Abstract
Objective
While previous research has shown that initiation of postmenopausal estrogen hormone therapy (HT) in late-life increases risk of dementia, animal studies and some observational studies have suggested that mid-life use of HT may be beneficial; however this hasn’t been rigorously investigated in large population-based studies. Our objective was to compare HT use in mid-life with that in late-life on risk of dementia among 5504 postmenopausal female members of an integrated health care delivery system.
Method
HT use was determined at mid-life (mean age 48.7) from a survey in 1964 and in late-life (mean age 76) using pharmacy databases from 1994–98. Risk of dementia diagnosis was evaluated with inpatient and outpatient diagnoses made in Neurology, Neuropsychology and Internal Medicine from 1999–2008. Cox proportional hazard models were used to examine HT use at different times on dementia risk with adjustment for age, education, race, body mass index, number of children, and co-morbidities.
Results
1524 women (27%) were diagnosed with dementia during the follow-up period. Compared to women never on HT, those taking HT only at mid-life had a 26% decreased risk (multivariate adjusted hazards ratio aHR=0.74, 95% confidence interval (CI , 0.58, 0.94), while those taking HT only in late-life had an 48% increased risk (aHR=1.48, 95% CI, 1.10, 1.98) and women taking HT at both mid and late-life had a similar risk of dementia (aHR= 1.02, 95% CI 0.78, 1.34).
Interpretation
These findings suggest that use of HT in mid-life only may protect against cognitive impairment, while HT initiation in late-life could have deleterious effects.
Introduction
Estrogen has been shown to be beneficial to many central nervous functions in animal models and several observational studies suggest that HT use is associated with a decreased risk of dementia. Meta-analyses of observational studies show a 39%–50% reduction in risk of dementia as compared to women not on HT 1. Nonetheless, several randomized controlled trials of HT use found an increased risk of developing dementia associated with initiation of estrogen-progesterone in late-life 2, 3. The discrepancy between the clinical trials, basic science data and numerous observational studies may be due to methodology. However, it is also possible that discrepancies in study results are due to timing of HT use, known as the “critical window” theory 4–6. The critical window theory posits that estrogen is most protective on neurocognition when it is taken only in the perimenopausal or early postmenopausal period, while estrogen taken many years after menopause has no benefit and may possibly be harmful. Data from animal studies demonstrate that endogenous estrogen administered during this critical period is associated with neuroprotection7–11. In observational studies, many women initiate HT use either shortly after menopause or during perimenopause. Most women initiate for a few years and then stop12. However, in most randomized clinical trials of HT, women tended to be older when they were assigned to HT with a mean age of 67 in HERS and 63 in WHIMS3, 13. One small observational study found that women who reported HT use in mid-life had a decreased risk of dementia14, yet another recent observational study found women starting HT in mid-life who stayed on throughout late-life had an elevated risk of dementia compared to women not using HT 15.
Thus far, no observational longitudinal studies have directly compared women on HT in mid-life only, with women either using HT both in mid-life and late-life, or women using HT in late-life only, and the potential association with dementia risk. It is not feasible to test the critical timing theory in a randomized clinical control trial setting due to the several decades required for the evaluation of an exposure in mid-life on a late-life outcome (dementia) as well as the potential harmful effects of HT in late-life. The goal of our current study therefore was to compare the association of HT use in mid-life versus HT use in late-life on risk of dementia in a large observational study of long-term female members of an integrated health care delivery system.
Methods
Study population
This is an observational cohort study of long-term female members of the Kaiser Permanente Medical Care Program of Northern California who participated in periodic multiphasic health checkups (MHC) that were part of routine medical care, in San Francisco and Oakland, CA, between 1964 and 1973 when they were 40–55 years old (N=7758). Kaiser Permanente of Northern California is a nonprofit, group-practice health integrated delivery system that covers more than one fourth of the population in the geographic areas served. Kaiser Permanente members are representative of the sociodemographics of the local population 16. Our analytic sample only included women who self-reported as being post-menopausal at time of the MHC exam, who were also alive and health plan membersin 1994; and without a diagnosis of dementia prior to 1999 (N=5504).
