Abstract
Objective
To investigate the relationship of systemic factors to the 15-year cumulative incidence of retinopathy in nondiabetic persons in the Beaver Dam Eye Study.
Methods
Included 4,699 persons, 43-86 years of age at baseline examination in 1988-90 and with follow-up in 1993-95, and/or 1998-2000, and/or 2003-05. Stereoscopic color fundus photographs were graded to determine the presence of retinopathy. The main outcome measure was cumulative incidence of retinopathy accounting for competing risk of death or diabetes.
Results
The 15-year cumulative incidence of retinopathy in the nondiabetic cohort was 14.2%. In multivariate analyses, older age (hazard ratio [HR] per age group 1.13, 95% confidence interval [CI] 1.01-1.27), higher systolic blood pressure (BP, HR per 10 mmHg 1.15, 95% CI 1.07-1.20), presence of chronic kidney disease (CKD, HR 1.51, 95% CI 1.12-2.00) and wider retinal arteriolar diameter (HR per 10 μm 1.17, 95% CI 1.10-1.26) at baseline were associated with the incidence of retinopathy. In a separate model, the 15-year incidence of retinopathy was higher in those with uncontrolled hypertension compared with those who were normotensive (HR per 10 mmHg 1.41, 95% CI 1.05-1.89). There were no associations of body mass index, lipids, glycosylated hemoglobin, smoking status, markers of inflammation, endothelial dysfunction and oxidative stress, and hematologic factors to retinopathy incidence.
Conclusions
These data show two modifiable factors, uncontrolled hypertension and CKD, are related to an increased incidence of retinopathy in nondiabetic persons and show that control of BP is associated with a lower risk of incident retinopathy compared to uncontrolled BP.
Introduction
Retinopathy is estimated to affect between 1 and 15% of people without diabetes.1-9 It usually involves the presence of a retinal microaneurysm or blot hemorrhage in one eye and is associated with older age and hypertension. Independent of age, hypertension and other risk factors, the presence of retinopathy in nondiabetic persons is associated with increased risk of incident hypertension, diabetes mellitus, myocardial infarction, congestive heart failure, stroke, impaired cognition, and kidney disease.10-20 There are few long-term epidemiologic data describing the incidence and disappearance of retinopathy and associated risk factors in the nondiabetic population.21 The purpose of this paper is to investigate the natural history and relationship of systemic factors to the 15-year cumulative incidence and disappearance of retinopathy in nondiabetic persons in the Beaver Dam Eye Study (BDES).
Methods
Population
Methods used to identify and describe the population have appeared in previous reports.22,23 In brief, a private census of the population of Beaver Dam, Wisconsin (99% white), was performed from fall 1987 to spring 1988 in people 43 to 84 years of age.22 Of the 5,924 eligible individuals, 4,926 participated in the baseline examination in 1988-1990.23 Of the 4,542 surviving participants, 3,684 (81.1%) participated in the 5-year follow-up examination in 1993-1995.24 Comparisons between participants and nonparticipants at baseline and the 5-year follow-up examination have appeared elsewhere.23,24 Of the 3,334 surviving participants in the baseline and second examinations, 2,764 (82.9%) participated in the 10-year follow-up examination between March 1, 1998, and June 9, 2000.25 Comparisons between participants and nonparticipants at baseline and the 10-year examination have appeared elsewhere.24 Of the 2,480 surviving participants examined at the baseline, 5-year, and 10-year follow-up examinations, 2,119 (85.4%) participated in the 15-year follow-up examination between March 31, 2003, and April 30, 2005.26 The mean and median times between the baseline and 15-year follow-up examination were 14.9 years (standard deviation = 0.5 year) and 14.8 years, respectively.
Comparisons between participants and nonparticipants at the 15-year follow-up have been presented elsewhere.26 In general, persons who did not participate in the 15-year follow-up were older at baseline than those who did. After adjusting for age, nonparticipants were more likely to have fewer years of education completed, higher systolic blood pressure, and more pack-years smoked than persons who participated. After adjusting for age and gender, nondiabetic participants with retinopathy at baseline were as likely to participate as those without retinopathy (data not shown).
Procedures
Similar procedures were used at the baseline and follow-up examinations. Informed consent was obtained and institutional review27-35 board approval was granted at the beginning of each examination.
Participants underwent a standardized interview and examination at each visit. Information on demographic characteristics, cigarette smoking, alcohol intake, self-reported history of physician-diagnosed diabetes, hypertension or cardiovascular disease (CVD), and medication use were obtained from the questionnaire. There were also questions regarding use of diet and oral hypoglycemic agents or insulin for the management of hyperglycemia and questions regarding history of cigarette smoking, hypertension, and use of antihypertensive medications for the management of high blood pressure. Questions were also asked about use of lipid lowering agents for the management of dyslipidemia and aspirin and other nonsteroidal anti-inflammatory agents.
Blood pressure was measured according to the Hypertension Detection and Follow-up Program protocol.30 Nonfasting serum glucose level was determined using the hexokinase method,31 and plasma glycosylated hemoglobin was determined using affinity chromatography (Isolab Inc., Akron, OH).32 White blood cell count, red blood cell count and platelet count were determined using a Coulter counter method.
At the baseline and follow-up examinations, additional blood samples were stored in freezers at -80°C until the time of laboratory analysis. Baseline frozen samples were analyzed for serum creatinine, cystatin C, high sensitivity C-reactive protein (CRP), interleukin-6 (IL-6) and isoprostane levels.
