Abstract
Addiction has been described as the pathological usurpation of the neural mechanisms normally involved in emotional processing. Event-related potentials (ERPs) can provide a noninvasive index of neural responses associated with the processing of emotionally relevant stimuli and serve as a tool for examining temporal and spatial commonalities between the processing of intrinsically motivating stimuli and drug cues. Before beginning a smoking cessation program, 116 smokers participated in a laboratory session in which dense-array ERPs (129 sensors) were recorded during the presentation of pictures with emotional (pleasant and unpleasant), neutral, and cigarette-related content. ERP differences among categories were analyzed with use of randomization tests on time regions of interest identified by temporal principal component analysis. Both emotional and cigarette-related pictures prompted significantly more positivity than did neutral pictures over central, parietal, and frontal sites in the 452–508 ms time window. During the 212–316 ms time window, both pleasant and cigarette-related pictures prompted less positivity than neutral images did. Cigarette-related pictures enhanced the amplitude of the P1 component (136–144 ms) above the levels measured in the emotional and neutral conditions. These results support the hypothesis that for smokers, cigarette-related cues are motivationally relevant stimuli that capture attentional resources early during visual processing and engage brain circuits normally involved in the processing of intrinsically emotional stimuli.
Keywords: attention, emotion, ERP, LPP, P1, smoking
INTRODUCTION
Tobacco smoking contributes to the death of more than half of its regular users (Mackay et al. 2006). Although the adverse effects of tobacco on human health have been widely disseminated since the 1964 Surgeon General Report (Smoking and Health 1964), approximately 20% of the U.S. population currently smokes (CDC 2007). Many of these smokers report wanting to quit smoking, but most of those who try (85%) relapse, often within the first week after the cessation attempt (NIDA 2009). Both the presence of cigarette cues and affective experiences have been cited by smokers in conjunction with smoking relapse (Shiffman et al. 2007). Thus, determining the underlying neural processes activated by cigarette and emotional (pleasant and unpleasant) cues is important for understanding the long-term neural adaptations associated with chronic smoking.
Several researchers have argued that cues predicting drug availability “hijack” the neural mechanisms that under normal circumstances shape survival behaviors related to the pursuit of reward (Everitt et al. 2001; Robinson and Berridge 2003; Hyman 2005). Although this hypothesis has received substantial support from animal studies, it has rarely been tested in humans (Leyton 2007; Robinson and Berridge 2008). Cue reactivity paradigms involving humans (i.e., experiments in which physiological responses in the presence of visual cues are recorded) usually include only drug-related and neutral cues (Warren and McDonough 1999; McDonough and Warren 2001; Littel and Franken 2007). Without measuring reactivity to other intrinsically emotional stimuli, it is difficult to argue that smokers (or those dependent on other substances) show similar brain reactions in the presence of drug-related and emotional cues.
Given their sensitivity to the neural responses elicited by affective stimuli, event-related potentials (ERPs) can provide an excellent noninvasive tool to evaluate whether humans process drug-related and emotional stimuli similarly. For example, the presentation of emotionally provocative images reliably increases the amplitude of the late positive potential (LPP). Both pleasant and unpleasant stimuli increase the LPP recorded over central and parietal sites between 400 and 700 ms after stimulus onset (Schupp et al. 2000; Cuthbert et al. 2000; Keil et al. 2002). This effect shows high temporal stability (Codispoti et al. 2007), is particularly pronounced for highly arousing pictures (e.g., erotica or mutilations) (Schupp et al. 2004a), and is resistant to manipulations affecting perceptual composition (De Cesarei and Codispoti 2006; Bradley et al. 2007) or exposure time (Codispoti et al. 2009) of the visual stimuli. These results support the idea that motivational significance is the main dimension affecting the LPP and that its presence indicates the ability of emotionally relevant visual stimuli to engage neural circuitry associated with motivation (Bradley 2009; Lang and Bradley 2010).
Another ERP component modulated by the presentation of emotional visual stimuli can be observed over occipitotemporal sites between 200 and 300 ms after stimulus onset. Within this time window, emotional images prompt less positivity than neutral ones (Schupp et al. 2003; Schupp et al. 2004b). Schupp and colleagues (Schupp et al. 2006) referred to this effect as early posterior negativity (EPN) and have suggested that it reflects the ability of emotional pictures to capture attention during the early stages of the visual processing. Unlike the LPP, this component is affected by manipulations that alter the perceptual features of the stimuli (e.g., picture size (Codispoti and De 2007) or stimulus complexity (Bradley et al. 2007)) but not by stimulus repetition (Codispoti et al. 2006; Codispoti et al. 2007). Together, these data suggest that EPN may reflect more obligatory perceptual processes needed to encode and recognize picture contents rather than emotional processing per se (Bradley et al. 2007).
On the basis of these findings, we hypothesized that in nicotine-addicted individuals, cigarette-related cues, a category of stimuli that acquired motivational significance by being repeatedly associated with nicotine delivery, will elicit ERPs comparable to those observed in the presence of other emotional stimuli. Specifically, in smokers we expect the presence of cigarette-related images to enhance LPP and EPN amplitudes to the levels observed when intrinsically emotional stimuli are viewed.
