Summary
A mild inhibition of mitochondrial respiration extends the lifespan of many organisms, including yeast, worms, flies and mice [1–10], but the underlying mechanism is unknown. One environmental condition that reduces rates of respiration is hypoxia (low oxygen). Thus it is possible that mechanisms that sense oxygen play a role in the longevity response to reduced respiration. The hypoxia-inducible factor HIF-1 is a highly-conserved transcription factor that activates genes that promote survival during hypoxia [11–12]. In this study, we show that inhibiting respiration in C. elegans can promote longevity by activating HIF-1. Through genome-wide screening, we found that RNAi knockdown of many genes encoding respiratory-chain components induced hif-1-dependent transcription. Moreover, HIF-1 was required for the extended lifespans of clk-1 and isp-1 mutants, which have reduced rates of respiration [1, 4, 13]. Inhibiting respiration appears to activate HIF-1 by elevating the level of reactive oxygen species (ROS). We found that ROS is increased in respiration mutants, and that mild increases in ROS can stimulate HIF-1 to activate gene expression and promote longevity. In this way, HIF-1 appears to link respiratory stress in the mitochondria to a nuclear transcriptional response that promotes longevity.
Results and Discussion
To identify genes that affect HIF-1 activity in C. elegans, we performed a genome-wide screen for RNAi clones that induced a HIF-1-responsive GFP reporter, Pnhr-57::GFP [14–15]. We found 248 RNAi clones targeting 245 genes that reproducibly increased the level of Pnhr-57::GFP under normoxic conditions (Table S1A). The screen was predicted to identify genes already known to regulate HIF-1. Under normal oxygen conditions, C. elegans HIF-1 is hydroxylated by the oxygen-dependent prolyl hydroxylase EGL-9, and this hydroxylation leads to HIF-1’s degradation in a process that involves the E3 ubiquitin ligase VHL-1 [12, 14, 16]. Under hypoxic conditions, this hydroxylation does not occur and the stabilized HIF-1 activates downstream target genes required for adaptive responses to hypoxia [12, 14]. We recovered RNAi clones targeting egl-9, vhl-1 and rhy-1 (which encodes a HIF-1-inhibitory transmembrane protein) [15], validating our screen (Table S1A).
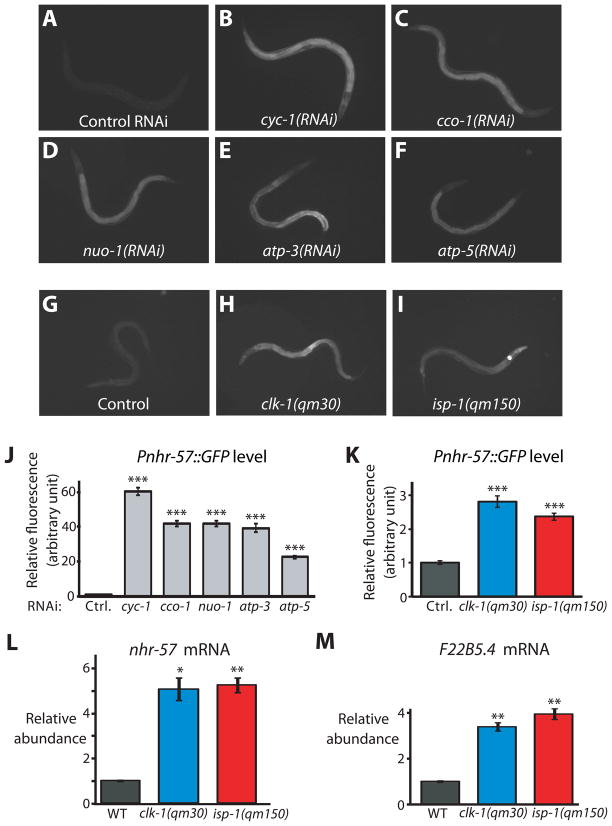
Remarkably, a highly significant fraction of the genes we identified encoded mitochondrial proteins (26 out of 113 gene-ontology (GO)-annotated cellular components, P<10−14, Table S1B), including components of the electron transport chain and ATP synthase such as cyc-1 [cytochrome c1], cco-1 [cytochrome c oxidase], nuo-1 [NADH:ubiquinone oxidoreductase], atp-3 [ATP synthase subunit δ], and atp-5 [ATP synthase subunit d]; Fig. 1A–F and J). These findings implied that impaired respiration increases HIF-1 activity under normoxic conditions. Consistent with this idea, we found that Pnhr-57::GFP was up-regulated in two mutants that are defective in respiration, clk-1(qm30) (which encodes a mitochondrial hydroxylase required for ubiquinone production) and isp-1(qm150) (which encodes an iron-sulfur protein in complex III) (Fig. 1G–I and K).
