Abstract
CD4+CD25+Foxp3+ T-regulatory cells (Tregs) accumulate in tumors, however little is known about how the tumor environment influences this process. Here we show that human melanomas express ICOS-ligand (ICOS-L/B7H) that can provide costimulation through ICOS for the expansion of activated Tregs maintaining high Foxp3 and CD25 expression as well as suppressive function. Thus, ICOS-L expression by melanoma tumor cells may directly drive Treg activation and expansion in the tumor microenvironment as another mechanism of immune evasion.
Keywords: Melanoma, ICOSL, Treg, Foxp3, ICOS
Introduction
CD4+CD25+Foxp3+ T regulatory cells (Tregs) mediate immune suppression to control unfavorable immune responses against self-antigens or pathogens (1, 2). Tregs are found in high numbers in tumors of different tissue origin and their presence correlates with poor anti-tumor immune response and poor prognosis (3–6). In melanomas it has been reported that 25% of the CD4+ T cells found in tumors are Foxp3+, with only about 7% found in the peripheral blood of the same patients (7, 8). These results indicate that melanomas attract and retain Tregs in the tumor, or that tumor-infiltrating Tregs can expand in the tumor microenvironment. In addition, it has been reported that the tumor microenvironment can generate inducible Tregs from CD4+CD25− T cells (9, 10), with the total pool of Tregs being composed therefore of both natural Tregs and newly arising induced peripheral Tregs (10–12).
Tregs, like other T cells, respond to TCR stimulation and can be induced to expand with cytokines such as IL-2 (13, 14). Upon activation Tregs also express costimulatory receptors, such as ICOS and CD28, that further boosts their activation, proliferation, and survival (15). ICOS costimulation of CD4+ T cells has been found to facilitate the production of Th2 cytokines such as IL-4, IL-13, and IL-10 (16). Recently, a number of normal tissues have been reported to express ICOS-L and regulate CD4+ T-cell activation and cytokine production (17–24). In several mouse models of autoimmunity the CD4+CD25+ Tregs expressing ICOS were shown to produce IL-10 and control auto-reactive T cells in the invaded organs such as the pre-diabetic pancreas (25, 26). Tregs present in melanoma have also been found to express ICOS on their surface (27), and ICOS+ Tregs have been shown to potently inhibit T-cell responses indirectly through suppressing antigen-presenting cells with IL-10 (26, 27). In cancer, the source of ICOS costimulation for Tregs is largely unknown. Although DC, pDC, and B cells can express ICOS-L (16), there are no reports that ICOS-L expressed by tumor cells can regulate CD4+ T-cell activation, and the expression of ICOS-L in melanoma has not been characterized yet.
In this work, we report that both cultured and freshly-isolated metastatic melanoma cells from Stage IV melanoma patients express ICOS-L on their surface and can co-stimulate Tregs to promote high expression of CD25, Foxp3 and ICOS.
Material and Methods
Cell lines
L cells transfected with CD32 and ICOS-L were a gift of Yong-Jun Liu. Melanoma tumor cell lines used in the study: WM35, WM35P1N1, WM35P2N1, A684, 888, 938, 624, WM793, WM793P1N1, WM793P2N1, A375, A375S2, 526, 2088, 2089, 2084, A681, A682, A687, 1007, MEMO and SK-MEL-1.
Antibodies
ICOS-L, B7-H1, ICOS, CD86, Foxp3, rat IgG, and hamster IgG isotypes antibodies were from eBioscience (La Jolla, CA). HLA class II, CD4, CD25, CD45RA, CD45, CD8, CD16, IL-10, and IFN-γ antibodies were from BD Biosciences (San Jose, CA). Anti-MCSP-1 was from Miltenyi Biotec (Auburn, CA). Cytokine analysis by intracellular staining was done with BD fixation/permeabilization kit.
Quantitative real-time RT-PCR
RNA was obtained using PureLink RNA mini-kit (Invitrogen, Carlsbad, CA) and RT was performed using Superscript RT First Strand (Invitrogen). Real-time PCR was then done using a Syber-Green based kit from BioRad (Santa Clara, CA). The primer sequences are: ICOS-L: Forward 5’-AGCGTTGAGGTTACAC TGCATGTGGC-3’, Reverse 5’-GCTGACCACGTCATACAAGCCCCGCA-3’; B7-H1: Forward 5’-GTCACGGTTCCCAAGGACC-3’, Reverse 5’-CAGATGACTTCGGCCTT GGG-3’; Actin: Forward 5’-TTCCTATGTGGGCGACGAGG-3’, Reverse 5’-ACTCCA TGCCCAGGAAGGAAG-3’.
Screening of melanoma cell lines and primary melanomas for B7 molecule expression by flow cytometry
Melanoma cell lines were harvested with protease free dissociation buffer (Invitrogen). Metastatic melanoma tissue was obtained by surgical resection with patient informed consent using clinical and laboratory protocols approved by the M.D. Anderson Cancer Center Institutional Review Board (see Table 1). Tumor samples were processed within 2 h of resection first by cutting them into 5–8 mm2 pieces followed by mechanical disruption using a Seeward Stomacher (Fisher, Pittsburgh, PA). The cell suspensions were applied over a 70% Ficoll-Isopaque layer in D-PBS. Tumor cells were collected from the gradient interface, washed and stain with antibodies against MCSP1, HLA class II, CD45, and either ICOS-L, B7-H1, or CD86. Before flow cytometry analysis, 7 amino-actinomycin D (7-AAD) was added to the cells. All samples were analyzed using a BD FACScanto II flow cytometer using FACSDiva 5.1 software (BD Biosciences).
Table 1.
