphor CRT monitor (Iyama HM204DT) at a resolution of 800x600 pixels and vertical refresh rate of 200 Hz in a dimly-illuminated room. A dental imprint bite bar and a head rest prevented movements of the head and kept subjects at a distance of 126 cm from the monitor. Stimuli were observed monocularly with the right eye, while the left eye was patched. Stimuli were rendered by means of EyeRIS14, a hardware/software system for gaze-contingent display control which enables precise synchronization between eye movement data and the refresh of the image on the monitor, as well as accurate spatial localization of the line of sight. Vertical and horizontal eye position data were sampled at 1 kHz and recorded for subsequent analysis. Subjects used different buttons on a joypad to control the x and y coordinates of the thread on the screen. Adjustments occurred in discrete steps; each button press moved the thread by 1.4'.
Stimuli and Procedure
Data were collected in separate experimental sessions, each of approximately 20 minutes. Every experimental session started with preliminary setup operations that lasted a few minutes and allowed the subject to adapt to the low level of light in the room. These preliminary operations included: (a) positioning the subject optimally and comfortably in the apparatus; (b) tuning the eyetracker; and (c) calibrating EyeRIS. Subjects were never constrained in the experimental setup for more than 30 minutes consecutively.
Accurate localization of the line of sight is necessary in order to examine the way eye movements scan the scene during high-acuity judgments. To optimize the transformation of the eye position measurements given by the eyetracker into screen coordinates, a dual-step calibration procedure, in which the subject observed and refined the estimated position of the preferred retinal location, preceded each block of trials. In the first phase of the calibration, the subject sequentially fixated on nine points evenly spaced within the working area of the display, as in standard calibration routines. For each point, the mean output voltage from the eye tracker was estimated over a period of 3.5 s. The mapping from eye-position coordinates to degrees of visual angle was determined by bilinear interpolation over the mean eye positions measured at these nine points. This transformation was made possible by virtue of the highly linear behavior of the DPI eyetracker within the central region of the visual field. In the second phase of the calibration procedure, subjects fine-tuned the gaze-to-pixel mapping using a gaze-contingent display technique. In this phase, subjects adjusted the position of a cross, displayed in real time on the screen at the estimated preferred retinal location, while sequentially fixating again on the nine points of the calibration grid. Subjects used the buttons on EyeRIS' joypad to finely adjust the position of the cross at each of these fixation points. These refinements were then incorporated into the offsets and gains of the bilinear interpolation. This method effectively reduces the dispersion of eye position measurements during calibration and improves the precision of the voltage-to-pixel mapping.
Subjects threaded a needle in a simulated virtual environment. The “needle” consisted of two aligned vertical bars (each 68' × 7') with a 4' gap (the “eye of the needle”) in the center. A horizontal bar (68' × 1.4') served as “thread” (see Fig. 1). The needle always remained immobile at the center of the screen. The thread started from a random location to the right of the needle and moved following the subject's commands (the initial distance from the needle was always 31' on the x-axis). With the exception of the experiment in Figure 1c, subjects only controlled the vertical position of the thread; on the horizontal axis, the thread approached the needle with constant speed of 1.4'/s and stopped 7' from the needle, so that each trial lasted for 17.5 s. In the experiment of Figure 1b, the thread followed a previously recorded trajectory, which varied randomly across trials. Subjects evaluated whether or not the thread was correctly aligned with the eye of the needle after the thread stopped moving. In the experiment of Figure 1c, the thread went through the needle until its tip reached the needle's central axis.