Fig. 1. Inhibiting respiration increases HIF-1 activity.
(A) Animals expressing the HIF-1-regulated Pnhr-57::GFP transgene displayed low levels of GFP when grown on control bacteria carrying the empty RNAi vector. (B–F) In contrast, RNAi of respiratory-chain or ATP synthase genes cyc-1 (B), cco-1 (C), nuo-1 (D), atp-3 (E), or atp-5 (F) induced the expression of Pnhr-57::GFP. RNAi treatment of cyc-1 or cco-1 only during adulthood did not increase the level of Pnhr-57::GFP (Fig. S1D-H). (G–I) Mutations in clk-1 (H) and isp-1 (I), which reduce respiration, elevated Pnhr-57::GFP expression (G). (J) Quantification of fluorescence in A to F (n>30); and (K), in G to I (n>42). mRNA levels of nhr-57 (L) and F22B5.4 (M), another HIF-1-regulated gene, were significantly increased in clk-1(qm30) and isp-1(qm150) mutants [Please see Fig. S1A-C for qRT-PCR data of other hypoxia-responsive genes]. Data were obtained from 3 independent quantitative RT-PCR analyses and error bars represent s.e.m (*P<0.01, **P<0.001, ***P<0.0001, two-tailed Student’s t-test compared to wild type).
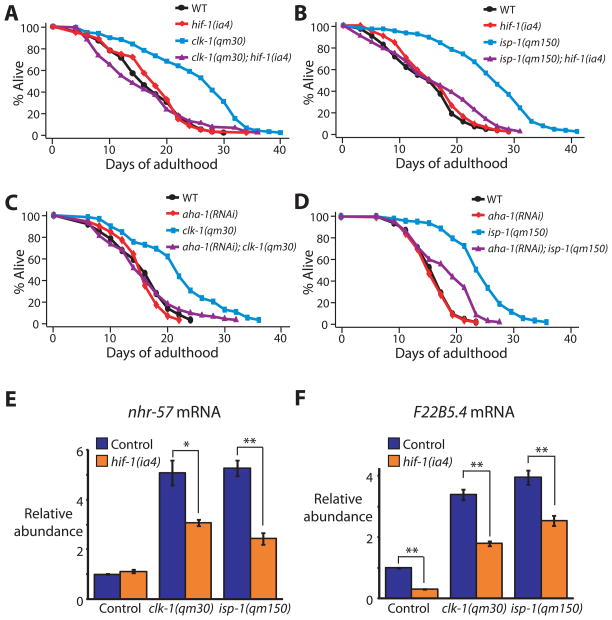
The finding that inhibiting respiration activates hif-1-dependent gene expression suggested that HIF-1 might be part of the pathway by which inhibition of respiration extends lifespan. We found that the long lifespans of clk-1 and isp-1 mutants were significantly suppressed by hif-1 loss-of-function mutations or hif-1 RNAi (Fig. 2A, B and Fig. S2A, B). hif-1 RNAi decreased the lifespans of these respiration mutants even if it was initiated during adulthood (Fig. S2C, D). This was noteworthy, as respiratory-chain RNAi only extends C. elegans’ lifespan when initiated during larval development [5, 17]. Perhaps HIF-1 acts in the adult to maintain the operation of a regulatory state initiated by respiration inhibition during development. We also found that RNAi knockdown of aha-1, which encodes the HIF1β subunit [18], shortened the lifespans of clk-1 and isp-1 mutants. (Fig. 2C, D). HIF-1 inhibition did not shorten lifespan indiscriminately, as it did not affect the lifespan of wild type (Fig. 2A–D and Fig. S2A–D) [19].