Tumor origin and expression of ICOS-L, B7-H1 and HLA class II
| Tumor number | Location of metastasis | ICOS-L | B7-H1 | HLA class II |
|---|---|---|---|---|
| 2033 | Neck subcutaneous superior and inferior cervical lesion | Negative | P | High |
| 2035 | Left serratus: muscle under arm | Negative | P | High |
| 2088 | Abdomen: local fungating melanoma | Negative | N | Negative |
| 2069 | Neck | Low | N | Low |
| 2089 | Axillary lymph node | Low | P | Low |
| 2097 | Left arm: tumor mass | Low | P | Low |
| 2016 | Lung wedge | High | P | Low |
| 2017 | Parathyroid superficial | High | P | Low |
| 2057 | Lung nodule | High | P | High |
| 2062 | Uterus | High | P | Low |
| 2063 | Occipital scalp mass | High | N | High |
| 2082 | Right leg: superficial | High | P | High |
N: negative, P: positive
T-cell activation and staining of isolated T cells after coculture with melanoma cells
Melanoma cell lines and L cells were irradiated with 200 Gy and 70 Gy, respectively, before plating. CD4+ T cells from PBMC were purified using Miltenyi microbeads (Auburn, CA) and further sorted into CD4+ and CD25− and CD4+ CD25+ (Treg-enriched) subsets in a FACSAria (BD Biosciences). Cells were cultured together with irradiated melanoma or L cell lines and soluble anti-CD3 IgE (CLB-T3/4.E) at 20 ng/ml, or 100 ng/ml OKT-3. IL-2 (20 U/ml final) was added to all cultures. Where indicated, sorted cells were labeled with 1 µM CFDA-SE (Invitrogen). Five to six days later the cells were analyzed for the expression of CD4, ICOS, CD25, and Foxp3. SB431542, a TGF-β-R1 kinase inhibitor, was used at 5 µM (Sigma-Aldrich, St. Louis, MO). IL-10 and IFN-γ production was evaluated after activating the cultured cells for 6 h with PMA+Ionomycin. Brefeldin A (Golgi-Stop; BD Biosciences) was added during the last hour of incubation. Cytokine staining was done using BD Fix/Perm reagents (BD Biosciences).
Blockade of ICOS-L in a tumor mouse model
C57BL/6 mice were injected i.v. with 105 B16-OVA cells (day 0) followed by i.p. transfer of 2 million CD4+ OT-II- Ly5.2 T cells on day 2. Starting on day -2 the mice were treated with 100 µg of anti-ICOSL antibody (28), or rat IgG, and thereafter for every two days until the end of the experiment on day 17. On day 17 after tumor challenge, Foxp3, CD25, CD4 and ICOS expression on T cells from lung tissues was evaluated by flow cytometry (29). C57BL/6 mice were purchased from the NCI (Bethesda, MD) and OT-II CD45.1 mice were from our colony.
T-cell suppression assay
For the T-cell suppression assays, the isolated Tregs from the co-cultures were washed and rested in culture medium with no IL-2 for 1 day. Autologous sorted CD4+CD25− cells labeled with CFDA-SE were cultured at a 1:1 ratio with the isolated and rested Tregs. An equal number of autologous irradiated PBMC pulsed with 10 ng/ml of anti-CD3 were added and the cultures incubated for 6 days. Cell division was analyzed by flow cytometry measuring CFDA-SE dilution.
Results
ICOS-L is expressed by human melanoma cells
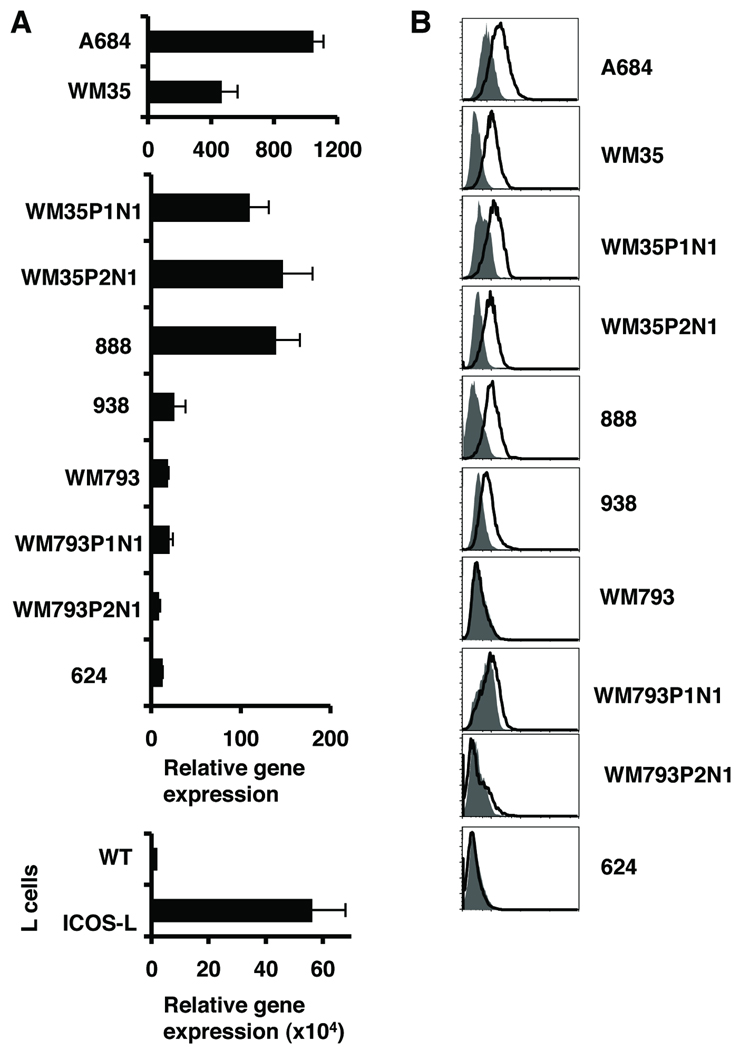
While searching for the expression of B7 molecules in mouse tumors we found that ICOS-L (also known as B7H, B7RP-1, GL50, or B7-H2), was expressed by the B16/F10 melanoma excised from C57BL/6 mice (Fig. S1). Then we checked whether human melanoma cells also express ICOS-L. We first analyzed mRNA expression of ICOSL on a panel of human melanoma cell lines and found that some had high ICOS-L mRNA expression (Fig. 1A) when compared to wild-type L cells. As a positive control, we used L cells stably transduced with human ICOS-L (Fig. 1A, bottom). The cell surface expression of ICOS-L was found in some melanoma cell lines, which correlated to the mRNA levels of expression (Figure 1B) and after the analysis of 22 melanoma cells lines we found that about 45% of the cells lines expressed ICOS-L (Supplementary Table 1). Some cells expressed high levels of ICOS-L such as WM35 and some only a discrete or low expression such as 938. Normal melanocytes showed low levels of ICOS-L mRNA and negative ICOS-L cell surface protein expression (data not shown). Thus, a significant number of human melanoma cell lines can express ICOS-L protein on the cell surface.
Figure 1. Melanoma cells express ICOS -L.