To test this hypothesis we recorded dense-array (129 sensors) ERPs from a large group of smokers during the presentation of neutral, emotional (pleasant and unpleasant), and cigarette-related pictures. Unlike previous cue reactivity studies (Warren and McDonough 1999; McDonough and Warren 2001; Littel and Franken 2007), the inclusion of intrinsically emotional stimuli, in addition to the cigarette-related ones, granted us the opportunity to interpret the ERP results within the theoretical frame provided by emotional neuroscience. The use of the dense array allowed us to investigate both temporal and spatial commonalities in the neural processing of intrinsically emotional and drug-related stimuli. To ensure the generalizability of our results to a clinically important population, we included a sizable and representative sample of smokers who were interested in quitting.
METHODS
Participants
Participants were recruited via local (Houston metropolitan area) radio and newspaper advertisements requesting volunteers who wanted to quit smoking and were willing to participate in a smoking cessation medication clinical trial. After screening for basic trial-related inclusion and exclusion criteria, eligible participants completed a baseline laboratory session, which was the source of our data. This session was conducted before any treatment randomization or intervention. Two additional laboratory sessions were conducted at follow-up after participants tried to stop smoking. The data from these latter two laboratory sessions, as well as the outcome of the clinical trial, will be the subject of future articles. The current study focused only on results from the baseline session.
A total of 154 participants met the basic inclusion criteria: age 18–65 years, smoke 5 or more cigarettes per day, have a baseline expired CO greater than 6 ppm, have fluent English skills, have a working telephone, not currently taking psychotropic medication, not have a current psychiatric disorder including substance abuse (except for smoking), not be involved in any smoking cessation activities, have no contraindications for bupropion or varenicline, and have no uncontrolled medical illness. Because of poor recording quality (21 participants) or technical errors (17 participants), laboratory data from 38 participants were discarded, yielding a total of 116 participants in this study. Table 1 provides the demographic characteristics of the sample used for data analyses. All participants provided informed consent before being subjected to any study procedure, and the research was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Table 1.
Demographics and Smoking Characteristics of the Sample
| Variable | Total (N = 116) |
|---|---|
| Race/ethnicity | % (N) |
| African-American, non-Hispanic | 24.1 (28) |
| White, non-Hispanic | 57.0 (66) |
| Hispanic | 11.2 (13) |
| Other | 7.7 (9) |
| Gender | |
| Male | 67.2 (78) |
| Female | 32.8 (38) |
| Mean (SD) | |
| Age (years) | 44.7 (11.1) |
| Education level (years) | 13.8 (2.0) |
| Years smoked | 24.3 (12.0) |
| Smoking rate (cigs/day) | 19.2 (8.6) |
| Expired carbon monoxide | 25.0 (13.8) |
| Time since last cigarette (hours) | 1.1 (1.7) |
| FTND score* | 4.6 (2.1) |
FTND = Fagerström Test for Nicotine Dependence
Material and design
Three equivalent picture sets were created by selecting pictures from the International Affective Picture System (Lang et al. 2005) and from cigarette-related picture collections previously used in our (Carter et al. 2006) and other (Gilbert and Rabinovich 1999) laboratories. Each set included 4 picture categories (pleasant, unpleasant, neutral, and cigarette-related) with 24 slides each (total, 96 slides per set). During the slide presentation, images were presented in pseudo-random sequences with no more than two pictures of the same category presented consecutively. Each picture was presented for 4 seconds and was followed by a random intertrial interval of 3–5 s, during which the screen had a black background with a white fixation cross. The entire picture presentation lasted approximately 30 min (the pictures were presented twice during the session, for a total of 192 pictures). Each session was divided into 8 equivalent blocks lasting 3.2 minutes each and separated by a 30-s interval, during which the subject was instructed to relax. During the picture presentation, 1/4 of the slides in each category were startle probed by presenting a burst of 100 dB white noise for 50 ms between 2.5 and 3.5 s after picture onset. Since the LPP peaks between 400 and 700 ms after picture onset, the presentation of the probes did not affect the results presented here. Stimuli were presented with a Pentium 4 computer using Psychology Tools’ E-prime software (version 1.4; Pittsburgh, PA) on a plasma screen placed approximately 1.5 m from the participant’s eyes. The images subtended approximately a 24° horizontal viewing angle.
Procedure
Participants were instructed to smoke normally before the laboratory session so as to be in a non-deprived state. Upon arrival at the laboratory, participants provided an expired carbon monoxide (CO) sample and completed the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al. 1991). In addition, they filled in other questionnaires aimed at assessing positive and negative affect. After completing the questionnaires, participants were escorted into the laboratory, and the recording sensors were applied. The slide presentation began after a 15-min adaptation period and delivery of six habituation startle probes.
During the slide presentation, the electroencephalogram (EEG) was recorded using a 129-channel Geodesic Sensor Net, amplified with an AC-coupled high input impedance (200 MΩ) amplifier (Geodesic EEG System 200; Electrical Geodesics Inc., Eugene, OR), and referenced to Cz. The sampling rate was 250 Hz, and data were filtered online by using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedance of each sensor was kept below 50 KΩ, as suggested by the manufacturer.