Mid-life Data collection
At the MHC, participants were interviewed and information on demographics, lifestyle, and medical history was collected including questions on medical conditions, menopausal status, and medication use 17. Women were asked if they were currently taking hormones. Women were considered to be taking mid-life HT if they answered yes to the question, and were not on thyroid hormones and did not have a self report of endocrine diseases. Women were also asked about menopausal status, number of children, history of miscarriages, and hysterectomy status. Systolic and diastolic blood pressure, weight, and height were measured according to standard procedures17 and body mass index was calculated (kg/m2). Blood was drawn for total serum cholesterol and fasting glucose. The participants were considered to have mid-life hypertension if they had one of the following: self-report of physician diagnosed hypertension, use of antihypertensive medication, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Mid-life diabetes was defined by self report of physician diagnosed diabetes, use of insulin or oral hypoglycemic agents, a fasting glucose (last food eaten in ≥8 hours) of ≥140 mg/dl, or a non-fasting (last food eaten in ≤4 hours) glucose of ≥200 mg/dl. Oophorectomy status from 1971–1974 was collected from hospitalization database using ICD-9 procedure codes : 65.3x, 65.4x, 65.5x, and 65.6x. From 1971 until the end of the study hysterectomy procedures were collected from our hospitalization database using ICD-9 procedure codes (68.0. 68.1, 68.2, 68.3, 68.4, 68.5, 68.6, 68.7, 68.8, 68.9).
Late- Life hormone therapy
From January 1,1994-December 31, 1998 KPNC pharmacy databases were searched for prescriptions of HT. The KPNC pharmacy database was implemented in all facilities in 1994, and contains prescription medications dispensed at KP hospitals, medical centers and medical offices. The pharmacy data contains medical record number, medicine name, date of prescription, dosage and refill information. All types of oral and patch HT were included, (estrogen only and estrogen/progesterone combinations). Vaginal creams were not included given the lack of data to suggest a systemic effect on the central nervous system. Those with two or more prescriptions or refills of HT during the four years were considered as late-life HT users. Each prescription is a 100 day prescription, thus the criteria of two or more prescriptions is equal to approximately six months of HT use.
Dementia diagnoses
Dementia diagnoses were ascertained through electronic medical records from a database that contains diagnoses from all outpatient and inpatient encounters at Kaiser Permanente medical centers and clinics. The form is completed by the treating clinician. Diagnoses considered in this study included AD (ICD 9 CM code 331.0) and VaD (ICD 9 CM code 290.4) from visits to Neurology and Neuropsychology and diagnoses of dementia (ICD 9 CM 290.0) from visits to Internal Medicine. Participants were considered to have dementia if they had any one of the diagnoses. Diagnoses were ascertained from January 1, 1999 to June 2008 when the MHC participants were between 75 and 84 at the commencement of dementia ascertainment, and between 84 and 93 years of age at the completion of the ascertainment. Those with diagnoses of dementia, cognitive impairment or general memory complaints prior to commencement of dementia ascertainment in January 1, 1999 were excluded from the study (N=343).
Latelife Comorbidities and Mortality
Stroke was recorded from hospital discharge diagnoses (ICD-9 codes for ischemic stroke, 433–438, hemorrhagic stroke, 430–432) from 1971 through the end of the study, June 2008. Late-life diabetes status was ascertained from our diabetes registry,18–20 a continuously maintained registry of KP patients with type 1 and type 2 diabetes, that is 99% specific, from 1994 though 2008. Hypertension (ICD-9 codes 401.x-405.x) and hyperlipidemia (ICD-9 codes 272.0, 272.1, 272.2) were recorded from our outpatient databases from 1994–2008. Mortality information was available on our cohort through the end of 2007.