A. ICOS-L gene expression analysis in mRNA from melanoma cell lines, wild type L cells and L cells expressing ICOS-L. RT-PCR was performed for ICOS-L and β-actin genes. Data was normalized to the β-actin gene (Mean ± SD). Three separate graphs are shown to clearly illustrate the different levels of ICOS-L expression in the different sets of all lines. The expression of L cells was considered 1 in all the analysis. B. Protein expression of ICOS-L on melanoma cell lines. White histograms show anti ICOS-L and gray histograms isotype control. Normal melanocytes do not express ICOSL (not shown).
B7 molecule expression on antigen presenting cells and other tissues is regulated by cytokines. For example, IFN-γ has been shown to increase B7-H1 expression in both APC and tissues (30). We then tested the influence of IFN-γ on ICOS-L expression by melanoma lines. Although, B7-H1 mRNA expression was increased by IFN-γ, ICOS-L mRNA expression was not influenced by IFN-γ in a number of melanoma cell lines (Fig. S2A). The ICOSL expression was confirmed by FACS staining where an increase in cell surface B7-H1 was found after IFN-γ pre-treatment, with only a slight or no induction of cell surface ICOS-L expression (Fig. S2B). We also tested other cytokines, such as TNF-α, IL-1β, and IL-6 and did not find any change in the ICOS-L expression (data not shown). Therefore ICOS-L expression by melanoma cells is not influenced by IFN-γ and these other inflammatory cytokines.
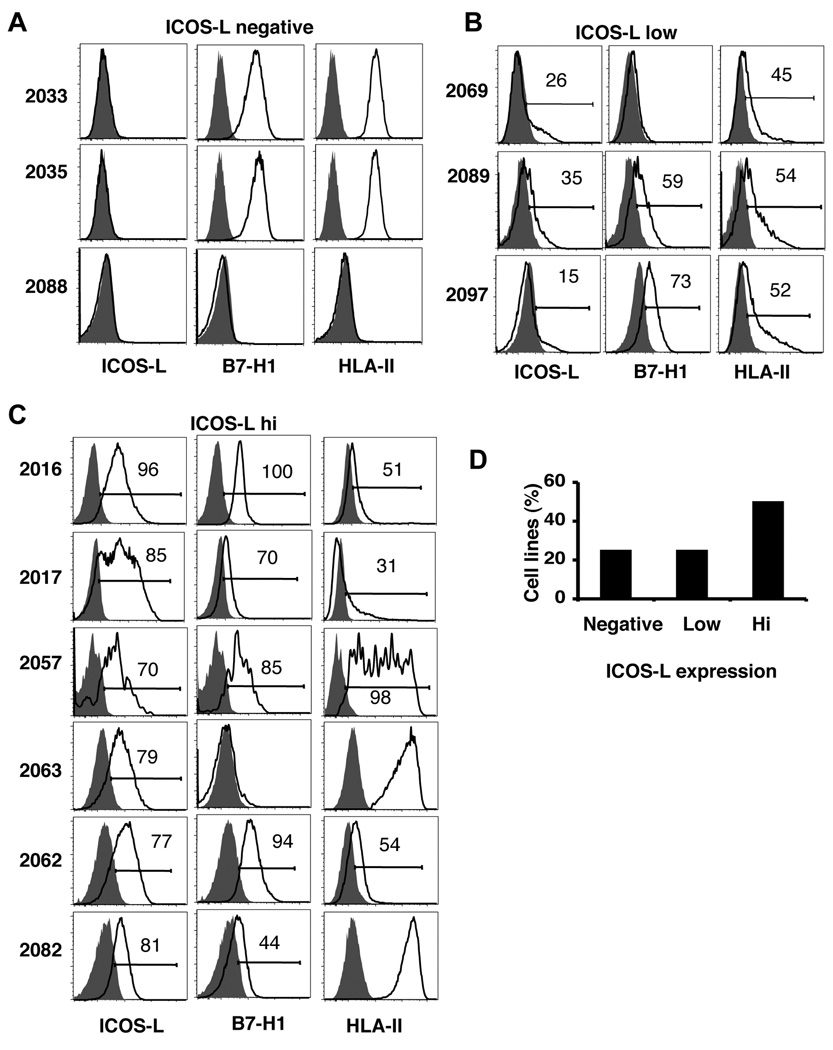
The expression of ICOS-L in melanoma cell lines could appear as result of long-term culture inducing somatic variation. Therefore, we screened fresh melanoma tumors for the expression of ICOS-L immediately after surgical excision from Stage IV melanoma patients. The tumors were obtained from different locations in the body and represented both non-lymph node-derived as well as lymph node-derived metastases (Table 1). After processing the tumors, a cell suspension enriched for melanoma cells was analyzed by FACS. After dead cell exclusion with 7-AAD, and exclusion of CD45+ leukocytes, melanoma cells were selected with the specific marker melanoma-associated chondroitin sulphate proteoglycan-1 (MCSP-1) for analysis of ICOS-L expression (see Fig. S3 for the gating strategy). Here, we also found that about 25% of the fresh human melanomas were negative for ICOS-L (Fig.2A), another 25% expressed low level of ICOS-L (Fig. 2B), while about 50% of metastases had relatively high levels of cell surface ICOS-L expression (Fig. 2C). A summary of the results of cell surface staining for ICOS-L on the 12 metastatic melanoma samples are shown in Fig. 2D. Therefore, human melanoma cells in vivo express ICOS-L and its expression is not simply induced by culture conditions. In addition, we have also cultured isolated tumor cells from patient material for up to 5 passages and confirmed the stable expression of ICOS-L in these new lines (data not shown). In addition to determining ICOS-L expression, we also analyzed B7-H1 and HLA class II on these freshly isolated metastases (Fig. 2A–C) and found that a number of tumors co-expressed ICOS-L and B7-H1 as well as HLA class II. As summarized in Table 1, 50% of freshly isolated melanoma samples expressed high levels of HLA class II with differential co-expression of ICOS-L, B7-H1, or both ICOS-L and B7-H1. This result indicates that tumor cells may be able to provide direct antigenic stimulation in context with ICOS costimulation.
Figure 2. Fresh melanoma cells express ICOS-L.
A–C. ICOS-L, B7-H1 and HLA class II expression analysis from fresh melanoma samples. A are melanomas that did not express ICOS-L, B shows the samples that have low expression of ICOS-L, and C the high ICOS-L expressing melanomas. White histograms show specific antibody staining and gray histograms show isotype. D. Summary of ICOS-L expression in fresh melanomas (n= 12).