Data reduction and statistical analyses
After data collection, a 30-Hz low-pass filter was applied off-line. Data were visually inspected, and channels contaminated by artifacts for more than 50% of the recording were interpolated with use of spherical splines. On average, approximately 2% of the channels met this criterion and were interpolated. Eye blinks were then corrected by using a spatial filtering method as implemented in BESA ver. 5.1.8.10 (MEGIS Software GmbH, Gräfelfing, Germany). After eye blink correction, the EEG data were transformed to the average reference, which was necessary for accurate topographic mapping and topographic waveform plots, and segmented into 900-ms segments starting 100 ms before onset of the picture. Baseline was defined as the 100-ms interval preceding the picture. Using the segmented data, artifacts affecting sensors within specific trials were identified. Artifacts were defined by the following criteria: EEG amplitude above 100 or below −100 μV; absolute voltage difference between any two data points within the segment larger than 100 μV; voltage difference between two contiguous data points above 25 μV; and less than 0.5 μV variation for more than 100 ms. A segment was excluded from the subsequent averages if more than 10% of the sensors within the segment were contaminated by artifacts. Overall, fewer than 5% of the segments were excluded. At the end of this process, the average ERPs were calculated at each scalp site for each category (i.e., pleasant, unpleasant, neutral, and cigarette-related).
Temporal regions of interest selection
Temporal regions of interest were identified by means of temporal principal component analysis (PCA). PCA is a factor-analytic statistical approach that accounts for patterns of covariance in the data extracting linear combinations of variables (i.e., latent components) (Dien and Frishkoff 2005). PCA was performed using JMP version 7.0.2. The voltages obtained from each electrode for each subject for each condition were used as cases, and the time points from −100 to 800 ms after picture onset were used as variables in the temporal PCA. The temporal PCA was computed on the covariance matrix, and factors accounting for more than 1% of the variance in the data were rotated by using an oblique procedure (Quartimin). The rotated factor loadings describing the time course of each factor were then rescaled to microvolts, and the peak of each factor was identified. Temporal regions of interest were defined as time points differing no more than 0.1 standard deviations from the peak of each factor. The mean voltage at each electrode within these temporal regions of interest was computed for each subject for each condition, and statistical analyses were computed on these time windows.
Statistical analyses
The presence of significant differences between each of the motivationally relevant conditions (i.e., pleasant, unpleasant, and cigarette-related) and the neutral one was calculated on the whole voltage topographies by using a randomization procedure (Maris 2004), which provided a means for controlling for Type I error inflation due to multiple comparisons from the 129-electrode configuration. The randomization procedure involved two steps: (a) computing a statistic (in this case an F statistic) for each sensor and (b) evaluating its P value under the randomization distribution. The randomization distribution was built by first randomly assigning to different data vectors the data matrix obtained for each participant within each experimental condition. The statistic of interest was then calculated for each sensor and the highest value stored. This process was repeated 10000 times to form a distribution for the F-statistic associated with the hypothesis of interest. After the construction of these randomly generated data vectors, the F-statistic was computed at each sensor by using the actual data obtained from the experiment. If the value of the F-statistic obtained during actual hypothesis testing exceeded the F-value marking the upper 5% of the distribution obtained from the random iterations, it was considered to be significant at the .05 level.
RESULTS
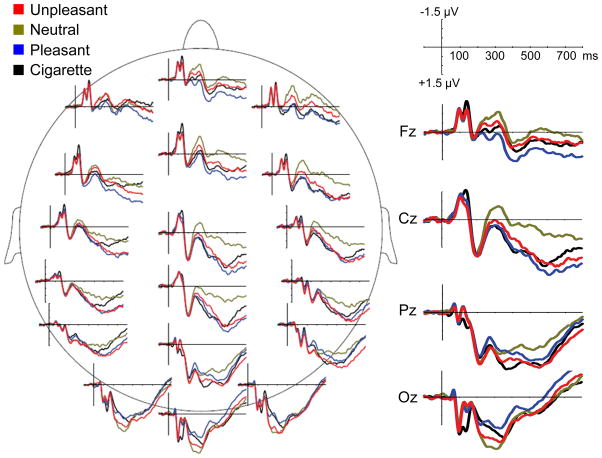
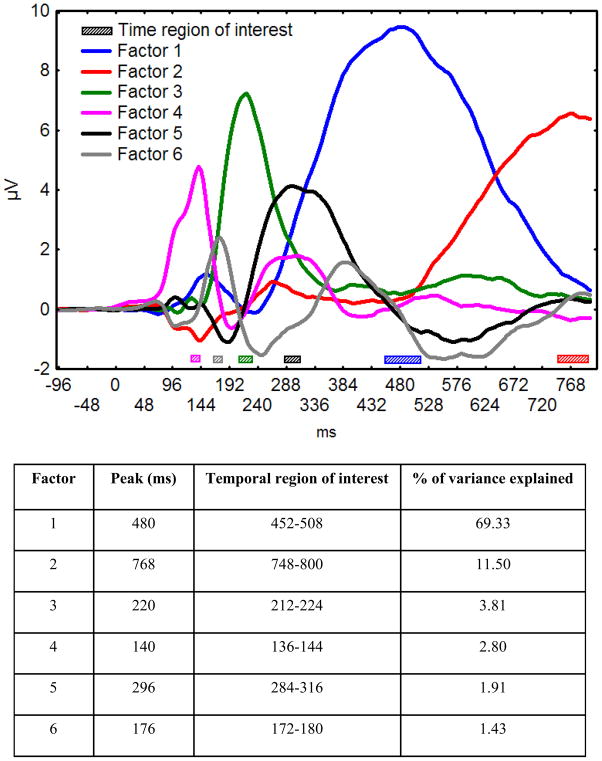
Figure 1 shows picture onset ERPs at selected sensors in the four conditions (i.e., pleasant, unpleasant, neutral, and cigarette-related). As expected, ERPs showed emotional modulation of both late and early components. The temporal PCA yielded six factors explaining 90% of the total variance in the ERP data; these six factors were retained for the rotation. Figure 2 shows the time course of the rotated factors (rescaled to μV) and indicates for each factor the amount of variance explained, its peak time, and the temporal region of interest used to compute the mean voltages in each condition for the subsequent statistical analyses. The statistical analyses indicated that Factor 2 (representing the increase of variance in the data across time) and Factor 6 did not show modulation in function of emotional content; hence, these two factors will not be discussed further.