Statistical analyses
All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Mid-life and late-life HT information was combined to examine 4 possible groups of women: those not on HT in mid or late-life (never), those on HT both in mid and late-life (both), those on HT only in mid-life (mid-life), those on HT only in late-life (late-life). Preliminary analyses included chi-square χ2 tests and t tests used to determine if demographic and clinical characteristics were significantly different by HT group (four groups). Since the majority of dementia cases occurred in women over age 80, we examined frequency of dementia cases stratified by median age in 1999 (80.4 years), across the four HT groups (never, mid-life, late-life and both). Unadjusted age as time scale Kaplan Meier survival curves of dementia risk were conducted to examine the likelihood of dementia over age and time by the four HT groups. We then used Cox proportional hazard regression models with age as time scale to investigate the independent relationship between HT use and risk of developing dementia. Follow-up time started with age at January 1, 1999 and participants were censored according to age at dementia diagnosis, age at date of death, age at date of lag in Kaiser membership (a lag in membership of 90 days or more), or age at the end of follow-up, June 1, 2008. Those in the never used HT category were the reference group in all models. We tested for a significant interaction of mid-life HT and late-life HT on dementia risk using a product term (midlife HT times late-life HT).
Models were adjusted for age (as time scale), education (high school, trade school, college 1–2 years, college 3–4 years and postgraduate, with grade school as reference), race (black, Asian, or other, with Caucasian as referent group), mid-life BMI (continuous variable), diabetes, hypertension, hyperlipidemia, stroke and hysterectomy status. Mid-life and late-life ascertainment of disease status for diabetes, hypertension, and hyperlipidemia (see midlife and latelife covariate section) were combined to create a time dependent covariate. Finally we performed a sensitivity analysis of HT and dementia risk stratified by stroke status, since stroke is a robust predictor of dementia, and prior studies have found HT use to increase risk of stroke. The study was approved by the Internal Review Board of Kaiser Permanente.
Results
The average age of the women in mid-life was 48.7, and 27% of the sample was non – white (Table 1). Forty-five percent of the women (n=2454) did not take HT in mid or late-life (never users, median age 50), 25% (n=1384) were taking HT in mid-life only (mid-life users, median age 50), 12% (n=673) were on HT in late-life only (late-life users, median age 47.3), and 18% (n=993) were on HT both in mid and late-life (both users, median age 49). Black women were more likely to be non-users, while white women were more likely to be on HT both in mid and late-life. Those on HT in late-life only were more likely to have more children, report fewer miscarriages, and to have a diagnosis of hyperlipidemia and stroke (Table 1). Twenty-three women had an oophorectomy at baseline.
Table 1.
Characteristics and Co-Morbidities of the Participants by Hormone Status
| No HT N=2454 | Mid-life HT N=1384 | Late-life HT N=673 | Both HT N=993 | Chi Square P value | |
|---|---|---|---|---|---|
| Age at midlife survey* | 49.0 (4.2) | 49.0 (3.9) | 47.3 (4.5) | 48.2 (4.0) | <.0001 |
| Race: | N (column %) | ||||
| Asian | 90 (3.7) | 26 (1.9) | 27 (4.0) | 24 (2.4) | |
| Black | 587 (23.9) | 283 (20.5) | 94 (14.0) | 128 (12.9) | |
| White | 1659 (67.6) | 1033 (74.6) | 518 (77.0) | 803 (80.9) | |
| Other | 117 (4.8) | 42 (3.0) | 34 (5.1) | 38 (3.8) | <.0001 |
| Education: | |||||
| Missing | 317(18.47) | 139(14.2) | 97(19.17) | 156(20.74) | |
| Trade school or college, | 556(32.4) | 323(32.99) | 198(39.13) | 269(35.77) | |
| High School | 804 (32.8) | 523 (37.8) | 208 (30.9) | 291 (29.3) | |
| Grade school | 432 (17.6) | 246 (17.8) | 82 (12.2) | 138 (13.9) | 0.0005 |
| Diabetes** | 490 (12.0) | 261 (18.9) | 115 (17.1) | 130 (13.1) | <.0001 |
| Hypertension** | 1809 (73.7) | 1005 (72.6) | 529 (78.6) | 828 (83.4) | <.0001 |
| Hyperlipidemia** | 880 (35.9) | 502 (36.3) | 296 (44.0) | 413 (41.6) | <.0001 |
| Stroke | 556 (22.7) | 324 (23.4) | 187 (27.8) | 269 (27.1) | 0.0050 |
| Had Hysterectomy | 81 (3.3) | 76 (5.49) | 52 (7.73) | 99 (9.97) | <0.0001 |
Mean ± standard deviation for continuous variables.