ICOS-L signaling delivered by melanoma cells promote sustained CD25 and Foxp3 expression by Tregs
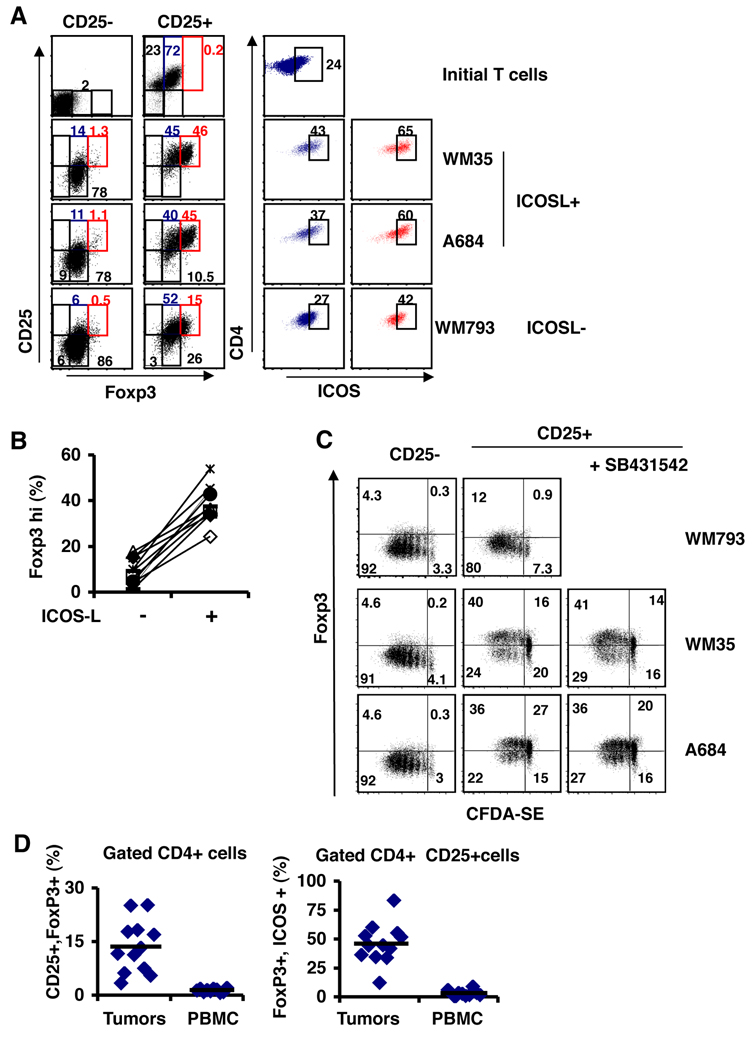
We next designed a series of experiments to test whether ICOS-L-expressing melanoma cells could provide costimulation to purified CD4+ T cell populations. We sorted CD4+CD25− (CD25−) and CD4+CD25+hi (Treg-enriched) T cells from peripheral blood of normal donors and cultured them with irradiated melanoma lines that were ICOS-L+ (WM35 or A684) or ICOS-L− (WM793) and soluble anti-CD3 IgE (that stimulates human T cells in a soluble fashion) (31) for 5 days. We used this IgE clone instead of OKT3 because the tumor cells do not express Fc receptors and can not induce cross-linking of the CD3 complex with OKT3 (31). Immediately after sorting and after the co-culture, the CD4+ T cells were analyzed for CD25, Foxp3 and ICOS expression. The initial CD25− cells did not express Foxp3, whereas the CD25+ cells contained about 72% of Foxp3+ cells which also had a fraction of 24% expressing ICOS (Figure 3A top panels). This is similar to recently published results showing that a significant fraction of Tregs in the periphery can have ICOS expression (26). The CD25− cells did not express ICOS (data not shown). After activation in the culture with all the melanoma lines, the CD25− cells up-regulated Foxp3 expression but only 6 to 14% up regulated CD25 and this expression was not influenced by the ICOSL expression of the melanomas. Also the CD25− cells only showed a 5 to 10% increased of the ICOS+ cells (data not shown). Since activated CD4+ T cells can express low levels of Foxp3 (32, 33), these results suggest that the CD25− cells have been recently activated and that our culture activation with the melanoma lines did not altered the capacity to induce Foxp3. For the CD25+ cells, although all the cells were CD25+Foxp3+, the expression of Foxp3 increased to a higher level in about 45% of the cells (Foxp3hi red gate in Figure 3A) in the co-cultures with ICOS-L+ melanoma lines, WM35 and A684 (Figure 3A left panels). Also the cells that were Foxp3hi had the higher expression of CD25. In contrast, CD25+ cells cultured with the ICOS-L− melanoma line, WM793, only showed a 15% increase of Foxp3hi cells and 29% of the cells started to lose CD25 expression by the end of the culture period. Therefore, ICOSL costimulation during activation of Treg cells induces the high expression of Foxp3 and sustains the expression of CD25. In contrast, non-Treg CD25− cells up-regulate Foxp3 to a lower level through activation of the TcR and independent of ICOS-L costimulation. We then analyzed the expression of ICOS in the Foxp3+ and Foxp3hi of the CD25+ cells (blue and red gates of figure 3A right panels) and found an increase level of ICOS+ cells in both Foxp3+ and Foxp3hi from the co-cultures with ICOS-L+ melanoma lines (40 and 60% respectively). Whereas the CD25+ cells cultured with ICOS-L− melanoma lines had only 27% ICOS+ cells in the Foxp3+ cells and 42% ICOS+ cells in the Foxp3hi cells (Figure 3A right panels). We tested several normal donors Treg in our co-cultures with melanoma lines and found that in every case there was an induction of Foxp3hi cells when the T regs were cultured with ICOS-L expressing melanoma lines (Figure 3B). Therefore, we concluded that ICOS-L costimulation during the activation of T regs positively induces the expression of Foxp3 and ICOS.
Figure 3. A. ICOS-L costimulation by melanomas promotes high Foxp3 and CD25 expression on Tregs.
