Abstract
Purpose
Using fMRI, we examined whether or not adolescents with low levels of nicotine exposure (light smokers) display neural activation in areas shown to be involved with addiction in response to smoking-related stimuli.
Design/Setting/Participants
Twelve adolescent light smokers (aged 13 to17, smoked 1 to 5 cigarettes per day) and 12 non-smokers (ages 13 to 17, never smoked a cigarette) from the San Francisco Bay Area underwent fMRI scanning. During scanning they viewed blocks of photographic smoking and control cues. Smoking cues consisted of pictures of people smoking cigarettes and smoking-related objects such as lighters and ashtrays. Neutral cues consisted of everyday objects and people engaged in everyday activities.
Findings
For smokers, smoking cues elicited greater activation than neutral cues in the mesolimbic reward circuit (left anterior cingulate (T=7.88, p<.001), right hippocampus (T=6.62, p<.001) and right parahippocampal gyrus (T=4.70, p<.001)). We found activation from smoking cues versus neutral cues within both the left and right frontal medial orbital regions (T=5.09, p<.001 and T=3.94, p=.001 respectively), which may be unique to adolescents. Non-smokers showed no significant difference in activation between smoking-related cues and neutral cues.
Conclusions
Our finding that smoking cues produced activation in adolescent light smokers in brain regions seen in adult and heavy teen smokers suggests that even at low levels of smoking, adolescents exhibit heightened reactivity to smoking cues. This paper adds to the existing literature suggesting that nicotine dependence may begin with exposure to low levels of nicotine, underscoring the need for early intervention among adolescent smokers.
Keywords: fMRI, adolescent nicotine addiction, adolescent smoking, brain imaging
INTRODUCTION
People who start smoking as teens are more likely to become life-long smokers than those who start smoking in their 20′s or later [1]. Evidence suggests that adolescents may be more susceptible to nicotine addiction than adults, experiencing addiction at significantly lower nicotine levels [2-4]. Despite the fact that most adult smokers began smoking as adolescents, we have relatively little understanding of the neurophysiological processes underlying adolescent nicotine addiction. Underscoring this issue, pharmacotherapy for adolescent smoking cessation has been largely ineffective [5]. Understanding the neurobiology of adolescent nicotine addiction may help the development of new treatment strategies for adolescent smoking cessation.
Recently, functional magnetic resonance imaging (fMRI) has been used in studies of adult smokers to study the neural pathways involved with nicotine craving and addiction [6-14].
Several of these studies have reported brain activation in specific areas within the mesolimbic dopamine system following smoking cues. Although these studies have identified brain regions that are associated with smoking cue reactivity in nicotine-addicted adults, there has been limited use of fMRI to study adolescent smokers [15-17]. One study by Lee and colleagues that used heavy, established adolescents smokers observed similar findings to those seen in adult smokers (e.g., activation of the anterior cingulate cortex) [17]. Investigating areas of brain activation in adolescents who are light smokers and have not established strong smoking habits may provide additional insight as to the brain regions affected early in the process of addiction.
This study focused on adolescent light smokers to identify brain regions important during the developing stages of addiction. Using fMRI, we examined whether or not adolescents with low levels of nicotine exposure (light smokers) display neural activation following exposure to smoking cues. In addition, we looked for activation within regions shown to be activated in adult smokers.
METHODS
Fourteen adolescent light smokers (aged 13 to17, smoked 1 to 5 cigarettes per day), and 12 adolescent non-smokers (ages 13 to 17, never smoked a cigarette) were recruited from San Francisco Bay area high schools and pediatric clinics using fliers and online advertising. Participants were screened to exclude those who were currently or previously reported using nicotine replacement, Zyban® (bupropion HCL), or psychiatric medication (e.g., dopamine antagonists) in the prior month. Participants were also excluded if they were pregnant. Finally, given the effects on concentration and dopamine activation, participants were excluded if they reported using substances such as marijuana, cocaine or methamphetamine in the prior 72 hours.