N and column percents for categorical variables.
P values were calculated using the chi square test.
Comorbidities and hysterectomy status combined from mid-life and late-life
Twenty –seven percent (N=1524) of the women were diagnosed with dementia between January 1,1999 and June 1, 2008 (Table 2) and had a median age of 80.4 years at start of dementia follow-up (January 1, 1999). Since the majority of dementia cases were in women above median age at start of follow-up, frequency of dementia by the 4 hormone groups was examined stratified by median age. Women on HT in late-life only had the highest prevalence of dementia diagnoses (Table 2, 23% of women on late-life HT for those < age 80.4 years, p<.01; 36% of women on late-life HT for those ≥ age 80.4 years, p<.01). For both age groups, those on HT in mid-life only had the lowest prevalence of dementia diagnoses (20.9% for women <80.4 years. p<.01, and 31.6% for women > 80.4 years, p<.01).
Table 2.
Frequency of Dementia Cases by Hormone Therapy Status Stratified by Median Age in 1999
| Age in 1999 | No HT | Mid-life HT | Late-life HT | Both | Chi Square P value |
|---|---|---|---|---|---|
| < 80.4 years | N (column %) | N (column %) | N(column %) | N (column %) | |
| No Dementia | 914(78.3) | 458(79.1) | 330(76.9) | 427(78.8) | |
| Dementia | 253(21.6) | 121(20.9) | 99(23.1) | 115(21.2) | .01 |
| ≥ 80.4 years | |||||
| No Dementia | 841(65.3) | 550(68.3) | 155(63.5) | 305(67.6) | |
| Dementia | 446(34.6) | 255(31.6) | 89(36.5) | 146(32.4) | .01 |
Age as time scale survival curves were performed to analyze the likelihood of dementia by HT timing. As seen in Figure 1, those on HT in late-life only were the least likely to be dementia free (58%), while those taking HT in mid-life only had the highest likelihood of being dementia free (78%). Those on HT at both time points had very similar dementia risk as those never taking HT at the two time points, approximately 70% of both groups were dementia free at the end of the follow-up period (Figure 1).
Figure 1.
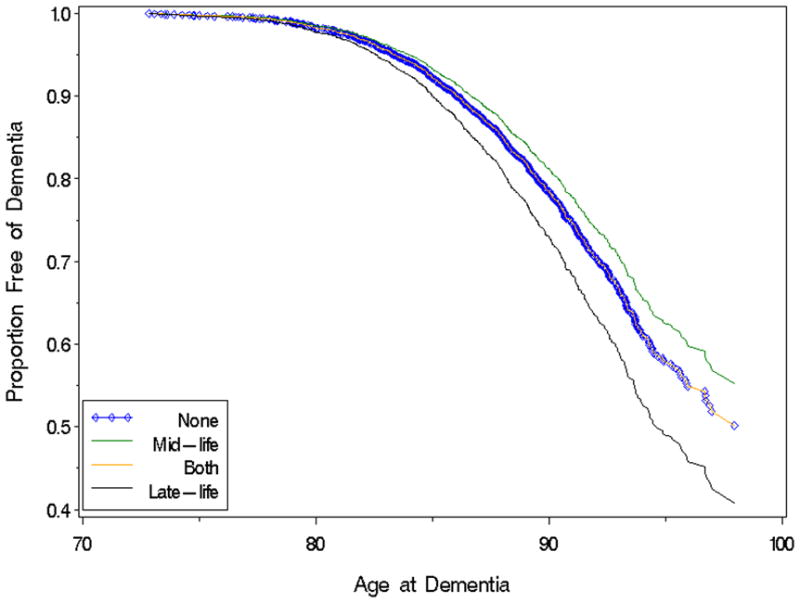
Age as Time Scale Kaplan Meier Survival Curves of Dementia Risk
There was a statistically significant interaction between mid-life and late-life HT use (p<.03) in association with dementia risk. Cox proportional hazard models with never HT users as the reference group showed significant associations between HT use and dementia risk. Those reporting HT use in mid-life only, had a reduced risk of dementia, while those reporting HT use in late-life only had an elevated risk of dementia. In a model fully adjusted for age (as time scale), education, race, BMI, number of children, diabetes, hypertension, hyperlipidemia, and stroke, mid-life HT use was associated with a 26% reduced risk of dementia, (HR=0.74, 95% CI, 0.58,0.94) (Table 3), while late-life HT use was associated with a 48% increased risk of dementia as compared to women not on HT (HR=1.48, 95% CI, 1.10,1.98). Additionally adjusting for hysterectomy status did not change the results by much (fully adjusted HRs: mid-life HT, HR=0.72, 95% CI 0.56, 0.91), late-life HT, HR= 1.45, 95% CI, 1.08,1.95), and both HT, HR=0.96, 95% CI 0.73, 1.26). Models excluding the twenty-three women with an oophorectomy were not different from the models that included them.