Fig. 2. The lifespan extension conferred by respiration mutants requires hif-1.
(A, B) hif-1(ia4) loss-of-function mutations decreased the longevity of clk-1(qm30) (A) and isp-1(qm150) (B) mutants significantly (in three out of four trials and four out of four trials, respectively; See Supplemental Table S2). (C, D) The long lifespan of clk-1(qm30) (C) and isp-1(qm150) (D) mutants was significantly shortened by aha-1 [HIF1β] RNAi. Neither the hif-1(ia4) mutation nor aha-1 RNAi affected the lifespan of wild type (WT). (See Supplemental Table S2 for statistical analysis.) [We note that whereas Mehta et al. and we both found that hif-1 mutations do not affect the lifespans of wild type [19], Chen et al. and Zhang et al. reported that hif-1 mutants live longer than wild type [37–38]. We carried out additional experiments to resolve this discrepancy, which are described in supplemental material (Fig. S2M, N)] (E, F) The increased mRNA levels of the HIF-1-dependent genes nhr-57 (E) and F22B5.4 (F) in clk-1(qm30) and isp-1(qm150) mutants were significantly decreased by hif-1(ia4) mutation. Error bars represent s.e.m (n=3, *P<0.05, **P<0.01, two-tailed Student’s t-test). See Fig. S2G, H for quantitative RT-PCR data of other hif-1-dependent hypoxia-inducible genes.
Unexpectedly, loss of hif-1 only partially reduced the lifespan extension caused by cyc-1 or cco-1 RNAi (Fig. S2E, F). This finding suggests that the mechanisms by which respiratory-chain RNAi and the clk-1/isp-1 respiration mutations lengthen lifespan may be at least somewhat distinct from one another.
Does inhibition of respiration activate the entire hif-1-dependent hypoxia response? Using quantitative RT-PCR, we found that four out of five C. elegans hif-1-dependent hypoxia-inducible genes we tested [14] (nhr-57 and F22B5.4, fmo-2, egl-9 and phy-2) were significantly up-regulated in clk-1 and isp-1 mutants (Fig. 1L, M, Fig. S1A-C). The expression of two of these, nhr-57 and F22B5.4 (Fig. 2E, F) was partially dependent on hif-1, but the other two up-regulated genes, fmo-2 and egl-9, were expressed independently of hif-1 (Fig. S2G, H). Thus hypoxia and respiration inhibition activate distinct patterns of hif-1-dependent gene expression. Mild hypoxia has been shown to extend C. elegans lifespan [20], so it will be interesting to learn what, if any, role hif-1 may have in that longevity response.
Defects in respiration in C. elegans not only extend lifespan, they also slow the rates of growth and behaviour [1, 4–6, 21], reduce brood size and delay reproduction [4, 21]. We found that inhibition of hif-1 or aha-1 had little or no effect on these phenotypes (Table S2). Thus, HIF-1 appears to influence only one aspect of the animal’s response to respiration inhibition, longevity. Interestingly, in flies and mice, respiration-inhibiting conditions that extend lifespan do not affect growth or behaviour. In C. elegans, respiratory-chain RNAi dose-response experiments indicate that lifespan and behaviours are affected co-ordinately [17]. These observations suggest the possibility that the growth, reproductive and behavioural responses to respiration inhibition evolved separately from the longevity response and are subject to a different mode of regulation. Consistent with this, the worm-specific growth and behavioural phenotypes can be suppressed significantly by inhibition of the C. elegans-specific genes fstr-1/2, which appears to have a smaller effect on lifespan [22].