Figure 1.
Event-related potentials (at selected locations) in response to cigarette-related, neutral, pleasant, and unpleasant pictures.
Figure 2.
(Top Panel) Time course of the rotated factors (rescaled to μV) produced by the temporal principal component analysis and temporal regions of interest used in statistical analyses. (Bottom Panel) Peak time, variance explained, and temporal regions of interest for each factor.
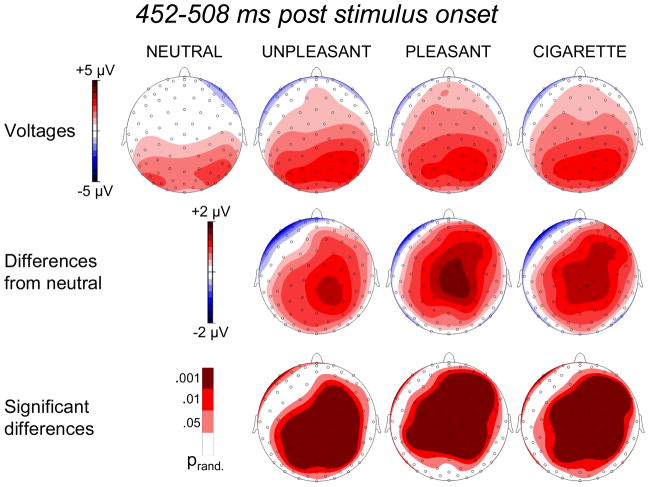
Factor 1: 452–508 ms
The time course of Factor 1 (Figure 2) is compatible with that of the late positive potential (LPP). When we plotted the distribution of the voltages within the 452–508 ms time window (Figure 3), it was evident that the presentation of emotional images increased cortical positivity well above the level observed when neutral pictures were shown. This effect was particularly pronounced over central and parietal sensors and was equally strong for pleasant and unpleasant conditions (no significant difference emerged when we performed this comparison). Importantly, the presentation of cigarette-related pictures enhanced the LPP to the same level observed when intrinsically emotional stimuli are shown. In fact, contrasting voltage topographies obtained in the presence of emotional and cigarette-related images did not yield any significant difference either in amplitude or space distribution. This result is consistent with our hypothesis and confirms that, in smokers, the presentation of cigarette-related and emotional stimuli engages the same neural emotional processes.
Figure 3.
Top row: Topographic distribution of the voltages in the 452–508 ms time region of interest during the presentation of neutral, unpleasant, pleasant, and cigarette-related pictures. Middle row: Topographic distribution of the voltage differences (motivationally significant minus neutral). Bottom row: Topographic distribution of statistically significant differences.
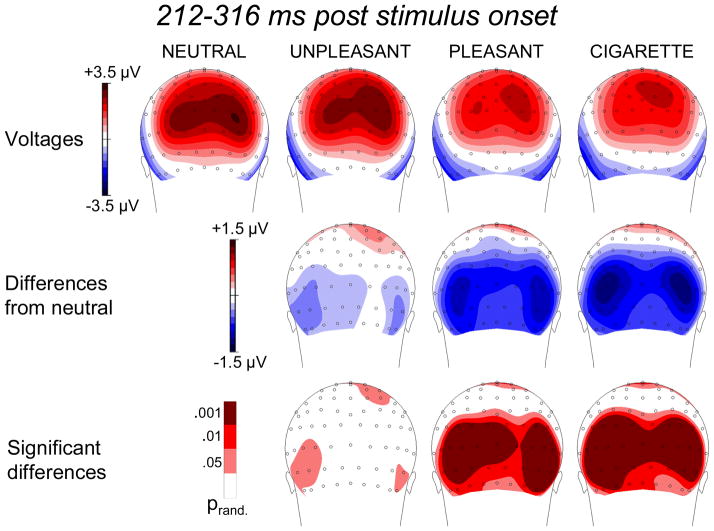
Factors 3 and 5: 212–316 ms
The statistical analyses showed that the ERPs within the time regions of interest derived from factors 3 and 5 (i.e., 212–224 and 284–316 ms after stimulus onset) showed the same pattern of emotional modulation even though they presented slightly different raw topographies. Within both time windows, emotional stimuli reduced cortical positivity over occipital areas relative to neutral ones. Therefore, to avoid redundancy, we collapsed together the results from the two time windows (Figure 4). Unlike what was observed in the 452–508 ms time window (i.e., the LPP), but in line with previous reports (Schupp et al. 2003; De Cesarei and Codispoti 2006), we observed a significant difference between emotional conditions: The reduction of cortical positivity over posterior areas was more pronounced for pleasant than for unpleasant stimuli. Moreover, the presentation of cigarette-related stimuli also led to a significant reduction of positivity over occipital sites relative to the neutral condition, and the topographical distribution of the effect observed in this condition was comparable to that observed for emotional (pleasant) stimuli. Similar to what was observed for pleasant stimuli, cigarette-related stimuli prompted less positivity over occipital sites than negative stimuli did.