Table 3.
Cox Proportional Hazard Models of Hormone Use and Risk of Dementia
| Timing of Hormone Use | Not on HT | Mid-life | Late-life | Both |
|---|---|---|---|---|
| Unadjusted* | 1.0 (reference group) | 0.86 (0.72, 1.03) | 1.30 (1.04, 1.63) | 1.00 (0.82, 1.22) |
| Adjusted for education, race, BMI, number of children | 1.0 (reference group) | 0.75 (0.59, 0.95) | 1.54 (1.15, 2.06) | 1.13 (0.86, 1.47) |
| Additionally adjusted for diabetes, hypertension, hyperlipidemia, stroke | 1.0 (reference group) | 0.74 (0.58, 0.94) | 1.48 (1.10, 1.98) | 1.02 (0.78, 1.34) |
adjusted for age as the time scale, reference group are those not on hormone replacement therapy
Stratification by stroke status revealed that the pattern of decreased risk of dementia associated with mid-life HT use was similar in magnitude for women regardless of stroke status, although not a significant effect for those without a stroke (Table 4). However the pattern of increased risk of dementia associated with late-life HT use was significantly stronger among women who had a stroke; a 63% fully adjusted increase in dementia risk for women with a stroke versus a 16% fully adjusted increase for those without a stroke (Table 4).
Table 4.
Cox Proportional Hazard Models of Hormone Use and Risk of Dementia Stratified by Stroke Status
| With a Stroke (n=1336) | ||||
|---|---|---|---|---|
| Timing of Hormone Use | Not on HT | Mid-life | Late-life | Both |
| Unadjusted* | 1.0 | 0.87 (0.71, 1.08) | 1.37 (1.05, 1.80) | 1.12 (0.89, 1.43) |
| Adjusted for education, race, BMI, number of children | 1.0 | 0.74 (0.55, 1.00) | 1.73 (1.22, 2.44) | 1.24 (0.90, 1.71) |
| Multivariate Adjusted** | 1.0 | 0.74 (0.55, 0.99) | 1.63 (1.16, 2.31) | 1.10 (0.80, 1.52) |
| Without a Stroke n=4168) | ||||
| Unadjusted* | 1.0 | 0.81 (0.59, 1.10) | 1.14 (0.76, 1.71) | 0.72 (0.50, 1.03) |
| Adjusted for education, race, BMI, number of children | 1.0 | 0.72 (0.47, 1.10) | 1.16 (0.65, 2.08) | 0.88 (0.54, 1.45) |
| Multivariate Adjusted** | 1.0 | 0.72 (0.47, 1.10) | 1.15 (0.64, 2.05) | 0.88 (0.54, 1.45) |
adjusted for age as time scale,
Multivariate models adjusted for age, education, race, BMI, number of children, diabetes, hypertension, hyperlipidemia
Discussion
To our knowledge, the present study is the first observational longitudinal direct comparison of HT status both in mid-life and late-life on risk of dementia. We found that women reporting use of HT in mid-life (but not late-life) had a 26% reduced risk of a dementia diagnosis, while women using HT only in late-life had a 48% elevated risk compared to women not using HT at either time point. Women reporting HT use both in mid and late-life were not statistically different in dementia risk from women not using HT in mid or late-life. The results were independent of sociodemographic differences, hysterectomy status, number of children, body mass index and a large number of vascular comorbidities and risk factors.