Stereoscopic 30° color fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) and macula (Diabetic Retinopathy Study standard field 2) and a nonstereoscopic color fundus photograph temporal to but including the fovea (modified Diabetic Retinopathy Study standard field 3) were taken in each eye.33 Additional fundus photographs were taken if any lesions were found outside these fields.
Retinopathy was defined using a classification derived from studies of diabetic retinopathy but used herein to describe the presence of such lesions in the absence of diabetes. The presence of retinal hemorrhages, microaneurysms, cotton-wool spots, hard exudates, intraretinal microvascular abnormalities, venous beading, new vessels on the disc and elsewhere, and preretinal and vitreous hemorrhages was graded in a masked fashion using an abbreviation of the modified Airlie House classification scheme.34,35 Retinal microaneurysms were defined as small (usually not larger than the width of a vessel at the disc margin [125 microns]), circular, hard-edged and evenly colored, while retinal blot hemorrhages were usually larger than microaneurysms (any red spot >125 microns in its longest dimension was considered a hemorrhage unless the shape, smooth margins and central light reflex suggested it was a MA), with uneven edges and coloring. The presence of other retinal disease, such as central and branch retinal arterial or venous occlusion, retinal cholesterol emboli, and surface wrinkling retinopathy was graded using a detailed protocol.
When two eyes of a participant were discrepant in the presence of a lesion, the grade assigned was that of the more severely involved eye. For example, in assigning the presence of retinal microaneurysms, if they were present in one eye but not the other, the participant would be considered to have retinal microaneurysms. When lesions could not be graded in one eye, the participant was assigned a score equivalent to that in the other eye.
After converting the field 1 photographs to digitized images, retinal measurements were carried out by trained graders masked to participant characteristics using computer-assisted software. All arterioles and venules coursing through a specified zone of 0.5–1 disc diameter surrounding the optic disc margin were measured and summarized as central retinal arteriolar equivalent (CRAE) or central retinal venular equivalent (CRVE) using a modification of the Parr-Hubbard formula36 as described by Knudtson et al.37 These equivalents are the projected diameters of the central retinal vessels, measured away from the optic disc.
Definitions
Age was defined as the age at the time of examination. Retinopathy was defined to include presence of microaneurysms and/or blot hemorrhages, and/or more severe retinopathy lesions (e.g., hard exudates, cotton-wool spots, intraretinal microvascular abnormalities, retinal new vessels). Retinal microaneurysms and blot hemorrhages were also analyzed separately. The mean systolic blood pressure was the average of the two systolic blood pressure determinations, and the mean diastolic blood pressure was the average of the two diastolic blood pressure determinations. A person was defined as having a positive history if he/she responded positively to the questions regarding CVD and stroke. Hypertension was defined as a mean systolic blood pressure of ≥140 mmHg, and/or a mean diastolic blood pressure of ≥90 mmHg, and/or a history of hypertension with use of antihypertensive medication. Hypertension was further characterized by antihypertensive treatment status as untreated uncontrolled, treated and controlled, or treated and uncontrolled. Diabetes was defined as a history of diabetes mellitus, treated with insulin, oral hypoglycemic agents, and/or diet. Newly diagnosed diabetes mellitus was defined by a glycosylated hemoglobin value that was greater than 2 standard deviations above the mean for a given age-sex group or a random blood glucose value >200 mg/dL. Primary care physicians were consulted whenever there was doubt about past diagnosis. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
The glomerular filtration rate was estimated from serum creatinine using the re-expressed Modification of Diet in Renal Disease equation defined as follows: estimated glomerular filtration rate (eGFR) = 175 × (serum creatinine in mg/dL)-1.154 × age-0.203 (× 0.742 for women).38 Chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m2, based on the US National Kidney Foundation Kidney Disease Outcome Quality Initiative working group definition.39
Incident retinopathy, microaneurysms, and blot hemorrhages were defined in the nondiabetic cohort without retinopathy at baseline who developed these lesions at a follow-up examination before developing diabetes or a retinal vein occlusion. Disappearance was defined in a nondiabetic cohort without retinal vein occlusion who had signs of retinopathy at baseline and in whom the lesion or lesions disappeared at a follow-up examination before diabetes or a retinal vein occlusion occurred.
Cigarette smoking status was defined as follows: subjects were classified as having never smoked if they reported having smoked fewer than 100 cigarettes in their lifetime; as ex-smokers if they had smoked more cigarettes than this in their lifetime but stopped smoking before the examination; and as current smokers if they had not stopped. Visual impairment was defined as a best corrected visual acuity determined by a modified Early Treatment Diabetic Retinopathy Study refraction of 20/40 or worse in the better eye.23
Statistical Methods
SAS (SAS Institute, Cary, NC) was used for statistical analysis.40 Cumulative incidence was estimated by the product-limit method,41 and age-adjusted rates were computed by the direct method. Tests for differences between rates were conducted by the log-rank test.42 Multivariable models were constructed by discrete logistic hazard regression.43 Time-varying covariates were employed as follows: for each separate 5-year follow-up interval, the value of each covariate at the beginning of the interval, or previous value if that value was missing, was included in the model. For example, the baseline value of hypertension was included for the interval between baseline and the 5-year examination. The value at the 5-year examination was included for the interval between the 5- and 10-year examinations, and the value at the 10-year examination was included for the interval between the 10- and 15-year examinations. Time-varying covariates for other parameters subject to change were defined similarly. Participants were censored at the point they developed diabetes or a retinal vein occlusion. For example, if they developed diabetes or a retinal vein occlusion between exams 2 and 3, they were censored at exam 3.