The thread and the needle were displayed at the same contrast level over a noisy background. The spectral density of the background declined as 1/f2 with the spatial frequency f and was low-pass filtered with cut-off frequency at 5 cycles/deg. The mean luminance of the background was 16 cd/m2. To normalize the difficulty of the task across subjects, the contrast of the stimulus was adjusted individually for each subject so that successful completion of the task occurred in approximately 85% of the trials in the main experiment (Figs. 2–6) and 50% of the trials in the experiment of Supplementary Figure 1. Contrast values were selected during preliminary experimental sessions. Figure 3 also shows data collected in the absence of background noise, i.e., when the stimulus was displayed at maximum contrast over a uniformly gray field (luminance 16 cd/m2). In this condition the needle and the thread were clearly visible and subjects always successfully accomplished the task.
Figure 2 compares microsaccade rates measured during threading to those observed during sustained fixation and free viewing. In the sustained fixation condition, subjects maintained fixation on a black dot (4' × 4') on a homogeneous gray background for 17.5 s. In the free viewing condition, subjects freely explored gray-scale images of natural scenes extracted from a public domain database26. Each image subtended a visual angle of 18.1° × 13.6° and was displayed for 10 s.
Data Analysis
Movements with maximum speed higher than 3°/s and amplitude larger than 1' were classified as saccades. Saccade amplitude was defined as the distance between the locations in which eye velocity became greater (saccade onset) and lower (saccade offset) than 2°/s. Classification of eye movements was performed automatically and then validated by human experts. Mean instantaneous rates and amplitudes were evaluated over consecutive, non-overlapping bins of 2.5-s duration. Periods of blinks were automatically removed from data analysis.
The conditional probabilities of Figure 6c,d and the distributions of Supplementary Figure 1d were calculated by selecting all the adjustments preceded by a microsaccade. To be included in the analysis, an adjustment had to occur within 1.5 s after the end of a microsaccade and no other saccade had to be present in between these two events. Similar criteria were applied to the interval between the time of an adjustment and the onset time of a later microsaccade in order to select microsaccades which followed adjustments and compute the conditional probabilities of Figure 6e,f.
Supplementary Material
Acknowledgments
The authors thank Antonino Casile and David Richters for helpful comments on the manuscript. This work was supported by grant EY18363 from the National Institute of Health and grants BCS-0719849 and IOS-0843304 from the National Science Foundation.
References
- 1.Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32(2):185–201. doi: 10.1016/s0896-6273(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 2.Collewijn H, Kowler E. The significance of microsaccades for vision and oculomotor control. J. Vis. 2008;8(14:20):1–21. doi: 10.1167/8.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolfs M. Microsaccades: Small steps on a long way. Vision Res. 2009;49(20):2415–2441. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Ditchburn RW, Ginsborg BL. Vision with a stabilized retinal image. Nature. 1952;170(4314):36–37. doi: 10.1038/170036a0. [DOI] [PubMed] [Google Scholar]
- 5.Ditchburn RW, Fender DH, Mayne S. Vision with controlled movements of the retinal image. J. Physiol. 1959;145(1):98–107. doi: 10.1113/jphysiol.1959.sp006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract fading during fixation. Neuron. 2006;49(2):297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Cornsweet TN. Determination of the stimuli for involuntary drifts and saccadic eye movements. J. Opt. Soc. Am. 1956;46(11):987–993. doi: 10.1364/josa.46.000987. [DOI] [PubMed] [Google Scholar]
- 8.Engbert R, Kliegl R. Microsaccades keep the eyes' balance during fixation. Psychol. Sci. 2004;15:431–436. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 9.Cunitz RJ, Steinman RM. Comparison of saccadic eye movements during fixation and reading. Vision Res. 1969;9:683–693. doi: 10.1016/0042-6989(69)90125-4. [DOI] [PubMed] [Google Scholar]