Is hif-1 required for other C. elegans longevity pathways [10]? hif-1 RNAi did not shorten the long lifespan of daf-2/insulin/IGF-1-receptor mutants or dietary-restricted eat-2 mutants (Fig. S2I, J), consistent with recent, independent reports [19]; nor did it shorten the long lifespans of chemosensory osm-5 or germline-defective glp-1 mutants (Fig. S2K, L). Thus HIF-1 specifically affects lifespan in response to the inhibition of respiration.
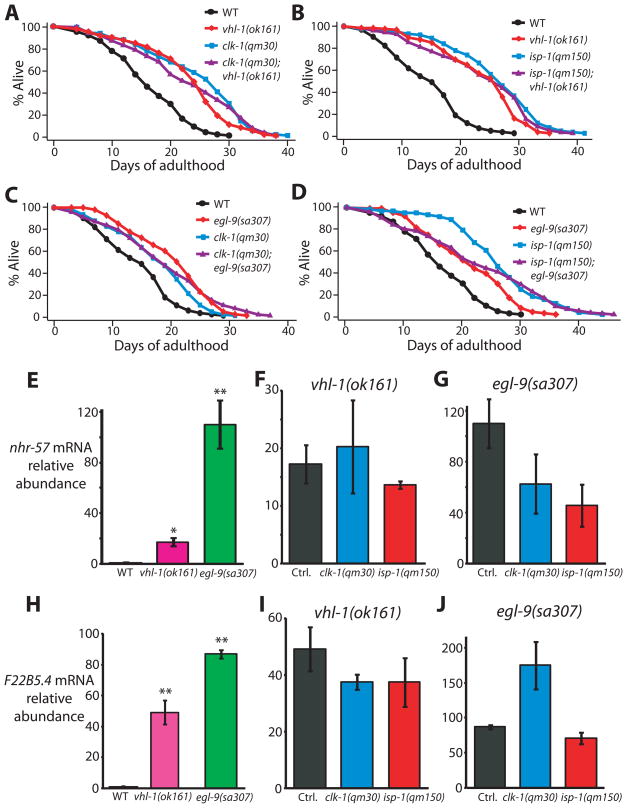
We also asked whether elevating HIF-1 in animals with wild-type respiration genes would be sufficient to extend lifespan. As shown independently by Mehta et al. [19], we found that conditions that stabilize HIF-1; that is, inhibition of vhl-1 or egl-9, significantly increased lifespan (Fig. 3A–D and Fig. S3A–D; see also Fig. S2M, N legend, and supplemental materials for discussion about recent studies of HIF-1 longevity). Importantly, clk-1 and isp-1 mutations did not further extend the long lifespans of vhl-1 or egl-9 mutants, arguing that vhl-1, egl-9 and respiration mutations all promote longevity by activating HIF-1 (Fig. 3A–D and Fig. S3A–D). Likewise, clk-1 or isp-1 mutations did not further increase nhr-57 and F22B5.4 mRNA levels in vhl-1 or egl-9 mutants (Fig. 3E–J).
Fig. 3. Activation of HIF-1 by vhl-1 or egl-9 mutations does not further lengthen the lifespan of respiration mutants.
(A, B) Mutations in vhl-1 increased the lifespan of wild type but did not further extend the lifespan of clk-1(qm30) (A) or isp-1(qm150) (B) mutants. (C, D) The egl-9(sa307) mutation did not further increase the lifespans of clk-1(qm30) (C) or isp-1(qm150) (D) mutants. (E) Consistent with previous reports [14, 39], mRNA levels of nhr-57 were significantly increased by vhl-1(ok161) and egl-9(sa307) mutations. (F, G) The increased mRNA levels of nhr-57 in vhl-1(ok161) (F) or egl-9(sa307) (G) mutants were not further augmented by clk-1(qm30) and isp-1(qm150) mutations (control, Ctrl.). (See Supplemental Table S3 for statistical analysis.) (H) Expression of F22B5.4 was highly induced in vhl-1(ok161) and egl-9(sa307) mutants as reported previously [14, 39]. (I, J) this induction was not significantly further increased by clk-1(qm30) and isp-1(qm150) mutations. Note that the increased F22B5.4 mRNA levels in egl-9(sa307) mutants by clk-1(qm30) mutation was marginally not significant (P=0.06). Error bars represent s.e.m (n=3, *P<0.01, **P<0.001, two-tailed Student’s t-test).