The hazard of developing retinopathy in the second eye compared to the first was modeled with a Markov multi-state model using the R software package.44 Age was used as the time scale and diabetes and death were modeled as absorbing states. The transition rate for developing retinopathy in the right eye was constrained to be equal to that in the left eye.
Results
Of the 4,699 persons examined at baseline who had information from at least one follow-up examination, we excluded 505 persons with confirmed diabetes, suspected diabetes, or no diabetes information at baseline and 32 persons with central or branch retinal venous or arterial occlusions or neovascular age-related macular degeneration at baseline. We also excluded 185 persons with no information regarding retinopathy at baseline, 481 persons lost to follow-up at the second exam, 168 persons missing retinopathy information at follow-up, and 55 with a retinal vein occlusion at the follow-up examination, leaving 3,273 persons for analysis.
The prevalence of any retinopathy at baseline in this group was 10.1% (7.8% at risk for disappearance); for retinal microaneurysms only it was 7.1% (5.5% at risk for disappearance), for retinal blot hemorrhages only it was 2.5% (1.3% at risk for disappearance), and for more severe retinopathy it was 1.1% (0.8% at risk for disappearance). Table 1 presents characteristics of the nondiabetic group who did or did not have retinopathy at baseline and was at risk for incidence or disappearance of retinopathy. Persons with retinopathy were older, had higher glycosylated hemoglobin, higher systolic and diastolic blood pressure, higher pulse pressure, higher white blood cell count, higher serum cystatin C, and higher frequency of hypertension present than those without retinopathy present at baseline.
Table 1.
Characteristics of Participants With and Without Retinopathy in the Beaver Dam Eye Study Baseline Examination, 1988-1990.
| Characteristic | No retinopathy | Retinopathy present* | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Age (yrs) | 3017 | 60.1 | 10.57 | 256 | 63.8 | 10.31 | <0.001 |
| Body mass index (kg/m2) | 3005 | 28.5 | 5.25 | 254 | 28.8 | 5.05 | 0.35 |
| Glycosylated hemoglobin (%) | 3017 | 5.7 | 0.68 | 256 | 5.8 | 0.87 | 0.01 |
| Serum total cholesterol (mg/dL) | 3017 | 232.4 | 43.27 | 256 | 233.2 | 44.30 | 0.77 |
| Serum HDL cholesterol (mg/dL) | 3012 | 52.9 | 17.54 | 256 | 50.8 | 17.33 | 0.07 |
| Systolic blood pressure (mmHg) | 3016 | 129.3 | 19.16 | 256 | 138.6 | 21.93 | <0.001 |
| Diastolic blood pressure (mmHg) | 3016 | 77.5 | 10.55 | 256 | 79.1 | 11.22 | 0.03 |
| Pulse pressure (mmHg) | 3016 | 51.8 | 16.11 | 256 | 51.8 | 20.42 | <0.001 |
| Pack-years smoked, in smokers | 1653 | 30.9 | 27.9 | 143 | 35.7 | 31.4 | 0.16 |
| CRAE (μm) | 3014 | 149.5 | 13.74 | 256 | 150.4 | 12.29 | 0.32 |
| CRVE (μm) | 3013 | 229.8 | 21.33 | 256 | 227.5 | 21.33 | 0.10 |
| Serum C-reactive protein (mg/L) | 3001 | 4.02 | 9.47 | 255 | 4.9 | 12.83 | 0.16 |
| Serum cystatin C (mg/L) | 2856 | 0.89 | 0.26 | 237 | 0.96 | 0.30 | 0.002 |
| Hematocrit (%) | 3017 | 43.0 | 3.80 | 256 | 43.4 | 4.11 | 0.08 |
| White blood cell count (k/μL) | 3016 | 7.22 | 1.97 | 256 | 7.6 | 2.75 | 0.01 |
| Serum isoprostane (pg/mL) ‡ | 1074 | 142.2 | 84.52 | 87 | 147.8 | 105.88 | 0.56 |
| Serum interleukin-6 (pg/mL) ‡ | 1076 | 3.8 | 15.87 | 87 | 4.3 | 8.55 | 0.76 |
| Sex (% male) | 3017 | 44.7 | 256 | 50.0 | 0.10 | ||
| History of CVD (% present) | 2983 | 11.6 | 255 | 15.3 | 0.08 | ||
| Chronic kidney disease (% present)‡ | 1093 | 14.5 | 88 | 17.1 | 0.50 | ||
| Hypertension (% present) | 3014 | 44.7 | 256 | 62.9 | <0.001 | ||
| Microaneurysm only (% present)*† | 256 | 69.0 | - | ||||
| Retinal hemorrhages only (% present)*† | 256 | 23.0 | - | ||||
| Retinopathy >=level 31 (% present)*† | 256 | 9.0 | - | ||||
Abbreviations: CRAE = central retinal arteriole equivalent; CRVE = central retinal venule equivalent; CVD = cardiovascular disease.
At risk for disappearance
Not mutually exclusive
Measured in subset of population only
The 15-year cumulative incidence of retinopathy, microaneurysms only, retinal hemorrhages only, and retinal microaneurysms and retinal hemorrhage or more severe retinopathy in nondiabetic persons was 14.2% (95% confidence interval [CI], 12.8 to 15.6%), 8.3% (95% CI 7.1 to 9.5%), 4.8% (95% CI 3.2 to 5.8%), and 1.2% (95% CI 0.8 to 1.6%), respectively. The hazard of developing retinopathy in the second eye when present in the other eye was 3.21 (95% CI 2.1 to 5.0) compared to developing it in the first eye.