Informed Consent
The research design and procedures were reviewed and approved by the University of California Institutional Review Board. Informed, written assent from the adolescent subject and consent from one parent were obtained for each subject before data collection.
Experimental Procedures
Participants were instructed to smoke ad libitum prior to scanning and were told that they could resume smoking following the scanning procedure. To verify and quantify participants’ smoking status, we measured exhaled carbon monoxide (CO) using the Vitalograph Breath CO monitor (Vitalograph, Inc, Lenexa, KS), and collected saliva for cotinine measurement. Cotinine was measured using liquid chromatography-tandem mass spectrometry with a lower limit of detection of 10 ng/ml. Participants completed confidential questionnaires that assessed their smoking behavior, addiction and other behavioral and demographic indices (see behavioral measures below). After the questionnaire, females gave a urine sample to verify a negative pregnancy status before the fMRI. All participants received a monetary payment of $100 for their participation.
Imaging Parameters
A Siemens 3 Tesla MAGNETOM Trio Tim scanner was used for fast echo-planar imaging. Following a localizer series, high-resolution T1-weighted structural images were obtained (TR/TE/TI= 10/4/300 ms, 15° flip angle, 1.0 × 1.0mm2 in plane resolution and 1 mm slab thickness). Next, functional imaging was obtained using an echo-planar pulse sequence with T2-weighted images sensitive to blood-oxygenation-level-dependent contrast (TR=2 s; TE=40 ms; flip angle=90, matrix = 64 × 64, FOV = 40 × 40 cm, 33 slices, 3 mm thickness).
Cue reactivity paradigm during fMRI scans
Cues consisted of photographs of smoking-related and neutral images. The smoking and neutral cue images were comprised from two standardized slide sets (see below). Images were an assortment of right and left orientation, and were selected for “teen friendliness”, such as younger-looking faces. The use of smoking-related visual cues has been behaviorally validated in samples of adolescent smokers [18] and allowed us to trigger measurable smoking-related neural activation without providing actual nicotine to our young participants. Smoking cues consisted of pictures of people smoking cigarettes and smoking-related objects such as lighters and ashtrays. The 36 smoking stimuli pictures were obtained from the International Smoking Image Series (ISIS)[19]. Neutral cues consisted of everyday objects (such as staplers and lamps) and people engaged in everyday activities (such as reading or writing). Sixteen Neutral cues were obtained from ISIS, and 20 neutral images were obtained from the International Affective Picture System [20]. Neutral cues were chosen to match the smoking cues in terms of size, color, and orientation.
All visual stimuli were presented onto an LCD screen behind the participant’s head, which the participant viewed through a mirror mounted on the head coil. Each functional MRI acquisition began with the presentation of a fixation cross for 18 seconds. Stimuli were presented in blocks lasting 18 seconds. Each block consisted of three images from the same condition (smoking or neutral), for a duration of 6 seconds per image. Between each block, the fixation cross was presented again for 18 seconds. Each fMRI acquisition run consisted of 3 blocks of each of the two conditions (smoking and neutral). The order in which blocks were presented as well as the order in which individual images appeared was randomized for each participant. There were a total of 3 runs. Stimuli were presented using E-prime 2.0 (Psychology Software Tools, Inc.).
Behavioral Measures
Craving
Before and after each fMRI acquisition run, participants were asked to rank their craving. The craving question “How much do you crave a cigarette right now?” was presented on the screen and participants responded by using a handheld controller which moved a cursor on the screen to select a score on a scale of 1 (“not at all”) to 10 (“extreme”).
Smoking Behavior
Prior to the fMRI session, smoking participants were asked about their frequency and quantity of cigarette smoking, at what age they had their first cigarette (puff and first whole cigarette), and at what age they began to smoke daily. Participants were also asked when they smoked their last cigarette prior to presenting for the study. The mean number of cigarettes participants reported smoking per day was calculated by averaging the number of cigarettes smoked for each day of the last week during which they smoked.