How might mutations in these respiration genes activate HIF-1? In cultured cells, hypoxia can increase the level of reactive oxygen species (ROS), which in turn activates HIF-1 by a mechanism that is not yet understood [23]. Because ROS are produced during electron transport, and because ROS increases when electron transport is reduced in isolated mitochondria [23–26], we hypothesized that ROS levels rise in respiration mutants, and that this rise in ROS, in turn, activates HIF-1.
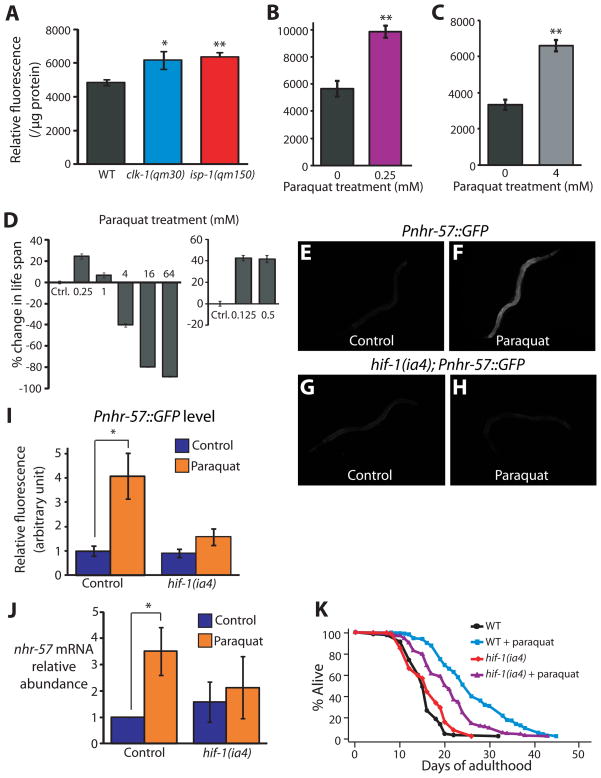
To measure ROS, we used a 2′,7′-dichlorofluorescein diacetate (DCF-DA) fluorescence assay that we found to reliably report ROS levels in C. elegans (Fig. 4A–C, Fig. S4A). We found that ROS levels were significantly increased in whole body lysates of clk-1 and isp-1 mutants (Fig. 4A). We observed a similar increase in fluorescence in vivo, using another fluorescent ROS-sensor, dyhydroxy ethidium (DHE) (Fig. S4B–F). These data indicate that the defective mitochondria in the clk-1 and isp-1 mutants generate elevated levels of ROS.
Fig. 4. Increased ROS causes HIF-1 to promote longevity in respiration-defective mutants.
(A) ROS levels, measured using a 2′,7′-dichlorofluorescein diacetate (DCF-DA) fluorescence assay, were significantly increased in clk-1(qm30) and isp-1(qm150) mutants (n=5). See also Fig. S4A for data using mev-1(kn1) mutant animals, which were previously shown to have increased ROS levels [40]. (B–C) A low dose (0.25 mM) (B) and high dose (4 mM) (C) of paraquat significantly increased the level of DCF-DA fluorescence. 4 mM paraquat was introduced from L4 to day 3 of adulthood because worms arrest as larvae if treated with 4 mM paraquat from hatching (n=3). Error bars represent s.e.m (*P<0.05, **P<0.01, two-tailed Student’s t-test). (D) Low doses of paraquat (0.125 mM, 0.25 mM, 0.5 mM and 1 mM) lengthened lifespan, whereas higher concentrations (4, 16, and 64 mM) shortened lifespan. Paraquat was introduced during adulthood. The lifespan measurements for 0.125 mM and 0.5 mM paraquat treatment were performed separately and therefore shown with different controls. See also Table S4. (E, F) Compared to untreated control Pnhr-57::GFP animals (E), animals treated with low levels of paraquat (0.25 mM) displayed increased GFP levels (F). Paraquat treatment further increased Pnhr-57::GFP levels in clk-1(qm30) and isp-1(qm150) mutant animals (Fig. S4J, K), suggesting that the induction was not saturated by either of the mutations or by the paraquat treatment. (G, H) The induction of Pnhr-57::GFP by paraquat treatment was significantly diminished in the hif-1(ia4) mutant. (I) Quantification of fluorescence in E to H (n >15). (J) The increased nhr-57 mRNA abundance caused by paraquat treatment, assayed using quantitative RT-PCR, was significantly decreased by hif-1(ia4) mutation. Data analysis was done from 10 independent quantitative RT-PCR experiments. [In two of our data sets, the levels of nhr-57 mRNA in paraquat-treated wild-type animals were increased by a very-large 681 and 755 fold compared to those in control animals. We excluded these two datasets from our analysis by using rejection analysis of QP-test for outliers (confidence level: 0.99) [41]]. (K) Mutations in hif-1 significantly decreased