1. Introduction
Convergent evidence suggests dysfunction of neural circuitry implicated in major depressive disorder (MDD) (Lockwood et al., 2002; Tekin and Cummings, 2002). Several brain regions, such as the frontal cortex, cingulate cortex, hippocampus, amygdala and parietal lobe have demonstrated involvement in this disorder by both morphometric (Andreescu et al., 2008; Egger et al., 2008; Koolschijn et al., 2009; Tang et al., 2007; Vasic et al., 2008) and functional (Dichter et al., 2009; Matthews et al., 2009; Wagner et al., 2008; Wang et al., 2008a; Wang et al., 2008b; Yang et al., 2009) magnetic resonance imaging (MRI) studies. Additional evidence from postmortem studies further supports the involvement of white matter which providessubstantial connections within those regions in the pathophysiology of MDD. For example, altered deep white matter myeline staining and hyperintensities were observed in the prefrontal cortex in MDD subjects (Regenold et al., 2007; Thomas et al., 2002). Taken together with the findings on oligodendroglial density in the prefrontal cortex in MDD (Uranova et al., 2004), abnormalities of white matter within those circuitries may be directly relevant to the pathophysiology of MDD.
Diffusion tensor imaging (DTI), an MRI technique, can provide information about white matter microstructure integrity in vivo by measuring the magnitude and direction of water diffusion and has been used successfully to investigate white matter abnormalities in several psychiatric disorders (White et al., 2008). Two DTI measurements (Le Bihan, et al., 2001), fractional anisotropy (FA) measuring the principle directionality of water diffusion and apparent diffusion coefficient (ADC) providing the overall evaluation of the water diffusion, have been widely used in recent studies. Increasing DTI studies have suggested that white matter abnormalities play a key role in MDD pathphysiology (Alexopoulos et al., 2002; Alexopoulos et al., 2008; Bae et al., 2006; Li et al., 2007; Ma et al., 2007; Nobuhara et al., 2004; Nobuhara et al., 2006; Taylor et al., 2008; Taylor et al., 2007; Taylor et al., 2004; Yang et al., 2007; Zou et al., 2008). However, results are inconsistent in these studies, probably due to differences in sample age, sex distribution, medication exposures, age of illness onset, illness duration and number of acute episodes. Additionally, different DTI methodologies, such as region of interest (Bae et al., 2006; Li et al., 2007; Taylor et al., 2008), fiber tracking (Malykhin et al., 2008), tract-based spatial statistics (Kieseppa et al., 2010) and voxel base DTI (Ma et al., 2007; Zou et al., 2008) were applied in these studies, possibly contributing to differences in results as well. In order to minimize chronicity-related confounds and treatment variables, we performed a voxel-based DTI study to examine whole brain white matter abnormalities in single-episode, medication-naive MDD participants with duration of illness less than 3 months in the present study.
2. Methods
2.1. Participants
We recruited 23 patients with diagnosed MDD from outpatients at the Department of Psychiatry, First Affiliated Hospital of China Medical University. All MDD participants were diagnosed by two trained psychiatrists individually using the Structured Clinical Interview for DSM-IV and met the following inclusion criteria: fulfilling DSM-IV criteria for major depressive disorder, single depressive episode; duration of illness less than 3 months; aged 18 to 45; no comorbid Axis I or II diagnosis; having a score of at least 17 on the 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960); as well as, no history of psychotropic medication, electroconvulsive therapy or psychotherapy.
We also recruited 21 healthy controls (HC) matched for sex, age and education by advertisements. The Structured Clinical Interview for DSM-IV confirmed the absence of DSM-IV Axis I or II disorders. Subjects with a history of mood disorders in their first-degree family members were excluded. Table 1 presents detailed demographic and clinical data of participants.
Table 1.