Nicotine Dependence
Prior to the fMRI session, nicotine dependence was assessed using the modified Fagerström Tolerance Questionnaire (mFTQ) [21]. Participants were also asked to rate how addicted to nicotine they felt using a scale from 0 (“not at all addicted”) to 100 (“extremely addicted”).
fMRI Data Analysis
Whole-Brain Analysis
Statistical analysis of fMRI data was performed using MATLAB (Mathworks Inc.) and SPM5 software (www.fil.ion.ucl.ac.uk/spm). Prior to analysis, the functional images were converted to 3-D Analyze format volumes. Images were corrected for motion artifacts using a 6-parameter rigid body affine transformation and corrected for differences in slice acquisition timing. The resulting images were normalized to a standard stereotaxic space (Montreal Neurological Institute (MNI) Template) using a 12-parameter affine/non-linear transformation and then spatially smoothed with a 8 mm full-width, half-maximum isotropic Gaussian kernel. For each subject, the fMRI data was analyzed using a general linear model in which blocks of each condition (Smoking and Neutral) were modeled by a separate regressor, consisting of the block onset and duration convolved with SPM’s canonical hemodynamic response function. Additional regressors were included to model motion correction parameters as covariates. Contrasts of interest were performed to compare smoking and neutral cue conditions. Second level random-effects analyses were performed to examine this contrast within and between the two groups. Each of these analyses was performed on the data from the whole brain. For the whole brain analyses, activations were considered significant at p <0.001 (uncorrected) with a minimum cluster size of 20 contiguous voxels.
Analyses were next performed to determine if activity within each significantly active voxel cluster in the whole brain analysis was correlated with specific behavioral measures known to be associated with level of addiction. First, percent signal change for each significantly active voxel cluster in the whole brain analysis was calculated. We then used the percent signal change to calculate Pearson correlation coefficients to see if activity within each of these clusters increased with increased nicotine exposure (i.e., number of cigarettes per day), level of addiction (measured by score on the mFTQ and self reported level of addiction), and level of craving (pre and post scan). Finally, we looked to see if activity increased with increased duration of time since last cigarette smoked.
A-priori ROI analysis
After a thorough review of the literature, we selected 10 brain regions of interest (ROIs) which have been shown to be activated by either nicotine or smoking cues in imaging studies of adult smokers [6, 8, 9, 13, 22-24]. The 10 ROIs included regions associated with the mesocorticolimbic reward circuits (orbitofrontal cortex, insula, anterior cingulate, posterior cingulate, hippocampus, thalamus, ventral striatum, amygdala, inferior temporal, and fusiform gyrus). Although the ventral tegmental area is also implicated in nicotine addiction, it is too small (~ 2 voxels) [25] to detect activation at the level of resolution we employed without incorporating surrounding tissues.
ROIs were defined using the MarsBar AAL ROI package, version 0.1 [26]. Planned comparisons were performed on each of these ROIs to test for significantly greater activation in Smoking versus Neutral cue blocks within the smokers using GLM analyses in the SPM5 MarsBar toolbox. For the analyses of the 10 ROIs in each hemisphere (10 right sided and 10 left sided), associations were regarded as significant if the probability of a Type I error was <0.05 after correcting for repeated analyses using the Benjamini and Hochberg False Discovery Rate (FDR). We then compared activation within these same ROIs between smokers and non-smokers.
RESULTS
Participant Characteristics
Two smokers were excluded from analysis: one subject was withdrawn from the study after he reported drinking alcohol in the prior 24 hours and reported falling asleep in the scanner. Another subject was excluded due to signal interference from glue in her hair extensions. Thus, the final sample consisted of 12 smokers and 12 non-smokers.
Characteristics of the 12 adolescent smokers and non-smokers are described in Table 1. The smoking sample was 42% female with a mean age of 16.3 years-old, reported smoking an average of 3.6 cigarettes per day (SD=1.3) and reported a mean duration of daily smoking of 1.9 years (SD=1.1). Mean score on the mFTQ addiction scale was 2.8 (SD=1.1, range= 2-5) reflective of a range of “no dependence” (1-2) to “moderate dependence (3-4).” The non-smokers were also 42% female with a mean age of 15.7 years (SD=1.6).