The modest protective association between mid-life HT and dementia in our study has been indirectly suggested by a study of self report of HT duration in which longer duration of HT use was associated with a reduced risk of dementia 6. Henderson and colleagues found in the MIRAGE study a reduced risk of AD associated with HT use in a small subgroup of women aged 50–63 years of age, but the sample size was too small to make direct comparisons of dementia risk with late-life HT users 14.
Results of our paper support prior observational and clinical trial studies showing an elevated risk of dementia with late-life use of HT13. This WHIMS trial of HT on dementia and cognition found that initiation of HT in postmenopausal women aged 65+ was associated with a two fold increase in risk of dementia over a four year period. Our results are consistent with the WHIMS trial. In the present study women using HT in late-life only had a 48% elevated risk of dementia over an eight year follow up period. Adjustment for hyperlipidemia, hypertension and stroke did not attenuate the magnitude of the effect of late-life HT use on increased risk of dementia, although in a sub-analysis conducted among women with a stroke, the risk of dementia associated with late-life HT use increased to 63%.
Women who reported HT use in mid-life and who also had a prescription for HT in late-life did not have a different risk of dementia compared to never users. If indeed earlier initiation and continual use of HT is neuroprotective then this group should also have a reduced risk of dementia. However, this was not the case in our study, and the reduced risk was limited to women who reported HT in mid-life but did not have a prescription for HT in late-life. The reduced risk of dementia associated with mid-life HT use only lends support to the notion that it is not only early postmenopausal use of HT that is protective, but that use should also be limited to a few years21, 22. It is also possible that in the both mid and late lift HT use group the potential benefit of midlife use was counteracted by a negative effect of late life use.
This hypothesis has been coined the ‘critical window’ theory and has ample support in animal models 15. This evidence suggests that estrogen given during the critical window is associated with neuroprotection through several mechanisms; among them : 1) the reduction of deposition of β-amyloid in the brain, 2) improvement of synapse formation in the hippocampus, 3) an increase of choline acetyltransferase activity in the basal forebrain, and 4) improved cerebral blood flow and glucose metabolism8, 9, 11. The ‘gold standard’ randomized clinical control trial design in humans to test the critical window theory is unfeasible, if not impossible due to ethical implications stemming from the WHI results that HT use in late-life is associated with increased stroke and cancer risk. Since dementia can be a chronic disease with an insidious onset that is over one decade, prospective mid-life information on risk and protective factors allows for evaluation of timing of risk factors23.
There are several strengths and weaknesses of the present study. This is a large diverse sample of women, with several decades of follow-up. We had the ability to report on the association between HT use in mid-life and risk of dementia. Since these women are all members of an integrated health care delivery system we could also ascertain late-life HT use with pharmacy records, and therefore had the statistical power to compare women on HT in mid-life versus late-life on over 1500 cases of dementia. However, our HT information at mid-life was self report, and therefore we don’t know dose or type of HT. Since our pharmacy database was initiated in 1994, we also don’t have information on duration of mid-life HT, however several studies indicate women who do initiate HT in midlife do so for one to three years 14, 24. Finally, our dementia outcome is from electronic medical records using diagnoses made by a neurologist or an internist. There is the possibility that not all individuals with dementia received a dementia diagnosis, however this would lead to a type 1 error, and bias the findings towards the null; likely showing an underestimation of the true effect of HT use on dementia risk. Although we were in a unique position to control for several potential confounders and co-morbidities when analyzing the association between timing of HT and dementia this is an observational study and potential effects of residual confounding from unmeasured variables cannot be ruled out 100%.