Table 2 shows the relationship of 15-year cumulative incidence of retinopathy, microaneurysms only, retinal hemorrhages only, and more severe retinopathy by age and sex. The cumulative incidence of retinopathy and lesions characterizing it, except microaneurysms only, increased with age and was similar in men and women.
Table 2.
15-year cumulative indence of any retinopathy, microaneurysms only, blot hemorrhages only and more severe retinopathy with competing risk of death or diabetes by age and sex in nondiabetic persons in the Beaver Dam Eye Study, 1988-1990 to 2003-2005
| Any Retinopathy | Microaneurysms Only | Retinal Hemorrhage Only | More Severe Retinopathy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cumulative Incidence (%) | N*† | Cumulative Incidence (%) | N*† | Cumulative Incidence (%) | N*† | Cumulative Incidence (%) | ||
| Age group, yr | |||||||||
| 43-54 | 1103 | 13.9 | 1088 | 8.9 | 1061 | 4.0 | 1049 | 0.4 | |
| 55-64 | 842 | 14.7 | 823 | 9.1 | 811 | 4.9 | 801 | 1.5 | |
| 65-74 | 746 | 16.2 | 723 | 8.0 | 722 | 6.6 | 707 | 1.9 | |
| 75-84 | 326 | 10.0 | 316 | 4.5 | 313 | 3.6 | 309 | 2.5 | |
| Sex | |||||||||
| Female | 1670 | 15.3 | 1628 | 8.4 | 1616 | 5.6 | 1591 | 1.5 | |
| Male | 1347 | 12.9 | 1322 | 8.3 | 1291 | 3.8 | 1275 | 0.8 | |
| Age | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Per 5 years | 1.24 | 1.11–1.39 | 1.08 | 0.93–1.26 | 1.43 | 1.18–1.75 | 2.38 | 1.60–3.55 | |
| Sex (Male vs Female) | 0.97 | 0.78–1.20 | 1.18 | 0.89–1.56 | 0.76 | 0.52–1.13 | 0.57 | 0.25–1.31 | |
Abbreviations: HR = hazard ratio; CI = confidence interval.
Cumulative 15-year incidence was calculated accounting for the competing risk of death or diabetes where time to the event of interest, incidence of retinopathy or retinopathy lesion(s) or competing events, death or developing diabetes, are modeled.
Not mutually exclusive. N at risk varies for each lesion because of incidence of other lesions between Beaver Dam 1 and Beaver Dam 2.
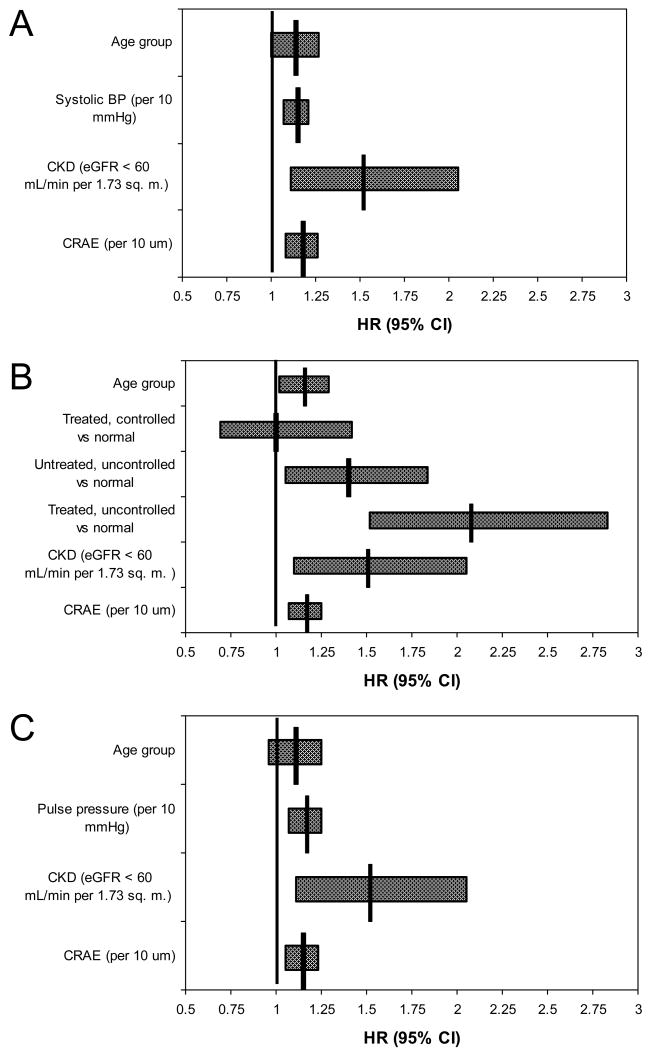
Table 3 shows the relationships, after age adjustment, of various characteristics at baseline to the hazard of developing retinopathy, microaneurysms only, retinal hemorrhages only, and more severe retinopathy over the 15-year period. Uncontrolled hypertension, higher systolic blood pressure, higher pulse pressure, presence of CKD, higher cystatin C, and greater CRAE were associated with the incidence of retinopathy. There were no associations of glycosylated hemoglobin, pack years smoked, markers of inflammation, endothelial dysfunction and oxidative stress, hematologic factors (Table 3), or history of use of lipid-lowering agents or of aspirin and other nonsteroidal anti-inflammatory agents (data not shown) with the incidence of retinopathy, retinal microaneurysms only, retinal hemorrhages only, or more severe retinopathy. In multivariable analyses (Figure 1) and in multivariable models with time-dependent covariates (data not shown), these associations with the incidence of retinopathy remained similar.