TABLE 1.
participant Characteristics
| Participant Characteristics | Smokers (n=12) |
Non-smokers (n=12) |
p value |
|---|---|---|---|
| Age | 16.3 ± .98 | 15.7 ± 1.6 | .235 |
| Duration of daily smoking (years) | 1.9 ± 1.1 | ||
| Mean cigarettes smoked per day | 3.6 ± 1.3 | ||
| Cotinine (ng/ml) | 63.2 ± 46.7 | 0 | <.001 |
| mFTQ1 score | 2.8 ± 1.1 | ||
| Self-reported addiction2 | 62.5% ± 19.7 | ||
| Craving score3 at baseline | 6.5 ± 2.0 | 1.0 ± 0 | <.001 |
| Craving score3 post scan | 7.0 ± 1.8 | 1.1 ± 0.3 | <.001 |
| % Female | 42% | 42% | |
| % White | 75% | 50% | |
| % Hispanic | 8% | 25% | |
| Alcohol use in past 3 months | 100% | 33% | .001 |
| Marijuana use in past 3 months | 83% | 33% | .005 |
mFTQ= modified Fagerström Tolerance Test (0-2= no dependence, 3-5= moderate dependence, 6-9= substantial dependence)
Baseline self-reported level of addiction on scale 0-100%
Craving score on scale 1-10
The median time smokers reported smoking their last cigarette was 1.9 hours prior to the scan (SD= 7.9 hours) but there was a wide range of 1-24.2 hours reflective of the ad libitum smoking by many of these adolescent light smokers. Pre-scan craving was highly correlated with baseline cotinine levels (r=.59, p=.004).
Whole brain fMRI Analyses
For smokers, smoking cues elicited greater activation than neutral cues in several brain regions in the mesocorticolimbic system including the left anterior cingulate (t=7.88, p<.001), right hippocampus (t=6.62, p<.001) and right parahippocampal gyrus (t=4.70, p<.001; see Figure 1 and Table 2). Other regions of activation to smoking cues versus neutral cues included the right middle occipital gyrus (t=6.62, p<.001) and the left middle occipital gyrus (t=6.03, p<.001).
FIGURE 1. Areas of greater activation after exposure to smoking cues than after neutral image exposure in adolescent light smokers.*.
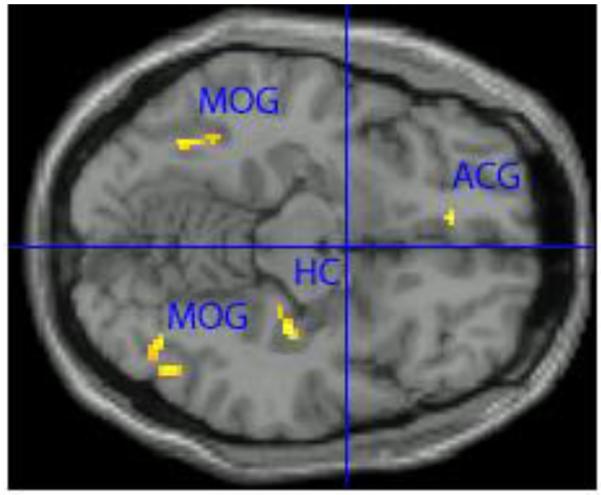
*Whole brain analysis, P<0.001, minimum cluster size ≥ 20 voxels. ACG= left anterior cingulate gyrus, MOG= right and left medial occipital gyrus, HC= right hippocampus.
TABLE 2.