Sorted CD4+CD25− (CD25−) and CD4+CD25+ (CD25+) cells were cultured with irradiated melanoma cell lines and anti-CD3. On day 5 the CD4+ cells were analyzed for the expression of Foxp3, CD25 and ICOS. ICOS-L+ tumors are WM35 and A684 and ICOS-L- cells are WM793. Foxp3 expression on sorted CD25− and CD25+ cells are shown in the left panels. ICOS expression on Foxp3+ (blue gate) and Foxp3hi cells (red gate) on CD25+ cells are shown in the right panels. B. Percentage of CD4+CD25+ cells expressing high levels of Foxp3 from different donors (n= 10) cultured with melanoma lines that express (+) or do not express ICOS-L (−). C. Sorted CD4+CD25− and CD4+CD25+ cells were labeled with CFDA-SE and activated with anti-CD3 over a monolayer of irradiated melanoma lines. After 5 days, the expression of Foxp3 and CFDA-SE dilution was analyzed. Where indicated the TGF-β-R1 kinase inhibitor SB431542 was added on day 1. D. Patients with melanoma have high levels of Foxp3+ ICOS + T cells in the tumor infiltrates. Tumor infiltrating lymphocytes (TIL) and PBMC from patients with stage IV metastatic melanoma were analyzed for the expression of CD4, CD25, Foxp3 and ICOS by flow cytometry. Shown are the percentages of CD25+Foxp3+ in the CD4+ population and the Foxp3+ ICOS+ cells in the CD4+CD25+ population.
To confirm the Foxp3 induction by ICOS-L costimulation driven by melanoma lines, we generated melanoma cell lines that could express ICOS-L and human CD32 (Fc receptor) on their surface, such that the CD3 complex could be cross-linked on the T cells by OKT3 after CD32 binding with or without ICOS-L costimulation (Fig. S4A). WM793, which does not express ICOS-L, was transfected with pcDNA3.2-CD32 with a empty pcDNA3.1 vector or with pcDNA3.1-ICOS-L and after selection and sorting, we obtained two cell lines designated as CD32+ WM793 and CD32+ ICOS-L+ WM793 (shown as ICOS-L+ WM793.CD32 in Fig. S4A). We used as reference L cells expressing CD32 and ICOSL (26) (donated of Dr. Yong-Jun Liu, M.D. Anderson Cancer Center). After co-culturing CD25− and CD25+ T cells with these set of cell lines we found that Foxp3hi induction in the CD25+ cells was only present when co-cultured with ICOS-L expressing cells (Figure S4B left panels) and that the Foxp3hi cells from these cultures had more than 80% ICOS+ cells (Figure S4B right panels). For the CD25− cells we found that activation with WM793 and ICOSL+WM793 generated about 18% Foxp3+ cells and 46% CD25+ cells with no influence of ICOS-L. Thus, activation of Tregs with melanoma cells providing ICOS costimulation promotes the expansion of ICOS+ CD4+CD25hiFoxp3hi T cells.
We next asked whether ICOS co-stimulation by ICOS-L+ melanoma cells could facilitate cell division of Tregs. Sorted CD4+CD25+ and CD4+CD25− T cells were labeled with CFDA-SE and cultured over ICOS-L+ (WM35 and A684) or ICOS-L− (WM793) melanoma cells. After five days in culture more than 90% of the CD25− T cells have divided and only 4% of the divided cells were Foxp3hi in both ICOSL+ and ICOSL− melanoma cell lines (Figure 3C left panel). Similarly, CD25+ T cells co-cultured with ICOS-L− melanoma line showed more than 90% cell division with 12% of Foxp3hi CFDA-SElow. In contrast, CD25+ T cells co-cultured with ICOS-L+ melanoma lines showed about 60% cell division with 40% of Foxp3hi CFDA-SElow cells (Fig.3C, middle panel). These results indicated that ICOS-L costimulation of Tregs slows the cell division rate and sustains Foxp3hi expression. Since TGF-β has been found to induce Foxp3 expression in non-Tregs, we then investigated the role of TGF-β signaling in our co-cultures. For this we used the TGF-β signaling inhibitor SB431542 (34) during the activation of CD25+ T cells with the ICOS-L+ melanoma cells. We found that the addition of SB431542 did not affect the cell division rate or the Foxp3 expression of the CD25+ T cells (Fig. 3C, right panel). Thus, the enhanced accumulation of divided CD4+CD25+Foxp3hi Tregs induced by ICOS-L+ melanoma cells was not due to TGF-β secreted by the melanoma cell lines. Moreover, real-time RT-PCR analysis found that although WM35 does express a low level of the TGF-β1 gene, A684 cells are TGF-β1-negative (data not shown). Thus, ICOS-L+ melanoma cells, although slowing down the cell division of Tregs, facilitated the maintenance of CD25 and high Foxp3 levels. When we studied 12 independent normal donor Tregs, we found that in all cases ICOS-L+ melanoma cell lines (WM35 and A684) facilitated increased expansion of Foxp3hi CFDA-SElow cells compared with ICOS-L− WM793 cells (Fig. S5A). We did not see a difference in viability of both CD25+ and CD25− T cells in these melanoma co-cultures.
To confirm the role of ICOS costimulation in driving the expansion of Tregs with sustained and higher Foxp3 expression we used OKT3 activation with ICOS-L-transduced L cells in CFDA-SE dilution assays. ICOS-L- L cells facilitated a much higher rate of cell division of the CD25+ T cells (52.5 to 90.8%) (Fig. S5B). More specifically 72% of the divided cells had high levels of Foxp3 expression compared to only 18% of the CD25+ cells that were cultured with L cells (Fig. S5B). Also the cells that have divided more showed the highest ICOS expression levels. As observed before, ICOS-L signaling did not alter the cell division or Foxp3 levels of CD25−T cells.
The favorable cell division rate of CD25+ cells observed with ICOSL+ L cells activation correlated with an increased in cell viability of about 19% in average (ICOS-L−: 59.9 +/− 1.83%, ICOS-L+: 79.35 +/− 7.56 %, n=5 experiments). Whereas the viability of the CD25− T cells was not affected by ICOS-L expression.
Next we asked whether patients with melanoma would have a CD25+Foxp3+ICOS+ T cell population in the tumor environment. We isolated tumor infiltrating lymphocytes (TIL) and PBMC from stage IV melanoma patients and analyzed T cell populations. We found that melanoma patients have ten times more CD4+CD25+Foxp3+ T cells in the TIL when compared to PBMC and they also have increased numbers of these cells expressing ICOS (Fig. 3D). This suggests that the tumor environment indeed favors CD25+Foxp3+ICOS+ T cell generation.