Demographic and Clinical Data of Subjects
| MDD patients (n=23) |
Healthy control subjects (n=21) |
||
|---|---|---|---|
| Age (years, mean±S.D.) | 31.4±8.8 | 30.4±8.2 | t=0.39 df=42 p=0.70 |
| Sex (male/female) | 10/13 | 9/12 | x2=0.002 df=1 p=0.97 |
| Education (years, mean±S.D.) | 12.3±3.2 | 12.7±3.4 | t=0.37 df=42 p=0.71 |
| HDRS score (mean±S.D.) | 21.8±3.8 | 0.9±0.8 | t=24.8 df=42 p=0.000 |
| Duration of illness (months, mean±S.D.) | 2.17±0.78 |
S.D.: standard deviation
MDD: major depressive disorder
HDRS: Hamilton Depression Rating Scale
Exclusion criteria for all participants included: any MRI contraindications; history of head injury or neurological disorder; any concomitant medical disorder. All participants were right-handed and were scanned within 48 hours of initial contact. The participants provided written informed consent after detailed description of the study. The study was approved by the Institutional Review Board of the China Medical University.
2.2. MRI acquisition and image processing
All MRI scans were performed on a GE Signa 1.5 T MR scanner (General Electric, Milwaukee, USA) at the First Affiliated Hospital of China Medical University, Shenyang, China. Head motion was minimized with restraining foam pads. A standard head coil was used for radiofrequency transmission and reception of the nuclear magnetic resonance signal. Diffusion tensor images were acquired using spin-echo planar imaging sequence, parallel to the anterior-posterior (AC-PC) plane. The diffusion sensitizing gradients were applied along thirteen non-collinear directions (b=1000 s/mm2), together with an axial acquisition without diffusion weighting (b=0). Scan parameters were repetition time (TR) = 12000 ms; echo time (TE) = 95 ms; image matrix = 256×256; FOV = 24×24 cm2; NEX = 5; 37 contiguous slices of 4 mm and no gap resulting in voxels of 0.94×0.94×4 mm3; scan time 14 min.
Diffusion tensor matrices, FA and ADC were calculated with DTI-Studio software (version 2.40, Johns Hopkins University, Baltimore, MD). The b=0 image was normalized to the standard Montreal Neurological Institute (MNI) space using nonlinear registration with Statistical Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm), then the transformation matrix was applied to the FA and ADC maps. All images were resampled with a final voxel size of 2×2×2 mm3 and spatially smoothed by a 6-mm full width half maximum Gaussian kernel.
2.3. Statistical Analysis
Independent-sample t tests and x2 tests were used to compare demographic data, HDRS scores between the MDD and HC group with SPSS 13.0 software (SPSS Inc, Chicago, Illinois). Two-sample t tests were performed in a voxel-by-voxel manner with SPM. The findings were considered statistically significant at a height threshold of p < 0.001 (two-tailed, uncorrected) and an extension threshold of 30 voxels.
3. Results
We included 23 MDD patients (10 men, 13 women) with a mean age of 31.4 (standard deviation [SD] 8.8, range 18–45) years and 21 healthy controls (9 men, 12 women) with a mean age of 30.4 (standard deviation [SD] 8.2, range 18–45) years. The mean number (and SD) of education years was 12.3 (3.2) years for patients and 12.7 (3.4) years for controls. Among patients, the mean duration of illness was 2.17 (SD 0.78) months and the mean HDRS score was 21.8 (SD 3.8). There were no significant differences in sex, age, education between the MDD group and the HC group. The MDD group had significantly higher HDRS scores than the HC group (Table 1).