Brain areas of greater activation after exposure to smoking cues than after neutral image exposure in smokers (n=12) and areas of greater relative activation (smoking cues minus neutral images) in smokers than in nonsmokers (n=12)
| Differences between smoking-related images and neutral images in smokers1 |
Differences between Smokers and Nonsmokers in relative activation2 |
||||||
|---|---|---|---|---|---|---|---|
| Side | Brain Area | MNI coordinates x, y, z |
Voxel Size |
T Statistic (df = 1, 11) |
p3 |
T Statistic (df = 1, 11) |
p3 |
| L | Anterior cingulate | −10 38 −10 | 39 | 7.04 | <0.001 | 2.35 | 0.014 |
| R | Hippocampus | 46 −68 −2 | 24 | 6.37 | <0.001 | 1.63 | 0.059 |
| L | Middle occipital gyrus |
38 −68 −10 | 172 | 6.58 | <0.001 | 0.91 | 0.186 |
| R | Middle occipital gurus |
46 −66 −10 | 240 | 5.95 | <0.001 | 1.66 | 0.056 |
Significant results represent regions in which activation in the smokers was greater after exposure to smoking-related images than after neutral images.
Significant results represent regions in which relative activation (smoking-related images minus neutral images) was greater in the smokers than in the nonsmokers.
Results for these whole brain analyses were considered significant at p<0.001.
The percent signal change (e.g., percent change in brain activation from baseline after viewing smoking cues) was not associated with the duration in time since last cigarette smoked (p values ranged from .23 - .78). Both pre and post scan craving were highly correlated with percent signal change in the right (r=.65, p=.02 pre-scan; r=.72, p=.01 post-scan) and left middle occipital gyrus (r=.74, p=.01 pre-scan; and r=.82, p<.01 post-scan). Additionally, the mean number of cigarettes smoked per day was highly correlated with percent signal change in the left anterior cingulate (r=.68, p=.02). Self-reported addiction (0-100% scale) was highly correlated with the percent signal change in left anterior cingulate (r=.61, p=.04). Mean score on the mFTQ was not correlated with percent signal change in any of the significantly active regions. Self-reported use of substances such as alcohol and marijuana by the participants was not correlated with percent signal change in any of the significantly active regions.
Non-smokers showed no significant activation difference between smoking-related cues and neutral cues. In the between group analyses, there were no regions with greater relative activation in response to smoking cues in smokers compared to non-smokers (see Table 2).
A-priori ROI analysis
In smokers, we found significant activation from smoking versus neutral cues within both the left and right frontal medial orbital regions (T=5.09, p<.01 and T=3.94, p=.01 respectively; see Table 3, non-significant ROIs not listed). We also found significant activation in the left amygdala (t=2.26, p=.05), left anterior cingulate (t=2.47, p=.04), left and right posterior cingulate (t=3.27, p=.03 and t=2.75, p=.03, respectively), right hippocampus (t=2.61, p=.03 respectively) and right inferior and middle temporal (t=2.80, p=.03 and t=3.01, p=.03). There were no significant activations in the non-smoker control group. In the between group analyses (e.g., smokers versus non-smokers), differences did not reach statistical significance.
Table 3.
Brain Areas of Greater Activation After Exposure to Smoking Cues Than After Neutral Cues in Smokers (N=12) using predefined ROI’s reported to be significant in previous studies of adult smokers
| Activations | x | y | z | T Value (df = 1, 11) |
p* |
|---|---|---|---|---|---|
| Amygdala | |||||
| Left | −24 | −2 | −18 | 2.26 | 0.05 |
| Anterior Cingulum | |||||
| Left | −4 | 34 | 13 | 2.47 | 0.04 |
| Right | 8 | 36 | 14 | 1.78 | 0.09 |
| Posterior Cingulum | |||||
| Left | −5 | −44 | 23 | 3.27 | 0.03 |
| Right | 7 | 43 | 20 | 2.75 | 0.03 |
| Medial Orbitofrontal | |||||
| Left | −5 | 52 | −9 | 5.09 | <0.01 |
| Right | 8 | 50 | −8 | 3.94 | 0.01 |
| Hippocampus | |||||
| Left | −25 | −22 | −11 | 2.08 | 0.06 |
| Right | 29 | −21 | −12 | 2.61 | 0.03 |
| Inferior temporal | |||||
| Right | 53 | −32 | −24 | 2.8 | 0.03 |
| Middle temporal | |||||
| Right | 52 | −39 | −3 | 3.01 | 0.03 |
corrected p significant at p<0.05.