Table 3.
Age adjusted relationships of various characteristics at baseline to the 15-year cumulative incidence of any retinopathy, retinal microaneurysms only, retinal hemorrhages only and severe retinopathy in the Beaver Dam Eye Study*
| Characteristics | Any Retinopathy | Microaneurysms Only | Retinal Hemorrhages Only | Severe Retinopathy | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Hypertension | ||||||||
| Treated, controlled vs normal | 0.93 | 0.64–1.34 | 0.92 | 0.56–1.51 | 0.97 | 0.52–1.80 | 1.11 | 0.34–3.58 |
| Untreated, uncontrolled vs normal | 1.27 | 0.96–1.69 | 1.45 | 1.02–2.08† | 0.77 | 0.43–1.39 | 2.12 | 0.86–5.22 |
| Treated, uncontrolled vs normal | 1.92 | 1.40–2.64† | 1.75 | 1.13–2.71† | 2.47 | 1.50–4.07† | 1.00 | 0.27–3.73 |
| Systolic BP (per 10 mmHg) | 1.10 | 1.04–1.17† | 1.12 | 1.04–1.21† | 1.04 | 0.93–1.15 | 1.26 | 1.05–1.51† |
| Diastolic BP (per 10 mmHg) | 1.09 | 0.98–1.21 | 1.11 | 0.96–1.28 | 1.07 | 0.88–1.29 | 1.15 | 0.79–1.68 |
| Pulse pressure (per 10 mmHg) | 1.13 | 1.05–1.22† | 1.15 | 1.04–1.27† | 1.03 | 0.89–1.18 | 1.35 | 1.07–1.69† |
| Pack years smoked (in smokers, per 10 packyears) | 0.94 | 0.88–1.01 | 0.96 | 0.88–1.04 | 0.93 | 0.82–1.04 | 0.99 | 0.75–1.31 |
| Serum total cholesterol (per 10 mg/dL) | 1.00 | 0.98–1.03 | 1.01 | 0.98–1.05 | 0.99 | 0.95–1.04 | 1.01 | 0.92–1.10 |
| Serum HDL cholesterol (per 10 mg/dL) | 0.98 | 0.92–1.04 | 0.95 | 0.88–1.04 | 0.98 | 0.88–1.10 | 1.18 | 0.98–1.43 |
| Body mass index (per 5 kg/m2) | 1.10 | 0.99–1.22 | 1.06 | 0.92–1.21 | 1.24 | 1.05–1.47† | 0.99 | 0.66–1.46 |
| Chronic kidney disease (present vs absent) | 1.49 | 1.10–2.02† | 1.48 | 0.98–2.23† | 1.37 | 0.81–2.34 | 1.40 | 0.53–3.70 |
| White blood cell count (per k/μL)‡ | 1.02 | 0.91–1.14 | 1.00 | 0.86–1.15 | 1.01 | 0.83–1.22 | 0.96 | 0.65–1.42 |
| Hematocrit (per 1%) | 1.02 | 0.99–1.05 | 1.03 | 0.99–1.07 | 1.01 | 0.96–1.06 | 1.05 | 0.94–1.17 |
| Serum C-reactive protein (per mg/L)‡ | 0.99 | 0.89–1.11 | 0.94 | 0.81–1.09 | 0.99 | 0.81–1.21 | 1.52 | 1.04–2.21† |
| Serum isoprostane (per pg/mL)‡ | 1.02 | 0.86–1.19 | 0.94 | 0.75–1.18 | 1.08 | 0.83–1.41 | 0.92 | 0.49–1.71 |
| Serum cystatin C (per mg/L) ‡ | 1.22 | 1.06–1.40† | 1.16 | 0.96–1.39 | 1.27 | 0.99–1.62 | 1.61 | 1.02–2.53† |
| History of cardiovascular disease (present vs absent) | 0.70 | 0.45–1.08 | 0.69 | 0.38–1.26 | 0.79 | 0.39–1.60 | 0.53 | 0.12–2.28 |
| Sex (male) | 1.00 | 0.80–1.24 | 1.19 | 0.90–1.58 | 0.80 | 0.54–1.18 | 0.67 | 0.29–1.55 |
| CRAE (per 10 μm) | 1.11 | 1.02–1.20† | 1.13 | 1.02–1.26† | 1.03 | 0.90–1.19 | 1.16 | 0.88–1.55 |
| CRVE (per 10 μm) | 1.01 | 0.96–1.07 | 1.02 | 0.95–1.09 | 0.95 | 0.86–1.04 | 1.11 | 0.93–1.33 |
| Glycosylated hemoglobin (per %) | 0.97 | 0.81–1.16 | 0.93 | 0.73–1.17 | 0.92 | 0.67–1.26 | 1.04 | 0.55–1.96 |
Abbreviations: HR = hazard ratio; CI = confidence interval; BP = blood pressure; HDL = high density lipoprotein; CRAE = central retinal arteriole equivalent; CRVE = central retinal venule equivalent.
Severe retinopathy defined as a Diabetic Retinopathy Study severity Level 31 or greater.
P-value significant at 0.05 level
Variable entered per SD on the log scale
Figure 1.