Blockade of ICOS-L reduces the generation of CD4+CD25+Foxp3+ICOS+ T cells
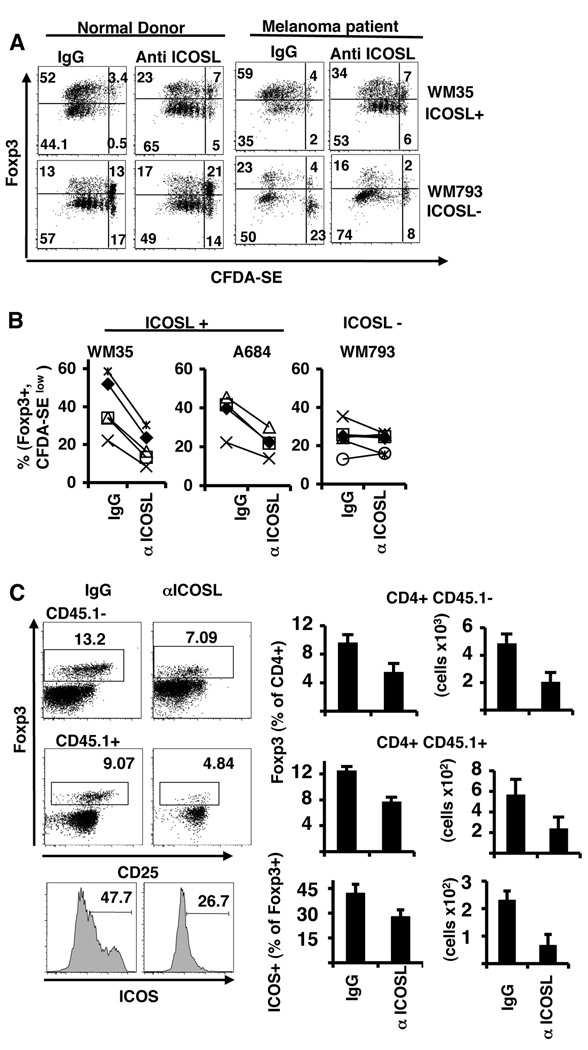
To confirm that Foxp3 induction in Treg cells is driven by ICOS-L stimuli, we sorted CD4+CD25+ T cells, labeled them with CFDA-SE and activated them with the tumors, as previously, but this time we added anti-ICOSL blocking antibody or mouse IgG. For these experiments, we used both normal donors and PBMC from Stage IV melanoma patients. We found that blockade of ICOS-L reduced the generation of Foxp3hiCFDA-SElow cells in normal donors by about 55% when the cells were cultured with ICOSL+ melanomas, but did not alter the percentage of cells generated by ICOSL− melanomas (Fig. 4A and 4B). Similar level of cell division and Foxp3 induction were found in cells from melanoma patients. These results indicate that part of the Foxp3hi Treg cells generated by activation with the tumors expressing ICOS-L is dependent on ICOS-L costimulation.
Figure 4. Blockade of ICOSL reduces the induction of Foxp3hi+CD25+ T-cells by melanomas.
A–B. Sorted CD4+CD25+ cells from normal donors or melanoma patients were labeled with CFDA-SE and activated with anti-CD3 over a monolayer of irradiated melanoma lines, ICOS-L+ WM35 or ICOSL− WM793. On day one mouse IgG or anti-ICOS-L antibody (MIH12) was added to the cultures. A. After 5 days cells were analyzed for Foxp3 expression and CFDA-SE dilution. B. Percentage of CD25+ cells expressing high levels of Foxp3 and CFDA-SElow from different donors (n=5) cultured with ICOS+ or ICOSL− melanomas and mouse IgG or anti ICOSL antibodies. C. Blockade of ICOS-L in vivo reduces the number of Foxp3+ ICOS+ T regs in the tumor tissue. Rat IgG or anti-ICOS-L was administered i.p. to C57BL/6 mice every 2 days for 16 days. Mice were i.v. injected with B16-OVA melanoma cells on day 2 and on day 7 mice were injected with 2 million OT-II-Ly5.1 CD4+ T cells. On day 17 lymphocytes from the lung were analyzed for the expression of CD45.1, CD4, CD25, Foxp3 and ICOS. Numbers in the plots are the percentage of Foxp3+ Tregs from Ly5.1− (recipient Treg) and Ly5.1+ (OT-II T reg) CD4+ T cells. Numbers in the histogram show the percentage of ICOS+ T cells from CD4+Foxp3+ cells. Graphs on the right show the percentage and total cell number of Foxp3+ cells from CD45.1− and CD45.1+ cells in the lungs of all mice analyzed (n=5).
To further confirm that ICOS-L expressed by the tumor is involved in the appearance of Foxp3+ cells in the tumor microenvironment, we made use of a mouse B16 melanoma model that expresses ICOS-L (see Fig. S1 in Supplementary Data). C57BL/6 mice were injected i.v. with B16 cells expressing recombinant ovalbumin (B16-OVA) that provokes the development of tumor colonies in the lung. After 5 days, we transfer CD4+ T cells from OT-II TCR transgenic mice recognizing OVA323–337 peptide in context of H-2b and that are congenic for CD45.1 (OT-II.CD45.1). Blocking antibodies against ICOS-L or rat IgG were administered every two days for 16 days starting 2 days before the tumor injection. We then evaluated Foxp3+ cells in the lung and found that both recipient CD45.1- and OVA- specific T cells had a reduced number of Foxp3+ cells in the mice treated with anti-ICOS-L antibody (Fig. 4C). Also these cells had reduced expression of ICOS. Therefore, ICOS-L expression in the melanoma tumors promoted the expansion of Treg in the tumor environment.