Non-significant ROI results are not shown. Coordinates in MNI.
DISCUSSION
Our findings show that adolescent light smokers exhibit brain activation in response to smoking cues and these areas of brain activation are similar to those observed in adult smokers. More specifically, the increased BOLD activations observed in the left anterior cingulate, bilateral middle occipital gyri and right hippocampus in the whole brain analysis of our light smokers have all been reported in adult and heavy teen smokers [7-9, 17]. Based on both animal and human studies, [27, 28] all of these regions are thought to play a significant role in nicotine addiction [29-32].
Activation of the hippocampus is thought to be involved with drug reward, drug-related memories and conditioned responses [33] whereas activation of the anterior cingulate gyrus is thought to be involved in compulsive drug use and poor inhibitory control [34]. In our study, activation within this region was highly correlated with both number of cigarettes smoked per day and self-reported addiction on a 0-100% scale. Interestingly, there was no association between activation in the anterior cingulate and score on the mFTQ. This finding is consistent with prior findings by our team [35] that the mFTQ may a less sensitive measure of early signs of addiction in adolescent light smokers than self-reported level of addiction. We also found a high correlation between craving and activation within the middle occipital gyri, again similar to studies in heavy smoking addicted adults. The middle occipital region is modulated by visual attention, and activation in response to smoking cues within this region has been correlated with both nicotine craving and addiction [6, 7, 9].
A significant number of our participants reported histories of other substance use in addition to smoking cigarettes. Although alcohol and marijuana act on similar substrates within the mesocorticolimic regions of the brain as nicotine, neither self-reported alcohol nor marijuana use was associated with increased activation to smoking cues. However, it is possible that marijuana smoking may affect cigarette-cue responsivity in ways which are beyond the scope of this study. Further research needs to be undertaken to tease apart these effects.
In our a priori ROI analyses we found significant activation to smoking cues in several key areas within the mesocorticolimic system including the amygdala, anterior cingulate, hippocampus and the medial orbital frontal region. All of these regions have been associated with responses to drug-related stimuli in adults [6, 8, 9, 13, 24, 36, 37] and are thought to play a role in reward-related learning [38], impulse control, salience attribution [34], and compulsive drug use [29, 34, 39]. However, we did not find significant activity in some other areas reported in adult smoking studies (e.g., insula, fusiform, thalamus, and temporal regions). Perhaps we found no significant associations with these additional areas because these other areas become activated at a later “stage” of addiction and thus are not activated in light smokers. Clearly, more research needs to be done in this area, including the direct comparison of adolescent light smokers to adult light and heavy smokers.
Importantly, non-smokers did not show activation in response to smoking cues in any areas in the whole brain analyses or in the ROIs selected as part of the a priori analyses. In the between group analyses, whole brain and a priori ROI, the contrasts between smokers and non-smokers did not reach statistical significance. We suspect that this lack of significance may stem from an increased variability of neural responsivity to smoking cues within the non-smoking group which may be teased out with a small larger size. Another limitation to this study was the necessity of allowing the smokers to smoke during the day prior to the scan, which may have blunted their cue reactivity. However, we found no correlation between activation and time since last cigarette. In addition, using a similar fMRI paradigm, McClernon et al. [6] found that abstinence did not result in larger responses to smoking cues.
CONCLUSIONS
Our finding that smoking cues produced activation in adolescent light smokers in brain regions seen in adult and heavy teen smokers suggests that even at low levels of smoking, some adolescents exhibit heightened reactivity to smoking cues. Clearly there is a need to directly compare adolescent light smokers with adolescent heavy smokers and adult light smokers to help understand the role of experience on activation in these areas. This paper adds to the existing base of literature which finds that nicotine dependence may begin with exposure to low levels of nicotine in some adolescents, underscoring the need for early intervention among adolescent smokers.