Multivariate models for hazard of developing retinopathy in persons without diabetes. All models include age, chronic kidney disease (CKD), and central retinal arteriole equivalent (CRAE) and one of the following: A: Systolic BP. B: Hypertension (expanded by level of control). C: Pulse pressure. Hypertension was defined as SBP ≥140 mmHg or DBP ≥90 mmHg or use of antihypertensive medication.
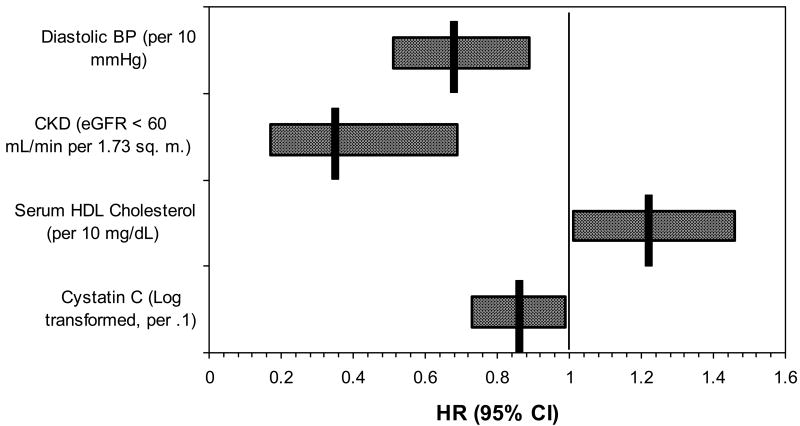
The 15-year cumulative disappearance of retinopathy, microaneurysms only, retinal hemorrhages only, and retinal microaneurysms and hemorrhage or more severe retinopathy in nondiabetic persons was 70.2% (95% CI 66.3 to 74.1%), 73.6% (95% CI 67.5 to 79.7), 75.9% (95% CI 68.1 to 84.7) and 46.9% (95% CI 28.9 to 64.9%), respectively. Table 4 shows the relationship of 15-year cumulative disappearance of retinopathy, microaneurysms only, retinal hemorrhages only, and more severe retinopathy with competing risks of diabetes and death by age and sex. The disappearance of retinopathy decreased with age. Uncontrolled hypertension, higher diastolic blood pressure, and the presence of CKD were associated with decreased disappearance of retinopathy (Figure 2). Higher serum HDL cholesterol was associated with increased disappearance of retinopathy (Figure 2).
Table 4.
15-year cumulative disappearance of retinopathy microaneurysms only, blot hemorrhages only and more severe retinopathy with competing risks of diabetes and death by age and sex in nondiabetic persons in the Beaver Dam Eye Study, 1988-1990 to 2003-2005.*
| Any Retinopathy | Microaneurysms Only | Retinal Hemorrhages Only | More Severe Retinopathy | |||||
|---|---|---|---|---|---|---|---|---|
| N | CD (%) | N*† | CD (%) | N*† | CD (%) | N*† | CD (%) | |
| Age group | ||||||||
| 43-54 | 57 | 90.9 | 49 | 93.5 | 6 | 83.3 | 1 | 0.0 |
| 55-64 | 75 | 70.7 | 54 | 73.4 | 13 | 76.9 | 5 | 40.0 |
| 65-74 | 85 | 67.8 | 47 | 66.8 | 23 | 91.3 | 7 | 57.1 |
| 75-84 | 39 | 41.0 | 19 | 31.6 | 16 | 50.0 | 3 | 0.0 |
| Sex | ||||||||
| Female | 128 | 77.6 | 82 | 86.9 | 34 | 76.5 | 8 | 25.0 |
| Male | 128 | 63.0 | 87 | 62.7 | 24 | 75.0 | 8 | 50.0 |
Abbreviations: CD = cumulative disappearance.
Cumulative 15-year disappearance was calculated accounting for the competing risk of death or diabetes where time to the event of interest, disappearance of retinopathy or lesion(s) or competing events, death or developing diabetes, are modeled.
Not mutually exclusive. N at risk varies for each lesion due to incidence of other lesions between Beaver Dam 1 and Beaver Dam 2.
Figure 2.
Multivariate models for hazard of disappearance of retinopathy in persons without diabetes. CKD = chronic kidney disease; CRAE = central retinal arteriole equivalent.
Controlling for age, there was no difference in incidence of visual impairment at a subsequent visit for individuals who had retinopathy at the previous visit compared to those who did not (p=0.77). Similarly, there was no significant decrease in mean number of letters read at a subsequent visit for individuals who had retinopathy at the previous visit compared to those who did not (p=0.45).
Discussion
There are few long-term epidemiological population-based data describing the appearance and disappearance of retinopathy and associated risk factors in persons without diabetes.21 Over a 15-year period, 14% of nondiabetic persons in the Beaver Dam Eye Study cohort developed retinopathy and it disappeared in 70%. While controlling for age, poorly controlled blood pressure, presence of CKD, and wider retinal arterioles at baseline were associated with the 15-year cumulative incidence and higher diastolic blood pressure, total serum cholesterol, and cystatin C and the presence of CKD were associated with the 15-year disappearance of retinopathy.