Implications and Future Directions
Our results indicate that use of HT in late-life increases risk of dementia, while use of HT in mid-life only may reduce risk. Our results also suggest that use of HT in late life elevated risk of dementia particularly among women with a stroke. These results confirm earlier suggestions that HT should not be initiated in late-life to reduce dementia risk13. Clinical trials evaluating initiation of HT in the perimenopausal or early postmenopausal period with surrogate markers of dementia risk, such as cognitive function and brain imaging outcomes will provide much needed insight into further investigation of the critical window hypothesis.
Acknowledgments
Grant Support
Kaiser Permanente Community Benefits (Whitmer), National Institute of Health, NIA AG021918 (Yaffe) and NIA AG 031155 (Yaffe)
Reference List
- 1.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279(9):688–95. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004 June 23;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 3.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002 November;113(7):543–8. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 4.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006 May;91(5):1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005 March;47(3):371–5. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002 November 6;288(17):2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 7.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007 February;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi F, Pluchino N, Stomati M, Pieri M, Genazzani AR. CNS: sex steroids and SERMs. Ann N Y Acad Sci. 2003 November;997:378–88. doi: 10.1196/annals.1290.041. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs RB. Estrogen and nerve growth factor-related systems in brain. Effects on basal forebrain cholinergic neurons and implications for learning and memory processes and aging. Ann N Y Acad Sci. 1994 November 14;743:165–96. doi: 10.1111/j.1749-6632.1994.tb55792.x. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997 May;48(5 Suppl 7):S8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS, Gould E, Orchinik M, Weiland NG, Woolley CS. Oestrogens and the structural and functional plasticity of neurons: implications for memory, ageing and neurodegenerative processes. Ciba Found Symp. 1995;191:52–66. doi: 10.1002/9780470514757.ch4. [DOI] [PubMed] [Google Scholar]
- 12.den TI, Oddens BJ. Determinants of long-term hormone replacement therapy and reasons for early discontinuation. Obstet Gynecol. 2000 April;95(4):507–12. doi: 10.1016/s0029-7844(99)00586-4. [DOI] [PubMed] [Google Scholar]
- 13.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003 May 28;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 14.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005 January;76(1):103–5. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petitti DB, Crooks VC, Chiu V, Buckwalter JG, Chui HC. Incidence of Dementia in Long-Term Hormone Users. Am J Epidemiol. 2008 Mar 15;167(6):692–700. doi: 10.1093/aje/kwm362. Epub 2008 Jan 23. [DOI] [PubMed] [Google Scholar]
- 16.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collen M, Rubin L, Neyman J, Dantzig G, Siegelaub AB. Automated Multiphasic Screening and Diagnosis. Am J Public Health. 1964;54:741–50. doi: 10.2105/ajph.54.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 19.Selby JV, Karter AJ, Ackerson LM, Ferrara A, Liu J. Developing a prediction rule from automated clinical databases to identify high-risk patients in a large population with diabetes. Diabetes Care. 2001 Sep;24(9):1547. doi: 10.2337/diacare.24.9.1547. PubMed -55 2001 September;24:1547–55. [DOI] [PubMed] [Google Scholar]
- 20.Karter AJ, Birner CR, Ackerson LM, et al. The epidemiology of self-monitoring of blood glucose in insulin taking diabetics: the Northern California Kaiser Permanente Diabetes Registry. Diabetes. 1997 [Google Scholar]
- 21.Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann N Y Acad Sci. 2001 December;949:203–14. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 22.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007 February;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 23.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005 June 11;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson VW. Estrogen therapy and the brain. In: Kelsey J, Marcus R, Lobo RA, editors. Menopause: Biology and Pathology. Academic Press; 2000. pp. 315–26. [Google Scholar]