ACKNOWLEDGEMENTS
The authors would like to thank Paul Keselman for technical support, data processing, problem solving and general assistance on this project. The authors would also like to acknowledge the time and effort of all the participants.
This study was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131 and NIH/NCI R01 CA140216. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work was performed at the University of California, San Francisco, San Francisco, CA.
Conflict of Interest: None of the authors have sources of funding, direct or indirect, and/or any connection with the tobacco, alcohol, pharmaceutical or gaming industries or any body substantially funded by one of these organizations.
REFERENCES
- 1.Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. N Engl J Med. 1991 Sep 26;325(13):968–969. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- 2.O’Loughlin J, DiFranza J, Tyndale RF, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. American Journal of Preventive Medicine. 2003 Oct;25(3):219–225. doi: 10.1016/s0749-3797(03)00198-3. [DOI] [PubMed] [Google Scholar]
- 3.DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tobacco Control. 2000 Sep;9(3):313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991-1993. Nicotine Tob Res. 2000 Aug;2(3):263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 5.Adelman WP. Nicotine replacement therapy for teenagers: about time or a waste of time? Archives of Pediatrics & Adolescent Medicine. 2004 Mar;158(3):205–206. doi: 10.1001/archpedi.158.3.205. [DOI] [PubMed] [Google Scholar]
- 6.McClernon FJ, Hiott FB, Huettel SA, et al. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005 Oct;30(10):1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClernon FJ, Kozink RV, Lutz AM, et al. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2008 Dec 24; doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Due DL, Huettel SA, Hall WG, et al. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002 Jun;159(6):954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 9.Smolka MN, Buhler M, Klein S, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006 Mar;184(3-4):577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- 10.McBride D, Barrett SP, Kelly JT, et al. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006 Dec;31(12):2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Brody AL. In vivo brain imaging of human exposure to nicotine and tobacco. Handb Exp Pharmacol. 2009;(192):145–171. doi: 10.1007/978-3-540-69248-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006 Aug;40(5):404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin TR, Wang Z, Wang J, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007 Nov;32(11):2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 14.Dagher A, Tannenbaum B, Hayashi T, et al. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009 Oct 13;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen LK, Picciotto MR, Heath CJ, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007 Dec 5;27(49):13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsen LK, Slotkin TA, Mencl WE, et al. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007 Dec;32(12):2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Lim Y, Wiederhold BK, et al. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005 Sep;30(3):195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- 18.Upadhyaya HP, Drobes DJ, Thomas SE. Reactivity to smoking cues in adolescent cigarette smokers. Addict Behav. 2004 Jul;29(5):849–856. doi: 10.1016/j.addbeh.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert D, Rabinovich N. International Smoking Image Series. 1999 [Google Scholar]
- 20.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS) University of Florida; Gainesville, FL.: 2008. [Google Scholar]
- 21.Prokhorov AV, De Moor C, Pallonen UE, et al. Validation of the modified Fagerström tolerance questionnaire with salivary cotinine among adolescents. Addictive Behaviors. 2000;25(3):429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SJ, Sayette MA, Delgado MR, et al. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res. 2005 Aug;7(4):637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein EA, Pankiewicz J, Harsch HH, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998 Aug;155(8):1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 24.Brody AL, Mandelkern MA, London ED, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002 Dec;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 25.D’Ardenne K, McClure SM, Nystrom LE, et al. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008 Feb 29;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005 Nov;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003 May;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Epping-Jordan MP, Watkins SS, Koob GF, et al. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998 May 7;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002 Oct;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 33.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988 Nov 4;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000 Mar;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein ML, Thompson PJ, Benowitz NL, et al. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine & Tobacco Research. 2007 Jan;9(1):129–135. doi: 10.1080/14622200601078517. [DOI] [PubMed] [Google Scholar]
- 36.Childress AR, Mozley PD, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999 Jan;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang GJ, Volkow ND, Fowler JS, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64(9):775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 38.May JC, Delgado MR, Dahl RE, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004 Feb 15;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Wang GJ, Ma Y, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005 Apr 13;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