Figure 4.
Top row: Topographic distribution of the voltages in the 212–316 ms time region of interest during the presentation of neutral, unpleasant, pleasant, and cigarette-related pictures. Middle row: Topographic distribution of the voltage differences (motivationally significant minus neutral). Bottom row: Topographic distribution of statistically significant differences.
Taken as a whole, these findings indicate that the presentation of cigarette-related cues modulates ERPs within the EPN window similarly to what intrinsically emotional stimuli do.
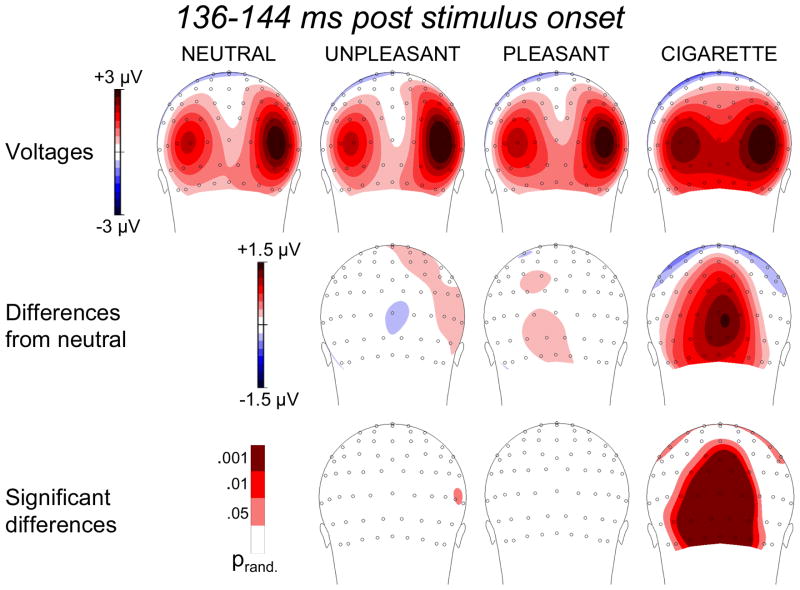
Factor 4: 136–144 ms
Factor 4 presented both a time course and a voltage distribution compatible with those associated with the P1 component (Figures 1 and 2). During passive picture viewing, this component is not typically modulated by emotional valence (Olofsson et al. 2008). Indeed, as shown in Figures 1 and 5, our data indicated that within such an early time window, brain potentials associated with both pleasant and unpleasant conditions did not differ from those obtained in the neutral condition. However, an interesting and provocative finding that emerged from this analysis was that over occipital sites the presence of cigarette-related pictures reliably enhanced the amplitude of the P1 component above the level observed when both intrinsically emotional and neutral images were presented (Figure 5).
Figure 5.
Top row: Topographic distribution of the voltages in the 136–144 ms time region of interest during the presentation of neutral, unpleasant, pleasant, and cigarette-related pictures. Middle row: Topographic distribution of the voltage differences (motivationally significant minus neutral). Bottom row: Topographic distribution of statistically significant differences.
Cigarette-cue reactivity and nicotine addiction
To investigate the relationship existing between amplitude of the ERP components evoked by cigarette-related images and nicotine addiction we used the number of cigarettes smoked per day by each participant at the moment of the baseline to divide the whole sample into three groups: “light” smokers (< 20 cigarettes per day, N=48), “moderate” smokers (20 cigarettes per day, N=37), and “heavy” smokers (> 20 cigarettes per day, N=30). For each participant we computed a “cue reactivity index” by subtracting the voltage of the neutral condition from the voltage of the cigarette-related one. The cue reactivity index was computed separately for each ERP component (i.e., P1, ERN, and LPP) and was used as the dependent variable in three separate ANOVAs. The factor “group” (light, moderate, and heavy) was the between subjects variable. In all three ANOVAs the same linear trend was present: the heavy smokers always showed the largest cue reactivity in the presence of cigarette-related images. The linear trend was significant for all three components (P1: F(1,113) = 5.10, P < .05; EPN: F(1,113) = 4.35, P < .05; LPP: F(1,113) = 7.02, P < .01).