The finding of a 14% cumulative incidence of retinopathy in nondiabetic persons is lower than the 16% 10-year cumulative incidence of retinopathy in nondiabetic persons 49 years of age or older reported in the Blue Mountains Eye Study (BMES).21 In both studies, retinopathy incidence was detected by assessment of stereoscopic color fundus photographs using the same grading protocols. The higher incidence of retinopathy in the BMES cohort may be due to the larger area of the fundus that was photographed (6 vs. 3 standard fields) in that study compared to the BDES. Based on our findings, we estimate that retinopathy will develop in 3,469,334 white nondiabetic persons 43-86 years of age in the US population over a 15-year period. This has public health implications because nondiabetic persons with retinopathy are at higher risk of developing systemic disease, e.g., ischemic heart disease, congestive heart failure, stroke, cognitive decline, and CKD than people without retinopathy.10-20
In the Beaver Dam Eye Study, uncontrolled blood pressure was associated with an increased likelihood of developing retinopathy and a decreased likelihood of its disappearance. Our findings are consistent with data from other studies and were not unexpected because of the known effects of blood pressure on the retinal microvasculature.1-4,6,8,42 This may represent a potential public health burden based on previous observations of increased risk of cardiovascular morbidity and mortality in persons with uncontrolled hypertension and signs of retinopathy.1,2,4,17,45
In the Beaver Dam Eye Study, independent of hypertension status, CKD was associated with an increased risk of incident retinopathy and a decreased risk of its disappearance. This is not unexpected because renal disease is associated with hypertension, inflammation and endothelial dysfunction, all hypothesized as pathogenetic factors for the development of retinopathy.46-48 However, this relation remained when controlling for these factors in multivariate analyses, suggesting that other unmeasured pathogenetic mechanisms associated with kidney disease may be involved in the development of retinopathy in nondiabetic persons. It is also possible that hypertension and perhaps elevated levels of inflammatory factors are causally associated with CKD and that we are measuring the residual effects of those relationships.
The relationship of wider retinal arterioles with increased risk of incident retinopathy was unexpected. We had hypothesized that narrower retinal arteriole diameters would be associated with hypertension and related to retinopathy and that wider venular diameters would be associated with systemic inflammation and endothelial dysfunction and related to incident retinopathy.7,49,50 Increased CRAE could be related to increased retinal blood flow, which has been posited as a candidate mechanism for retinopathy pathogenesis through hemodynamic injury in people with diabetes.51-53
In the BDES, retinopathy was transient when it appeared, with disappearance in 70% of nondiabetic persons over the 15-year period, usually within the five-year interval between visits and with no effect on vision. This is unlike the presence of minimal retinopathy in persons with diabetes, which is likely to progress and less likely to disappear.54,55 This brings into question whether a single retinal microaneurysm or retinal hemorrhage is a valid diagnostic criterion for defining the presence of diabetes in the general population. These findings also raise the question of whether the presence of a single retinal microaneurysm or blot hemorrhage in the eye of a hypertensive person with type 2 diabetes mellitus should be considered diabetic retinopathy.
A number of hypothesized risk factors, e.g., smoking status and glycemic, hematologic and inflammatory factors were not found to be associated with incident retinopathy in our study. With the exception of smoking, these factors have been shown to be associated with incident retinopathy in persons with diabetes.56 The association of hypertension and the lack of an association of glycosylated hemoglobin in nondiabetic persons with incident retinopathy suggests that the pathogenesis of these lesions is more likely due to the effects of high blood pressure itself on the retinal microvasculature, and less likely due to hyperglycemia related pathways.
Any conclusions or explanations regarding associations described herein must be made with caution. For example, a possible reason for not finding a relation between smoking and the incidence of retinopathy is that persons who smoked and developed retinopathy may have died before their follow-up examination was performed. Second, the retinopathy might have resulted from conditions in nondiabetic persons that were not asked about or determined in the study, such as acquired immune deficiency syndrome or aplastic anemia.57 These conditions are rare in this population and would not be expected to account for the high incidence of the retinal lesions found in the nondiabetic group. Third, misclassification of hypertension status may have occurred, because the classification was based, in part, on two measurements of the blood pressure during a single examination. This type of misclassification would likely weaken the significant relationship found between hypertensive status and the presence of retinal lesions in nondiabetic persons.
In summary, the data from this study show a 14% 15-year cumulative incidence of retinopathy in nondiabetic persons. The findings also show that two modifiable risk factors, uncontrolled hypertension and CKD, are related to incident retinopathy in nondiabetic persons. While retinopathy in nondiabetic persons is usually transient and in itself does not affect visual function, its clinical significance is as a marker for increased risk of CVD morbidity and mortality and other systemic diseases. A report by Wong et al. illustrates this, showing a multiplicative effect of jointly having retinopathy and cerebral white matter lesions with the 5-year relative risk of incident stroke.11 The latter varied from 1.4% when neither were present to about 4% when either was present to nearly 20% (RR 20) when both were present in the general Atherosclerosis Risk In Communities study population 43-72 years of age. At present, the finding of retinopathy in nondiabetic hypertensive persons should be thought of as a possible indicator of increased risk of systemic conditions such as ischemic heart disease and stroke. While control of blood pressure has been shown to reduce the risk of cardiovascular disease, it is not known whether such control would have greater impact on hypertensive persons with retinopathy present than in those in whom it is absent.
Acknowledgments
The National Institutes of Health grant EY06594 (R Klein, BEK Klein) and, in part, the Research to Prevent Blindness (R and BEK Klein, Senior Scientific Investigator Awards), New York, NY, provided funding for entire study including collection and analyses and of data. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Footnotes
Dr. Ronald Klein has full access to the data in the study and takes full responsibility for the integrity and the accuracy of the data.
Financial Disclosures: None reported.
Author contributions: Conception and design (RK, BEKK), acquisition of data (RK, BK), analysis and interpretation of data (RK, BEKK, KEL, CEM), drafting of the manuscript (RK), critical revision of the manuscript for important intellectual content (BEKK, KEL), statistical expertise (KEL, CEM), obtaining funding (RK, BEKK), administrative/technical/material support (RK, BEKK,CEM).