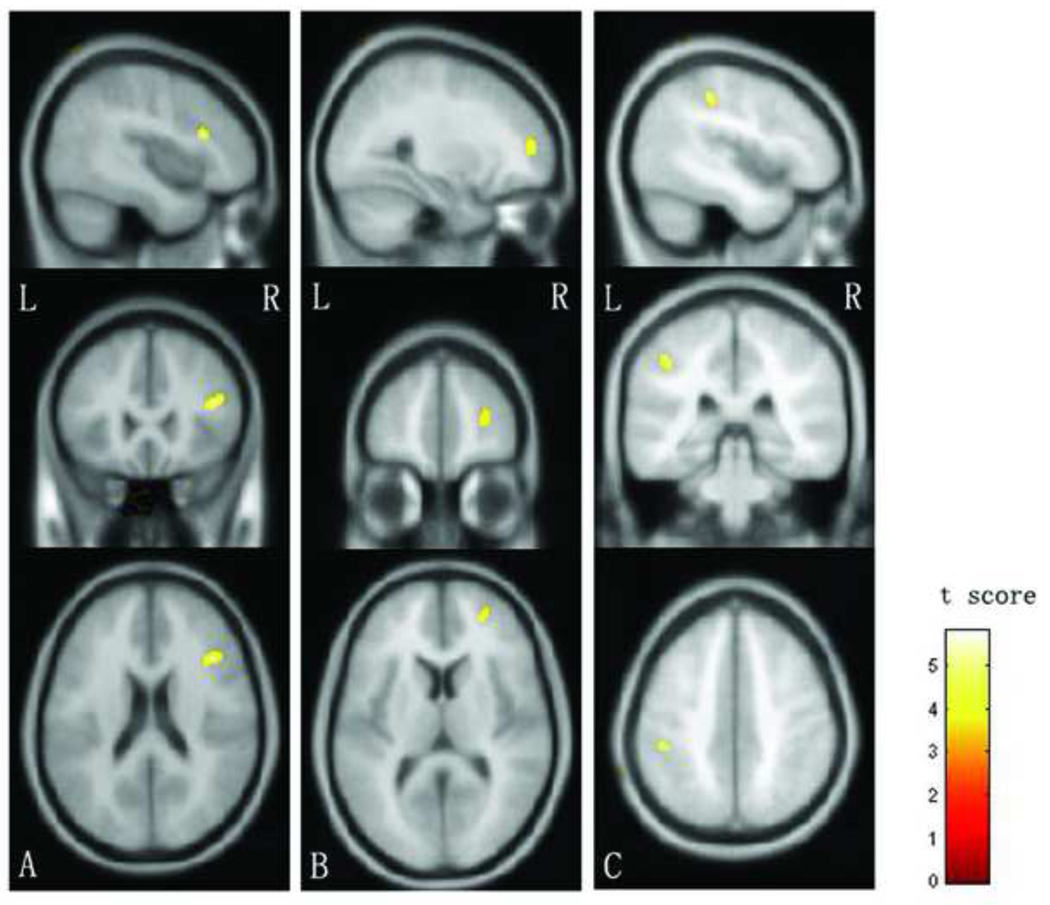
Compared with the HC group, the MDD group had lower FA values in the right superior longitudinal fasciculus within the frontal lobe (MNI coordinates: x=42, y=24, z=20, 71 voxels, t =4.37, df=42, p<0.001, uncorrected), right middle frontal white matter (MNI coordinates: x=28, y=52, z=10, 42 voxels, t =4.05, df=42, p<0.001, uncorrected) and left inferior parietal lobe (MNI coordinates: x=−42, y=−33, z=41, 39 voxels, t =4.45, df=42, p<0.001, uncorrected) (Figure 1). Analogously, the MDD group had higher ADC values in above three regions: the right superior longitudinal fasciculus within the frontal lobe (MNI coordinates: x=42, y=22, z=22, 142 voxels, t =5.77, df=42, p<0.001, uncorrected), right middle frontal white matter (MNI coordinates: x=28, y=50, z=12, 65 voxels, t =4.96, df=42, p<0.001, uncorrected) and left inferior parietal lobe (MNI coordinates: x=−44, y=−34, z=44, 61 voxels, t =4.66, df=42, p<0.001, uncorrected). There was no region showing significantly increased FA values or decreased ADC values in the MDD group, compared to the HC group.