DISCUSSION
To test the hypothesis that in addicted individuals, the presence of drug-related cues sparks brain processes similar to those induced by naturally emotional stimuli, we recorded dense-array ERPs from a large group of smokers during the presentation of pleasant, unpleasant, neutral, and cigarette-related images. Using temporal PCA to select the regions of interest confirmed the results obtained by Foti and coworkers (Foti et al. 2009). These authors, using a similar technique, showed that emotional processing independently modulates early and late components of the ERPs. As we hypothesized, viewing cigarette-related pictures increased the amplitude of the late positive potential (LPP) to the same level observed when emotional pictures (both pleasant and unpleasant) were presented. The LPP modulation is considered the ERP signature of emotional processing: the amplitude of this component increases when stimuli with high motivational value are viewed (Bradley 2009; Lang and Bradley 2009). Enhancement of the LPP is interpreted as a brain correlate of the mobilization of processing resources to facilitate rapid and appropriate reactions in the presence of stimuli holding evolutionary significance (Lang et al. 1997; Low et al. 2008).
Our results replicate findings obtained from non-addicted individuals and extend them to a new category of stimuli: cigarette-related cues. These stimuli do not hold intrinsic significance: they acquired it by being repeatedly paired to the cascade of neurophysiological events associated with nicotine self-administration brought about by smoking cigarettes. Our results show that the smoker’s brain reacts similarly to the presence of cigarette-related cues and intrinsically motivating stimuli. This finding, together with those from studies that showed increased autonomic and reflexive activation in the presence of drug-related cues (Geier et al. 2000; Cinciripini et al. 2006), allows us to conclude that in smokers, cigarette-related cues indeed trigger a pattern of brain activation comparable to that observed in the presence of other intrinsically emotional stimuli. Emotions have been described as action dispositions to effectively respond to events that threaten or sustain life (Lang 1979; Lang et al. 1997;Lang and Bradley 2009). Thus, we can hypothesize that the engagement of the brain appetitive system, as indexed by the LPP in the presence of drug-associated stimuli, might be the precursor of the active drug pursuit and consumption that characterize addicted individuals in the natural environment (Robinson and Berridge 1993).
Our results also show that the presence of cigarette-related images influences ERP components that precede the development of the LPP. As with emotional images, cigarette-related images increase the amplitude of the so-called early posterior negativity (EPN). Since the modulation of this component has been attributed to the ability of emotional stimuli to automatically capture attentional resources (Schupp et al. 2006), our results indicate that cigarette-related cues capture smokers’ attention. Given that the efficiency of cognitive processing is constrained by the availability of attentional resources, we can hypothesize that the automatic orienting of attention triggered by cigarette-related cues might play a pivotal role in undermining the addict’s ability to implement the cognitively onerous and attentionally demanding cognitive strategies necessary to resist cravings and inhibit the automatic behaviors that characterize drug addiction (Tiffany 1990). Such interpretation is in line with the results of a recent experiment in which we found that the P3 evoked by an acoustic startle probe was reduced in the presence of cigarette-related cues, suggesting that these cues do indeed reduce the smoker’s capacity to process other stimuli competing for attentional resources (Versace et al. 2010). However, the sensitivity of the EPN to extra-emotional features (De Cesarei and Codispoti 2006; Bradley et al. 2007) complicates its interpretation, and future studies will be required to establish the meaning and the usefulness of this component in the study of nicotine addiction.
Further studies will also be needed to replicate and interpret the unpredicted enhancement of positivity that peaked 140 ms after stimulus onset at occipital sensors and was observed exclusively in the presence of cigarette-related stimuli. We found this result very intriguing: Given its timing and location, we speculate that it represents an amplitude enhancement of the P1 component. Previous studies in which ERPs were recorded during passive viewing of emotional pictures have not typically reported P1 amplitude changes as a function of picture content (Olofsson et al. 2008). Our data confirm these findings clearly showing that only the presentation of cigarette-related stimuli enhanced P1 amplitude.
This result might be explained by considering the results from studies that investigated visual selective attention. It has been reported that changes in the P1 amplitude reflect the amplification of sensory information flow in the visual pathways by spatial attention (Hillyard and Anllo-Vento 1998; Luck et al. 2000). A recent report (Zhang and Luck 2009) complemented these results showing that, in addition to spatial attention, changes in P1 amplitude also index the influence of feature-based attention on early feedforward sensory activity. Zhang and Luck (2009), presenting stimuli under conditions of simultaneous competition between attended and ignored features, observed larger P1 amplitude for stimuli holding the attended feature even at unattended locations. This result indicates that when attended and ignored features compete (an ordinary situation when humans explore the natural environment or a pictorial representation of it; see Zhang and Luck [2009] supplementary discussion), feature-based attention operates throughout the visual field in a global manner.