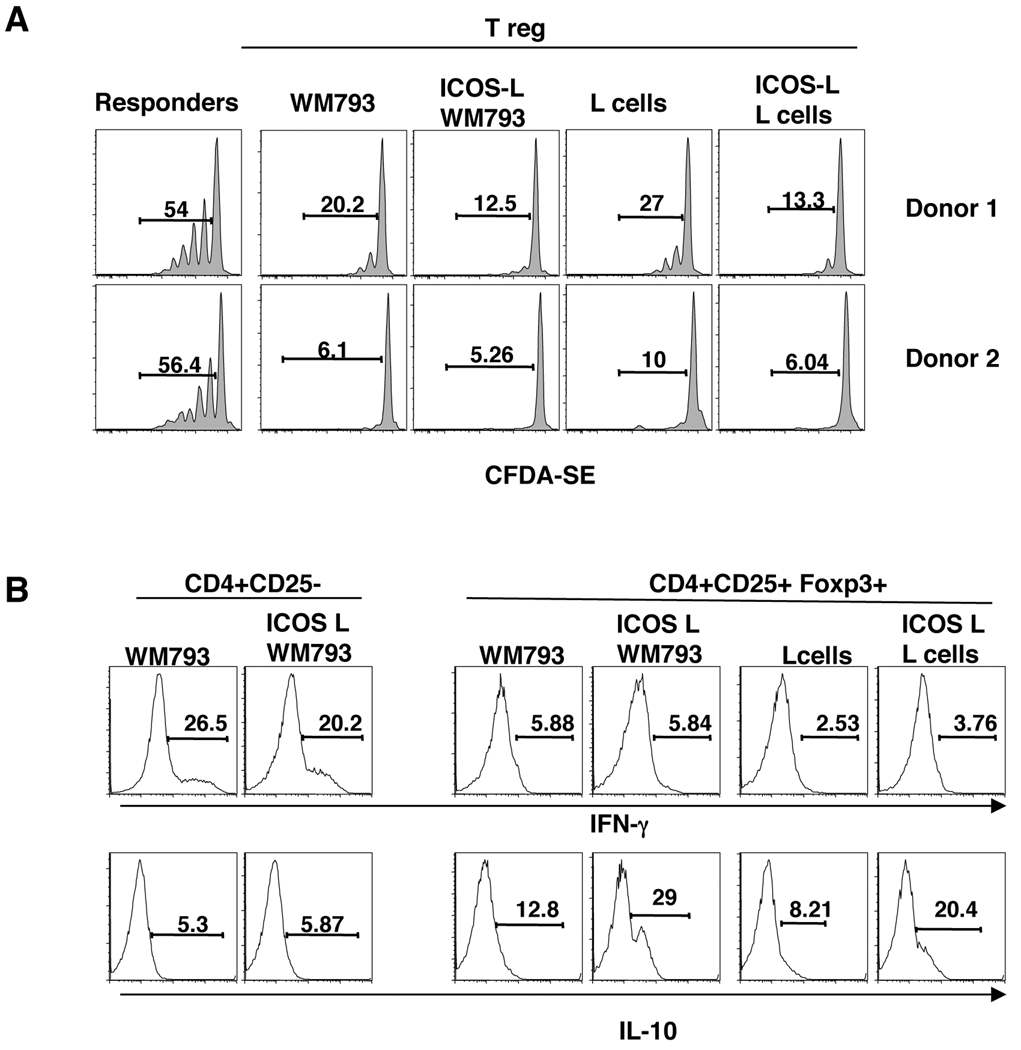
We next tested the suppressive activity of the CD4+CD25+ T cells form the co-cultures with ICOS-L+ WM793 or ICOS-L− WM793 melanoma cells. Autologous CD4+CD25− T cells were CFDA-SE-labeled and cultured for 6 days with the activated CD4+CD25+ T cells at a 1:1 ratio. As shown in Fig. 5, CD4+CD25+ T cells activated with ICOS costimulation by ICOS-L+ WM793 cells had a similar (albeit slightly enhanced) suppressor cell activity as CD4+CD25+ T cells activated with ICOS-L− WM793 cells, as indicated by the inhibition of CFDA-SE dilution in the CD4+CD25− responder cells. Similar results were found when the CD4+CD25+ T cells were activated with ICOS-L L cells or control L cells (Fig. 5A).
Figure 5. Tregs activated through ICOS-L+ melanomas have suppressive capacity and produce IL-10.
A. Tregs activated with ICOS-L-expressing WM793 cells or L cells can suppress effector T cells similarly as those activated over ICOS-L− WM793 and L cell controls. Sorted CD4+CD25+ T cells that were co-cultured with irradiated ICOS-L+ and ICOSL− WM793 or L cells were rested in media with no IL-2 and used at 1:1 ratio in a suppression assay with isolated autologous CD4+CD25− T cells pre-labeled with CFDA-SE. After 6 days, cell division by CFDA-SE dilution was analyzed. B. Tregs activated with ICOS-L-expressing melanoma cells produce IL-10. Activated CD4+CD25+ and CD4+CD25− cells co-cultured with the indicated cell line were intracellular stained for IL-10 and IFN-γ.
ICOS costimulation has been shown to promote the expansion of Tr1-like CD4+ T cells and ICOS+ Tregs capable of IL-10 production (26). We found that ICOS-L-expressing melanoma cells and L cells induced the expansion of Tregs that produce IL-10, (Fig. 5B, bottom right panel). These cells produced little IFN-γ, which indicated to us that the co-cultures with the tumors kept their Treg phenotype intact (Fig. 5B, top right panel). In contrast activated CD4+CD25− T cells from the same donors were found to produce IFN-γ, but less IL-10 when cultured with WM793 melanoma cells and ICOS costimulation did not alter the cytokine profile (Fig. 5B, left hand panel). Thus, these results indicate that ICOS-L+ melanoma cells can provide costimulation through ICOS on activated Tregs promoting the expansion of ICOS+, IL-10-secreting Tregs with T-cell suppressive properties.
Discussion
Costimulatory molecules of the B7 family control the activation of T cells during antigen recognition on APC and other tissues. In cancer, some of these B7 molecules can be immune suppressive and facilitate immune evasion. For example, the expression of B7-H1 in tumors, the ligand for PD1, inhibits the expansion and survival of anti-tumor T cells and is associated with worse prognosis (30, 35). The results presented in this paper demonstrate that in addition to B7-H1, a high proportion of human metastatic melanomas, as well as B16, a well-known murine melanoma, also express cell surface ICOS-L (B7H). It still remains unknown how ICOS-L expression is regulated in melanomas or normal melanocytes. We did not find ICOS-L by a number of pro-inflammatory cytokines (e.g., IL-6, IL-1β, TNF-α, IFN-γ) we tested in melanomas and we found that cultured normal melanocytes although having low mRNA levels of ICOS-L do not express ICOS-L protein. All the tumors analyzed were stage IV with no common tissue origin, which indicates that there may be some genetic component that determines ICOS-L expression.
ICOSL expression in the tumor environment is a potential source of costimulation for the tumor infiltrating lymphocytes. Some reports have shown that ICOSL expressed on normal renal tubule epithelial cells (36), and gastric cancer cells, can induce the activation of IL-10-producing CD4+ T cells (17). In this study we found that ICOS-L expressed by melanoma cells could costimulate Tregs to induce high levels of Foxp3, CD25 and ICOS. Our results suggest that tumor cells themselves may act as direct APC, since they can express HLA class II and provide self-antigen presentation and costimulation through ICOS-L. Interestingly, ICOS signaling allowed the expression of high levels of Foxp3 during cell division and also production of IL-10. In contrast, blockade of ICOS-L during T cell activation reduced Foxp3 expression but did not eliminate it, indicating that there are some other factor influencing Foxp3 expression, such as TCR signals (37) and perhaps other tumor factors. These results also indicate that in a more settle Tcr activation the T regs would require ICOS costimulation. Our results coincide with the finding that ICOS costimulatory signaling pathway drives Treg survival and expansion (26).