Figure 1.
The images display the regions where fractional anisotropy values were lower (p<0.001) in the major depressive disorder group, compared to the healthy control group, in the right superior longitudinal fasciculus (A) (71 voxels), right middle frontal white matter (B) (42 voxels) and the left inferior parietal white matter (C) (39 voxels).
4. Discussion
In this study, we found lower FA and higher ADC values in the right SLF within the frontal lobe, right middle frontal, and left inferior parietal white matter in single-episode, medication-naive MDD participants with duration of illness less than 3 months, compared to HC subjects. To our knowledge, this study provides the first evidence of white matter abnormalities in the MDD sample with minimized influences by chronicity-related confounds and treatment variables; notably, these deficits emerge in the early stages of illness (the illness duration less than 3 months). Interestingly, a previous DTI study with medication-naive MDD participants with longer duration of illness (3–24 months) showed similar findings of reduced FA values in frontal white matter(Ma et al., 2007). While our study found the differences between the groups both in FA values and in ADC values, suggesting that MDD patients may exhibit not only abnormalities in orientation or organization of the fiber tracts but also in the overall diffusion (Le Bihan et al., 2001). Taken together, these findings suggest frontal and parietal white matter abnormalities identified by DTI may play an important role in the development of MDD pathophysiology.
The SLF is a major intrahemispheric fiber tract. The major bundle originates from the dorsal lateral prefrontal cortex (DLPFC) and terminates in the parietal lobe, which comprises the DLPFC-parietal circuitry (Schmahmann et al., 2007). The SLF also provides connection between the frontal cortex and the occipital and temporal lobes (Makris et al., 2005). Convergent evidence suggests that dysfunctions of the DLPFC and its relative circuitries can lead to abnormal cognition and emotional regulation in MDD (Tekin and Cummings, 2002). Recent fMRI studies further support this hypothesis (Aizenstein et al., 2009; Fales et al., 2008; Halari et al., 2009; Hooley et al., 2009; Schlosser et al., 2008; Vasic et al., 2009). The current findings of lower FA values in the SLF, middle frontal and parietal lobes provide further evidence that dysfunction of the DLPFC-parietal circuitry may play an important role in MDD pathophysiology. Since our sample consists of medication-naive patients at first episode, our findings suggest that white matter abnormalities may be present in the early stages of MDD and are not the result of medication exposure.
Previous DTI studies in geriatric MDD mainly found frontal and temporal white matter abnormalities (Bae et al., 2006; Nobuhara et al., 2006; Taylor et al., 2004; Yang et al., 2007). However, findings differ in MDD studies of younger samples, which reported white matter abnormalities in more wide-spread regions. For example, the middle frontal, the lateral occipitotemporal and the parietal white matter abnormalities were identified in our current study (23 medication-naive MDD participants aged 18–45, with single depressive episode, duration of illness less than 3 months), as well as Ma’s study (14 medication-naive MDD participants aged 20–41, with comparatively longer duration of illness, 3–24 months) (Ma et al., 2007). Zuo et al found abnormalities in the internal capsule and the inferior parietal portion of SLF in forty-five MDD participants aged 18–55 (Zou et al., 2008). This may suggest different pathophysiology in younger adults with MDD compared to geriatric MDD.
This study was not without limitations. Firstly, the right SLF, middle frontal, and left inferior parietal white matter survived an uncorrected threshold of p<0.001, and similarly, this threshold was applied in previous study (Ma et al., 2007). However, if the analyses were performed with false discovery rate whole brain corrected, no significant difference was detected between the two groups. Therefore, these findings are preliminary and further investigation with larger sample size is required. Secondly, only voxel-based DTI analyses were performed in the study to explore whole brain white matter abnormalities in MDD participants; however, there are some potential problems with this technique. For example, for the traditional voxel-base DTI analyses, there is no satisfactory solution to aligning FA images from multiple subjects (Smith et al., 2006). Additionally, Jones et al. demonstrated that the applications of different spatial smoothing extent can give rise to varying results (Jones, et al. 2005). Further investigation with complementary DTI techniques, such as combining tract-based spatial statistics, would be important.