the longevity caused by paraquat treatment. Some long-lived mutants require the daf-16/FOXO transcription factor gene for their longevity, but respiration mutants do not [1, 4, 10]. We found that 0.25 mM paraquat treatment increased the lifespan of daf-16(mu86) null mutants (Fig. S4L). See Supplemental Table S4 for statistical analysis. Error bars represent s.e.m (*P<0.05, **P<0.01, two-tailed Student’s t-test). Previously, Rea et al. observed an increased trend in protein carbonylation levels at doses of respiratory-chain RNAi that increased lifespan, and also at higher RNAi doses, which did not extend lifespan [17]. On this basis, they concluded that ROS did not play a role in this longevity. One way to reconcile their findings with ours is to suggest that a sharp reduction in respiration prevents lifespan extension in spite of elevated ROS because it compromises the animals’ health.
Consistent with our results, Yang et al. showed that isolated submitochondrial particles from clk-1(qm30) mutants produce more H2O2 than do those from wild type [27]. We note that recently, Dingley et al. reported that mitochondrial superoxide level measured by using the fluorescent MitoSOX dye was slightly decreased in isp-1(qm150) mutants [28]. However, they also showed that mitochondria of respiration mutants, including isp-1 mutants, have prominent defects in the uptake of fluorescent dyes. Therefore, as they themselves speculated, it is possible that mitochondrial ROS levels in the isp-1 mutants, as measured by MitoSOX in their study, were underestimated.
To test whether ROS can extend C. elegans lifespan, we measured the lifespan of animals treated chronically with various concentrations of paraquat, which generates superoxide in mitochondria [29]. Low paraquat levels (0.125 mM, 0.25 mM, 0.5 mM and 1 mM) increased lifespan significantly, whereas, as expected, higher concentrations of paraquat (4, 16, and 64 mM) decreased lifespan in a dose-dependent manner (Fig. 4D and Table S4). Using the two ROS indicators, we confirmed that high concentrations of paraquat increased ROS (Fig. 4C and Fig. S4H). Unexpectedly, whereas one of the dyes showed a significant increase in ROS levels in animals treated with low levels of paraquat, the other dye reported reduced ROS levels at low paraquat concentrations (Fig. 4B and Fig. S4G). One possible explanation is that the two dyes sense different types of ROS, and that the animal’s protective response to paraquat can actually decrease the levels of certain ROS species relative to untreated controls. Although these findings raise new questions, overall the data suggest that moderate levels of ROS can extend lifespan. Consistent with these findings, low levels of juglone, another ROS-generating chemical, also extends C. elegans lifespan [30].
Next, we asked whether paraquat increases lifespan by activating HIF-1. We found that animals chronically treated with low levels of paraquat (0.25 mM) displayed an increase in nhr-57 expression (Fig. 4E, F, I and J) that was largely hif-1 dependent (Fig. 4G–J). Moreover, hif-1 was partially required for paraquat to increase lifespan (Fig. 4K). Together these data suggest that ROS generated by defects in respiration activate HIF-1, which in turn can promote longevity.