In light of the results reported by Zhang and Luck (2009), our findings suggest that smokers extract very early from the perceptual stream some visual feature that is systematically present within the cigarette-related images. At first, given that like all other categories, cigarette-related images depict a large variety of scenes in which human beings and objects are shown from various distances and points of view, this explanation might seem unlikely. Actually, it is exactly because of the presence of a single feature that each cigarette-related image is different from its neutral counterpart: the presence of the cigarette. We believe that the enhancement of the P1 observed exclusively in the presence of cigarette-related stimuli shows that these stimuli act as powerful attentional magnets able to bias visual processing. Some cautionary notes are necessary. Despite our efforts to equate stimuli for higher level perceptual features (e.g., presence of objects vs. people, complexity of the scenes) and luminosity, we cannot exclude the possibility that lower level perceptual features might have contributed to the modulation of the P1. Moreover, unlike Zhang and Luck (2009), we did not use a task aimed at testing feature-based attention under conditions of high competition. One could argue that in natural scenes relevant and irrelevant features naturally compete for attentional resources; nevertheless, future studies, in which attended and ignored features compete for attentional resources and perceptual parameters are carefully controlled, will be necessary to replicate our finding and validate its interpretation.
Another limitation of our study is the lack of a non-smoker control group: its absence does not allow us to exclude that cigarette-related cues might have prompted the same results even in non-addicted individuals. This possibility seems unlikely, though. Previous studies showed that drug-related and neutral cues lead to similar brain activation in non-addicted individuals (Due, Huettel, Hall, & Rubin, 2002; Littel and Franken 2007; Wolfling, Flor, & Grusser, 2008). Moreover, our data show that the level of nicotine dependence influences the amplitude of the ERPs evoked by cigarette-related images. and the heavy smokers always show the most pronounced cue reactivity in the presence of cigarette-related images.
Considering our aim of generalizing the results to a clinically relevant population, excluding smokers who were not interested in quitting cannot be necessarily considered a limitation. Nevertheless, in light of studies showing greater cue reactivity in non-treatment seekers than in drug users undergoing treatment (Wertz and Sayette 2001; Wilson et al. 2004), one could hypothesize that including smokers not interested in quitting might lead to larger ERPs in the presence of cigarette-related images than those recorded here. On the other hand all our participants were still smoking at their regular rate when the data were collected (for each participant the individual quit date was scheduled not less than 2 weeks after the baseline). This fact should have minimized the effects due to the intention to quit smoking. Certainly, the influence that both drug availability and cognitive strategies aimed at resisting cravings exert on neurophysiological responses in the presence of drug-related cues deserves further investigation. We have just begun a study to investigate the effects of cognitive appraisal on ERPs in the presence of emotional and drug-related cues; hopefully data from this new study will help clarify the interactions between cognitive and emotional processing in drug addiction.
The sequence of brain events highlighted by our results indicates that, in a natural and visually rich environment, smokers detect the presence of a cigarette-related cue within a few milliseconds after its appearance and that these cues immediately engage attentional resources and ignite an emotional reaction by exploiting the same brain mechanisms involved in the processing of intrinsically emotional stimuli. This rapid cascade of events in the presence of a motivationally significant stimulus is usually adaptive, as it allows for the rapid detection of sources of potential reward, the subsequent recruitment of attentional resources to magnify and preferentially select these stimuli among competing but less relevant ones, and the preparation for the appropriate consummation action. Unfortunately, for a smoker engaging in a quit attempt, the same cascade of events likely paves the slippery slope toward relapse by amplifying the salience of conditioned drug-related cues and depleting the attentional resources necessary to efficiently implement the cognitive strategies that are essential for inhibiting the preponderant conditioned responses triggered by such cues and successfully avoiding relapse.
The similar amplitude observed in the LPP for pleasant and cigarette-related conditions suggests that smokers attribute the same incentive value to natural and drug rewards. This result seems to be at odds with the idea that one of the consequences of drug addiction is the enhancement of the incentive value of drug-related stimuli at the expense of natural rewards (Koob and Volkow 2010). For example, Lubman and co-workers (Lubman et al. 2008) showed emotional and drug-related cues to individuals addicted to opiates and observed that the P300 amplitude was larger for drug-related cues than for emotional ones. Unfortunately, methodological issues (i.e., data were filtered using settings that prevented the analysis of slow-wave ERP components) and the small size and heterogeneity of the sample (9 recently detoxified patients and 7 addicts still receiving opiate therapy) suggest that caution is warranted in interpreting their results. Another study in which ERPs from heavy cannabis users were recorded during the presentation of emotional and drug-related images showed no ERP differences between these two categories of stimuli (Wolfling et al. 2008). Moreover, consistent with the findings presented here, when we evaluated smokers’ ability to process competing stimuli in the presence of cigarette-related and emotional stimuli, we found that the presentation of emotional and cigarette-related cues reduced available attentional resources to the same degree (Versace et al. 2010).
One explanation for the lack of differences between pleasant and cigarette-related cues might be the smoking status of our participants. When ERPs were recorded, all participants were still smoking at their regular rate and the satiated state could have “normalized” the reactivity of the appetitive brain mechanisms in the presence of intrinsically pleasant stimuli. Given the scarce number of studies that compared the incentive value of drugs with that held by natural rewards (in this case represented by the pleasant stimuli in our picture set), it is difficult to make comparisons. Recently, Goldstein and co-workers (Goldstein et al. 2010) obtained self-report data from cocaine-addicted individuals who rated “liking” and “wanting” for natural (food and sex) and drug rewards. Study participants “in general” attributed higher value to natural rewards than to drug-related ones, but this pattern was reversed when they were asked to provide the same judgments imagining a hypothetical “under the influence” condition. The differences between our study and the Goldstein et al. study (e.g., nicotine vs. cocaine, facing a cue vs. imagining one, physiological data vs. self-reports) make comparisons very difficult.