In summary, our results demonstrate abnormalities in SLF, middle frontal and parietal white matter in the early stage of MDD and suggest that these abnormalities may play a key role in the pathophysiology of MDD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, Becker JT, Reynolds CF, 3rd, Carter CS. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. American Journal of Geriatric Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. American Journal of Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. American Journal of Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, Reynolds CF, 3rd, Aizenstein H. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biological Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. Journal of Affective Disorders. 2009;114:131–142. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger K, Schocke M, Weiss E, Auffinger S, Esterhammer R, Goebel G, Walch T, Mechtcheriakov S, Marksteiner J. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Research. 2008;164:237–244. doi: 10.1016/j.pscychresns.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. Journal of Child Psychology and Psychiatry. 2009;50:307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun-Todd DA. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Research. 2009;172:83–91. doi: 10.1016/j.pscychresns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on the outcome of VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Eerola M, Mantyla R, Neuvonen T, Poutanen VP, Luoma K, Tuulio-Henriksson A, Jylha P, Mantere O, Melartin T, Rytsala H, Vuorilehto M, Isometsa E. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. Journal of Affective Disorders. 2010;120:240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffution tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Li L, Ma N, Li Z, Tan L, Liu J, Gong G, Shu N, He Z, Jiang T, Xu L. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Research. 2007;1168:124–128. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. American Journal of Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- Ma N, Li L, Shu N, Liu J, Gong G, He Z, Li Z, Tan L, Stone WS, Zhang Z, Xu L, Jiang T. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. American Journal of Psychiatry. 2007;164:823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Malykhin N, Concha L, Seres P, Beaulieu C, Coupland NJ. Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Research. 2008;164:132–142. doi: 10.1016/j.pscychresns.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Matthews S, Simmons A, Strigo I, Gianaros P, Yang T, Paulus M. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Research. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Minami T, Takase K, Yoshida T, Yagyu T, Tajika A, Sugimoto T, Tamagaki C, Ikeda K, Sawada S, Kinoshita T. Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology. 2004;50:48–53. doi: 10.1159/000077941. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biological Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Research. 2007;151:179–188. doi: 10.1016/j.psychres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Schlosser RG, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage. 2008;43:645–655. doi: 10.1016/j.neuroimage.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Research. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Macfall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. American Journal of Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Macfall JR, Gerig G, Krishnan RR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatric Disease and Treatment. 2007;3:669–674. [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Kuchibhatla M, Payne ME, Macfall JR, Sheline YI, Krishnan KR, Doraiswamy PM. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One. 2008;3:e3267. doi: 10.1371/journal.pone.0003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophrenia Research. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Hose A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. Journal of Affective Disorders. 2008;109:107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychological Medicine. 2009;39:977–987. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Sauer H, Schlosser Md RG. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. Journal of Psychiatry and Neuroscience. 2008;33:199–208. [PMC free article] [PubMed] [Google Scholar]
- Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. American Journal of Psychiatry. 2008a;165:863–871. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research. 2008b;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Topics in Magnetic Resonance Imaging. 2008;19:97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- Yang Q, Huang X, Hong N, Yu X. White matter microstructural abnormalities in late-life depression. International Psychogeriatrics. 2007;19:757–766. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Lansing AE, Wu J, Brown GG, Paulus MP. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20:440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Huang X, Li T, Gong Q, Li Z, Ou-yang L, Deng W, Chen Q, Li C, Ding Y, Sun X. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. Journal of Psychiatry and Neuroscience. 2008;33:525–530. [PMC free article] [PubMed] [Google Scholar]