Historically, it has been believed that ROS generated in mitochondria are one of the main determinants of aging [31]. However, several recent studies suggest that modest increases in ROS levels can have beneficial effects on lifespan by triggering the expression of cell-protective pathways [25, 30, 32–33]. For example, 2-deoxyglucose activates AMP kinase and extends lifespan in C. elegans in a ROS-dependent fashion [32]. In addition, we showed previously that antimycin A, a respiration inhibitor that increases ROS [23], extends C. elegans’ lifespan [5]. Here we showed that ROS generated when respiration rates are reduced in C. elegans increase HIF-1 transcriptional activity, which in turn is sufficient to lengthen lifespan.
These findings reinforce the emerging idea that a little ROS may be beneficial [25, 33]. However, the finding that low and high levels of ROS have opposite effects on lifespan makes the interpretation of experiments in which antioxidant proteins are eliminated a bit complicated. Specifically, unless loss of an antioxidant produces high ROS levels, one might expect to find an increase rather than decrease in lifespan, even if high levels of ROS do accelerate the normal aging process.
It was noteworthy that clk-1 and isp-1 mutants exhibited comparably-elevated ROS levels, as the overall respiration defect of isp-1 (complex III—defective) mutants appears to be greater than that of clk-1 (ubiquinone-defective) mutants [2, 4, 13]. This would be consistent with our interpretation that reactive oxygen species, rather than, say altered oxygen consumption rate, which is reduced in isp-1 but not clk-1 mutants [2, 4, 13], trigger longevity.
In summary, in this study we showed that respiration mutations in clk-1 and isp-1 extend lifespan by increasing hif-1-dependent gene expression, and that increased hif-1 activity is sufficient for longevity. HIF-1 is a transcription factor, implying that HIF-1 extends longevity via changes in downstream gene expression. How ROS in the mitochondria impact HIF-1 and cause it to influence nuclear gene expression is not clear, nor is it clear which genes HIF-1 activates to extend lifespan. Several lines of evidence suggest that increased expression of nhr-57 is not sufficient to increase lifespan. First, 4 mM paraquat treatment shortened lifespan although this condition increased Pnhr-57::GFP. In addition, we found that nhr-57 level was higher in egl-9 and vhl-1 mutant animals than clk-1 and isp-1 mutant animals in spite of the fact that they all have similar longevity phenotypes. Presumably, the activity of a group of HIF-1 regulated genes, which may or may not include nhr-57, is responsible for increasing lifespan in clk-1 and isp-1 mutants. Inhibition of respiration is known to trigger a conserved gene expression response called the retrograde response that activates alternative energy pathways and cell protective mechanisms [3, 34]. It will be interesting to learn to what extent HIF-1 influences the expression of these genes.
It is interesting to speculate that the longevity response to reduced respiration played a role in the evolution of mammalian lifespan, as larger species of mammals tend to have lower rates of respiration and to live longer than smaller mammals [10]. A hif-1-dependent response to the inhibition of a fundamental oxygen-dependent process like respiration might well have arisen early during evolution. Like the C. elegans respiration mutants described here, long-lived mClk-1+/− mice, which lack one copy of the mouse ortholog of C. elegans clk-1, have increased levels of mitochondrial ROS [35]. While this manuscript was in preparation, the Hekimi lab reported that the elevated ROS in mClk-1+/− mice activate HIF-1 to affect the immune response [36]. This is intriguing, as it suggests a possible conservation of mechanism from worms to mammals. It would be interesting to test whether increased HIF-1 activity contributes to the extended lifespan of these mice as well.
Highlights.
Reduced mitochondrial respiration extends lifespan in many species.
Inhibition of mitochondrial respiration increases HIF-1 activity in C. elegans.
HIF-1 is required for the longevity caused by mutations that inhibit respiration.
HIF-1 is likely activated by reactive oxygen species that are generated when respiration is inhibited.