As found here and by others (27), Tregs co-expressing ICOS are elevated in tumor infiltrating lymphocytes of melanoma patients, indicating an active presence of these cells in the tumor environment. Moreover, ICOS expression has been found in subsets of activated CD25+Foxp3hi T regs (38) from peripheral blood. Taken together, these data indicate that ICOS+ Tregs are a subset of recently activated Tregs as a result of contact with tumor self-antigens and are critical in maintaining self-tolerance. This is in keeping with recent data showing an association of ICOS expression and IL-10-producing capacity in human Tregs and Tr1 cells (15, 26, 39). Data showing reduced ICOS+ Treg frequencies in the blood and tissues of patients with autoimmune diseases such as type I diabetes further support this contention (40).
It will be important to determine the levels of ICOS-L expression in a larger number of primary and metastatic melanoma samples and determine whether ICOS-L+ tumor cells is associated with increased infiltrating Tregs and poorer overall survival. From a clinical standpoint, inhibition of ICOS expression or blocking ICOS costimulation may be of therapeutic benefit. We found that blockade of ICOS-L in vivo can in fact reduced Tregs in the tumor environment, however careful dissection of the role of ICOS costimulation blockade on Tregs versus effector T cells needs to be addressed.
Supplementary Material
Acknowledgments
We thank the melanoma TIL therapy team, D. Kentor for blood processing, and D. He, K. Dwyer and K. Ramirez for cell sorting. We thank the Melanoma and Immunology Department members for help and discussion. This work is supported in part by a NIH Melanoma SPORE Developmental grant (5 P50 CA093459-05-DRP14) to LR and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center (to CD and NMO). LR also acknowledges support from the Miriam and Sheldon Adelson Medical Research Foundation (AMRF). CD also acknowledges support from the Trust Fellowship of the M.D. Anderson Cancer Center.
References
- 1.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 5.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 6.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 9.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 11.Vence L, Palucka AK, Fay JW, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earle KE, Tang Q, Zhou X, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med. 2009;206:1009–1017. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong C, Juedes AE, Temann UA, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 17.Chen XL, Cao XD, Kang AJ, Wang KM, Su BS, Wang YL. In situ expression and significance of B7 costimulatory molecules within tissues of human gastric carcinoma. World J Gastroenterol. 2003;9:1370–1373. doi: 10.3748/wjg.v9.i6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klingenberg R, Autschbach F, Gleissner C, et al. Endothelial inducible costimulator ligand expression is increased during human cardiac allograft rejection and regulates endothelial cell-dependent allo-activation of CD8+ T cells in vitro. Eur J Immunol. 2005;35:1712–1721. doi: 10.1002/eji.200425727. [DOI] [PubMed] [Google Scholar]
- 19.Qian X, Agematsu K, Freeman GJ, Tagawa Y, Sugane K. The ICOS-ligand B7-H2, expressed on human type II alveolar epithelial cells, plays a role in the pulmonary host defense system. Eur J Immunol. 2006;36:906–918. doi: 10.1002/eji.200535253. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt J, Rakocevic G, Raju R, Dalakas MC. Upregulated inducible co-stimulator (ICOS) and ICOS-ligand in inclusion body myositis muscle: significance for CD8+ T cell cytotoxicity. Brain. 2004;127:1182–1190. doi: 10.1093/brain/awh148. [DOI] [PubMed] [Google Scholar]
- 21.Schreiner B, Wischhusen J, Mitsdoerffer M, et al. Expression of the B7-related molecule ICOSL by human glioma cells in vitro and in vivo. Glia. 2003;44:296–301. doi: 10.1002/glia.10291. [DOI] [PubMed] [Google Scholar]
- 22.Wahl P, Wuthrich RP. Role of the B7RP-1/ICOS pathway in renal tubular epithelial antigen presentation to CD4+ Th1 and Th2 cells. Nephron Exp Nephrol. 2004;98:e31–e38. doi: 10.1159/000079930. [DOI] [PubMed] [Google Scholar]
- 23.Wiendl H, Mitsdoerffer M, Schneider D, et al. Muscle fibres and cultured muscle cells express the B7.1/2-related inducible co-stimulatory molecule, ICOSL: implications for the pathogenesis of inflammatory myopathies. Brain. 2003;126:1026–1035. doi: 10.1093/brain/awg114. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Li ZL, Zhu XX, et al. [Expression of ICOSL on human coronary artery endothelial cells and its being interferentialed by ox-LDL.] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:819–821. [PubMed] [Google Scholar]
- 25.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol. 2008;180:2967–2980. doi: 10.4049/jimmunol.180.5.2967. [DOI] [PubMed] [Google Scholar]
- 28.Liang L, Porter EM, Sha WC. Constitutive expression of the B7h ligand for inducible costimulator on naive B cells is extinguished after activation by distinct B cell receptor and interleukin 4 receptor-mediated pathways and can be rescued by CD40 signaling. J Exp Med. 2002;196:97–108. doi: 10.1084/jem.20020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 31.Van Lier RA, Boot JH, De Groot ER, Aarden LA. Induction of T cell proliferation with anti-CD3 switch-variant monoclonal antibodies: effects of heavy chain isotype in monocyte-dependent systems. Eur J Immunol. 1987;17:1599–1604. doi: 10.1002/eji.1830171112. [DOI] [PubMed] [Google Scholar]
- 32.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahl P, Schoop R, Bilic G, et al. Renal tubular epithelial expression of the costimulatory molecule B7RP-1 (inducible costimulator ligand) J Am Soc Nephrol. 2002;13:1517–1526. doi: 10.1097/01.asn.0000017901.77985f. [DOI] [PubMed] [Google Scholar]
- 37.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med. doi: 10.1084/jem.20100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Vieira PL, Christensen JR, Minaee S, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 40.Luczynski W, Wawrusiewicz-Kurylonek N, Stasiak-Barmuta A, et al. Diminished expression of ICOS, GITR and CTLA-4 at the mRNA level in T regulatory cells of children with newly diagnosed type 1 diabetes. Acta Biochim Pol. 2009;56:361–370. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.