- 10.Winterson BJ, Collewijn H. Microsaccades during finely guided visuomotor tasks. Vision Res. 1976;16(12):1387–1390. doi: 10.1016/0042-6989(76)90156-5. [DOI] [PubMed] [Google Scholar]
- 11.Bridgeman B, Palca J. The role of microsaccades in high acuity observational tasks. Vision Res. 1980;20(9):813–817. doi: 10.1016/0042-6989(80)90013-9. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM, Cunitz RJ, Timberlake GT, Herman M. Voluntary control of microsaccades during maintained monocular fixation. Science. 1967;155(769):1577–1579. doi: 10.1126/science.155.3769.1577. [DOI] [PubMed] [Google Scholar]
- 13.Poletti M, Rucci M. Fixational eye movements under various conditions of image fading. J. Vis. 2010;10(3):1–18. doi: 10.1167/10.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santini F, Redner G, Iovin R, Rucci M. EyeRIS: A general-purpose system for eye movement contingent display control. Behav. Res. Methods. 2007;39(3):350–364. doi: 10.3758/bf03193003. [DOI] [PubMed] [Google Scholar]
- 15.Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447(7146):852–855. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- 16.Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. J. Exp. Psychol. 1950;40(6):687–701. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- 17.Ditchburn RW, Ginsborg BL. Involuntary eye movements during fixation. J Physiol. 1953;119:1–17. doi: 10.1113/jphysiol.1953.sp004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putnam NM, et al. The locus of fixation and the foveal cone mosaic. J. Vis. 2005;5(7):632–639. doi: 10.1167/5.7.3. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM. Effect of target size, luminance and color on monocular fixation. J. Opt. Soc. Am. 1965;55:1158–1165. [Google Scholar]
- 20.Hafed Z, Goffart L, Krauzlis R. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009;323:940–943. doi: 10.1126/science.1166112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timberlake GT, Wyman D, Skavenski AA, Steinman RM. The oculomotor error signal in the fovea. Vision Res. 1972;12:1059–1064. doi: 10.1016/0042-6989(72)90027-2. [DOI] [PubMed] [Google Scholar]
- 22.Haddad GM, Steinman RM. The smallest voluntary saccade: Implications for fixation. Vision Res. 1973;13:1075–1086. doi: 10.1016/0042-6989(73)90145-4. [DOI] [PubMed] [Google Scholar]
- 23.Snodderly DM. Effects of light and dark environments on macaque and human fixational eye movements. Vision Res. 1987;27(3):401–15. doi: 10.1016/0042-6989(87)90089-7. [DOI] [PubMed] [Google Scholar]
- 24.Kagan I, Gur M, Snodderly DM. Saccades and drifts differentially modulate neuronal activity in V1: Effects of retinal image motion, position, and extraretinal influences. J. Vis. 2008;8(14):1–25. doi: 10.1167/8.14.19. [DOI] [PubMed] [Google Scholar]
- 25.Epelboim J, Steinman RM, Kowler E, Edwards M, Pizlo Z, Erkelens CJ, Collewijn H. The function of visual search and memory in sequential looking tasks. Vision Res. 1995;35:3401–3422. doi: 10.1016/0042-6989(95)00080-x. [DOI] [PubMed] [Google Scholar]
- 26.Ballard DH, Hayhoe MM, Pelz JB. Memory representations in natural tasks. J. Cogn. Neurosci. 1995;7:66–80. doi: 10.1162/jocn.1995.7.1.66. [DOI] [PubMed] [Google Scholar]
- 27.Land M, Mennie N, Rusted J. The roles of vision and eye movements in the control of activities of daily living. Perception. 1999;28:1311–1328. doi: 10.1068/p2935. [DOI] [PubMed] [Google Scholar]
- 28.Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J. Neurosci. 2001;21:6917–6932. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rucci M, Desbordes G. Contributions of fixational eye movements to the discrimination of briefly presented stimuli. J. Vis. 2003;3(11):852–864. doi: 10.1167/3.11.18. [DOI] [PubMed] [Google Scholar]
- 30.van Hateren JH, Ruderman DL. Independent component analysis of natural image sequences yields spatio-temporal filters similar to simple cells in primary visual cortex. Proc. Biol. Sci. 1998;256(1412):2315–2320. doi: 10.1098/rspb.1998.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.