Supplementary Material
Acknowledgments
We thank Dr. J. A. Powell-Coffman, and the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources, for providing strains, and Kenyon and Lee lab members for helpful discussions. S.J.L. received postdoctoral support from the American Heart Association. This research was supported by NIH Merit Award #AG011816 to C.K. (who is director of the Hillblom Center for the Biology of Aging at UCSF and an American Cancer Society Research Professor) and by the World Class University program through the Korea Science and Engineering Foundation, funded by the Ministry of Education, Science and Technology (Project No. R31-2008-000-10100-0) and by the POSTECH Basic Science Research Institute Grant to S.J.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakowski B, Hekimi S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science. 1996;272:1010–1013. doi: 10.1126/science.272.5264.1010. [DOI] [PubMed] [Google Scholar]
- 2.Braeckman BP, Houthoofd K, De Vreese A, Vanfleteren JR. Apparent uncoupling of energy production and consumption in long-lived Clk mutants of Caenorhabditis elegans. Curr Biol. 1999;9:493–496. doi: 10.1016/s0960-9822(99)80216-4. [DOI] [PubMed] [Google Scholar]
- 3.Kirchman PA, Kim S, Lai CY, Jazwinski SM. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics. 1999;152:179–190. doi: 10.1093/genetics/152.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 5.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 6.Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dell’agnello C, Leo S, Agostino A, Szabadkai G, Tiveron C, Zulian A, Prelle A, Roubertoux P, Rizzuto R, Zeviani M. Increased longevity and refractoriness to Ca2+-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet. 2007;16:431–444. doi: 10.1093/hmg/ddl477. [DOI] [PubMed] [Google Scholar]
- 9.Copeland JM, Cho J, Lo T, Jr, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 11.Shen C, Powell-Coffman JA. Genetic analysis of hypoxia signaling and response in C. elegans. Ann N Y Acad Sci. 2003;995:191–199. doi: 10.1111/j.1749-6632.2003.tb03222.x. [DOI] [PubMed] [Google Scholar]
- 12.Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci. 2009;66:3539–3554. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felkai S, Ewbank JJ, Lemieux J, Labbe JC, Brown GG, Hekimi S. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans. EMBO J. 1999;18:1783–1792. doi: 10.1093/emboj/18.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J Biol Chem. 2005;280:20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- 15.Shen C, Shao Z, Powell-Coffman JA. The Caenorhabditis elegans rhy-1 gene inhibits HIF-1 hypoxia-inducible factor activity in a negative feedback loop that does not include vhl-1. Genetics. 2006;174:1205–1214. doi: 10.1534/genetics.106.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 17.Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci U S A. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda S, Ishii N, Suzuki K, Matsuo M. Oxygen-dependent perturbation of life span and aging rate in the nematode. J Gerontol. 1993;48:B57–61. doi: 10.1093/geronj/48.2.b57. [DOI] [PubMed] [Google Scholar]
- 21.Wong A, Boutis P, Hekimi S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1995;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C. A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000450. doi: 10.1371/journal.pgen.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 24.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010 doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang YY, Gangoiti JA, Sedensky MM, Morgan PG. The effect of different ubiquinones on lifespan in Caenorhabditis elegans. Mech Ageing Dev. 2009;130:370–376. doi: 10.1016/j.mad.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingley S, Polyak E, Lightfoot R, Ostrovsky J, Rao M, Greco T, Ischiropoulos H, Falk MJ. Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans. Mitochondrion. 2010;10:125–136. doi: 10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan HM, Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979;196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 30.Heidler T, Hartwig K, Daniel H, Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 31.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 32.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- 35.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem. 2008;283:26217–26227. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J Immunol. 2010;184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS One. 2009;4:e6348. doi: 10.1371/journal.pone.0006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bishop T, Lau KW, Epstein AC, Kim SK, Jiang M, O’Rourke D, Pugh CW, Gleadle JM, Taylor MS, Hodgkin J, et al. Genetic analysis of pathways regulated by the von Hippel-Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2004;2:e289. doi: 10.1371/journal.pbio.0020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman PS, Ishii N. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J Biol Chem. 2001;276:41553–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- 41.Efstathiou CE. A test for the simultaneous detection of two outliers amoung extreme values of small data sets. Analytical Letters. 1993;26:379–390. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.