By using ERPs, we are more likely to derive a framework showing how neural adaptations in response to chronic drug use might change when going from sated to deprived states and whether the relative value of intrinsic vs. drug-related cues changes during cessation. This could have important implications for predicting response to various treatments (i.e., those least affected by deprivation might quit more easily) as well as treatment development (i.e., finding medications that reduce the incentive value of drug use while leaving the incentive value of natural rewards intact). The data that we are currently collecting in smokers undergoing treatment and in various stages of nicotine withdrawal using the same experimental paradigm presented here my help clarify this issue and may improve clinical practices.
Regardless of these future outcomes, the current study provides data that clearly show how drug-related cues receive preferential processing and induce a maladaptive emotional reaction by exploiting the same brain systems that are engaged by intrinsically emotional stimuli. Given the high temporal resolution provided by ERPs, we demonstrated also that drug-related cues effectively capture attentional resources within a few milliseconds after their onset, an effect that probably limits the ability of the addicted individual to resist cravings and facilitates relapse. Given the large sample size, these data provide a reliable baseline that will be used in future studies to investigate the effects of successful detoxification intervention on electrocortical indices of affective modulation.
Acknowledgments
This work was supported in part by the National Institute on Drug Abuse through grant 1R01DA017073-01 to Paul Cinciripini, by a faculty fellowship from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment to Francesco Versace, and by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672. The authors wish to thank John Avalos, Jennifer Canul, Janeene Frerking, Erin Glueckert, Beda Jean-Francois, Christine Jeria, Silky Joshi, Katie McMahon, Samuel Miller, Kevin Mulpur, Natalie Nathan, Blanca Navarro, Tiffany Rattler, and Rudel Rymer for their help in data collection.
Footnotes
AUTHORS CONTRIBUTION
All authors were responsible for the study concept and design. FV, JDR, and VLB contributed to the data collection. FV and JAM carried out the data reduction. FV performed the statistical analyses and drafted the manuscript. All authors revised the manuscript and intellectually contributed to it, critically reviewed its content, and approved the final version for publication.
Dr. Cinciripini has served on the scientific advisory board of Pfizer Pharmaceuticals, has received grant support from Pfizer, and has conducted educational talks sponsored by Pfizer on smoking cessation for physicians. The other authors declare no conflict of interest.
References
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Low A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, et al. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarette smoking among adults, United States, 2007. Morbidity and Mortality Weekly Report. 2007;56(44):157–1161. [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, et al. The Effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Codispoti M, De CA. Arousal and attention: picture size and emotional reactions. Psychophysiology. 2007;44:680–686. doi: 10.1111/j.1469-8986.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Res. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: Distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Mazzetti M, Bradley MM. Unmasking emotion: exposure duration and emotional engagement. Psychophysiology. 2009;46:731–738. doi: 10.1111/j.1469-8986.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. When does size not matter? Effects of stimulus size on affective modulation. Psychophysiology. 2006;43:207–215. doi: 10.1111/j.1469-8986.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principal Component Analysis of ERP data. In: Handy TC, editor. Event-related potentials: a methods handbook. The MIT Press; Cambridge, Massachusetts: 2005. pp. 189–209. [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. The international smoking image series (with neutral counterparts), v. 1.2. Department of Psychology, Southern Illinois University; Carbondale, IL: 1999. [Google Scholar]
- Goldstein RZ, Woicik PA, Moeller SJ, Telang F, Jayne M, Wong C, et al. Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J Psychopharmacol. 2010;24:257–266. doi: 10.1177/0269881108096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Lawrence Erlbaum; Mahwah, NJ: 1997. pp. 97–136. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no. A-6. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. The effects of prolonged abstinence on the processing of smoking cues: an ERP study among smokers, ex-smokers and never-smokers. J Psychopharmacol. 2007;21:873–882. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- Low A, Lang PJ, Smith JC, Bradley MM. Both predator and prey: emotional arousal in threat and reward. Psychol Sci. 2008;19:865–873. doi: 10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. J Psychopharmacol. 2008;22:836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends Cogn Sci. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Mackay CJ, Erickson M, Shafey O. The Tobacco Atlas. American Cancer Society; Washington, D.C: 2006. [Google Scholar]
- Maris E. Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology. 2004;41:142–151. doi: 10.1111/j.1469-8986.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology (Berl) 2001;154:282–291. doi: 10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- NIDA. NIDA Research Report Series. NIH Publication Number 09-4342. 2009. Tobacco Addiction. [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychol. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 2004a;18:593–611. [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: Event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychol Sci. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004b;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoking and Health. Report of the Advisory Committee to the Surgeon General. US Department of Health, Education, and Welfare; Washington, DC: 1964. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, et al. Cigarette cues capture smokers’ attention: Evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CA, McDonough BE. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999;110:1570–1584. doi: 10.1016/s1388-2457(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Experimental & Clinical Psychopharmacology. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luck SJ. Feature-based attention modulates feedforward visual processing. Nat Neurosci. 2009;12:24–25. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]