References
- 1.McDonough JJ, Garrison GE, Hames CG. Blood pressure and hypertensive disease among negroes and whites; a study in Evans County, Georgia. Ann Intern Med. 1964:61208–228. doi: 10.7326/0003-4819-61-2-208. [DOI] [PubMed] [Google Scholar]
- 2.Svardsudd K, Wedel H, Aurell E, Tibblin G. Hypertensive eye ground changes. Prevalence, relation to blood pressure and prognostic importance. The study of men born in 1913. Acta Med Scand. 1978;204(3):159–167. [PubMed] [Google Scholar]
- 3.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980;24(Suppl):335–610. [PubMed] [Google Scholar]
- 4.Klein R. Retinopathy in a population-based study. Trans Am Ophthalmol Soc. 1992:90561–594. [PMC free article] [PubMed] [Google Scholar]
- 5.Stolk RP, Vingerling JR, de Jong PT, et al. Retinopathy, glucose, and insulin in an elderly population. The Rotterdam Study. Diabetes. 1995;44(1):11–15. doi: 10.2337/diab.44.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Yu T, Mitchell P, Berry G, Li W, Wang JJ. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116(1):83–89. doi: 10.1001/archopht.116.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20(6):1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Sharp PS, Chaturvedi N, Wormald R, McKeigue PM, Marmot MG, Young SM. Hypertensive retinopathy in Afro-Caribbeans and Europeans. Prevalence and risk factor relationships. Hypertension. 1995;25(6):1322–1325. doi: 10.1161/01.hyp.25.6.1322. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Wu SY, Hyman L, et al. Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology. 1999;106(10):1893–1899. doi: 10.1016/s0161-6420(99)90398-6. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 11.Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288(1):67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke. 2002;33(6):1487–1492. doi: 10.1161/01.str.0000016789.56668.43. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86(9):1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tekin O, Cukur S, Uraldi C, et al. Relationship between retinopathy and cognitive impairment among hypertensive subjects. A case-control study in the ankara-pursaklar region. Eur Neurol. 2004;52(3):156–161. doi: 10.1159/000081855. [DOI] [PubMed] [Google Scholar]
- 16.Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293(1):63–69. doi: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama T, Date C, Yokoyama T, Yoshiike N, Yamaguchi M, Tanaka H. A 15.5-year follow-up study of stroke in a Japanese provincial city. The Shibata Study. Stroke. 1997;28(1):45–52. doi: 10.1161/01.str.28.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Mohamed Q, Klein R, Couper DJ. Do retinopathy signs in non-diabetic individuals predict the subsequent risk of diabetes? Br J Ophthalmol. 2006;90(3):301–303. doi: 10.1136/bjo.2005.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006:10498–107. [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46(2):214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Rochtchina E, Kaushik S, Kifley A, Wong TY, Mitchell P, et al. Long-term incidence of isolated retinopathy lesions in older persons without diabetes: The Blue Mountains Eye Study. Abstract 1236 presented at: 2010 ARVO Annual Meeting Conference; Presented Monday, May 03, 2010; Fort Lauderdale, FL. 2010. Forthcoming. [Google Scholar]
- 22.Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134(12):1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103(8):1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period : The Beaver Dam Eye Study. Ophthalmology. 2001;108(10):1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Klein BE. The Beaver Dam Eye Study. Manual of Operations. US Department of Commerce; Springfield, VA: 1991. NTIS Accession No. PB91-149823. [Google Scholar]
- 28.Klein R, Klein BE. The Beaver Dam Eye Study II. Manual of Operations. US Department of Commerce; Springfield, VA: 1995. NTIS Accession No. PB95-273827. [Google Scholar]
- 29.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. US Department of Commerce; Springfield, VA: 1991. NTIS Accession No. PB91-184267. [DOI] [PubMed] [Google Scholar]
- 30.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 31.Stein M. D-glucose determination with hexokinase and glucose-6-phosphate dehydrogenase. 1963:117–123. [Google Scholar]
- 32.Klenk DC, Hermanson GT, Krohn RI, et al. Determination of glycosylated hemoglobin by affinity chromatography: comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin Chem. 1982;28(10):2088–2094. [PubMed] [Google Scholar]
- 33.Diabetic Retinopathy Study Research Group. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1, Part 2):210–226. [PubMed] [Google Scholar]
- 34.Klein BE, Davis MD, Segal P, et al. Diabetic retinopathy. Assessment of severity and progression. Ophthalmology. 1984;91(1):10–17. doi: 10.1016/s0161-6420(84)34374-3. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Klein BE, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93(9):1183–1187. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 37.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 39.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 40.SAS Institute Inc. SAS/STAT User's Guide, Version 8. Cary NC: SAS Institute Inc; 1999. [Google Scholar]
- 41.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 42.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369(9559):425–435. doi: 10.1016/S0140-6736(07)60198-6. Erratum in: Lancet. 2007 Jun 23;369(9579):2078. Wong, Tien [corrected to Wong,Tien Yin] [DOI] [PubMed] [Google Scholar]
- 43.Gudmundsdottir H, Taarnhoj NC, Strand AH, Kjeldsen SE, Hoieggen A, Os I. Blood pressure development and hypertensive retinopathy: 20-year follow-up of middle-aged normotensive and hypertensive men. J Hum Hypertens. 2009 doi: 10.1038/jhh.2009.94. [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team [computer program] R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 45.Keith NM, Wagener HP, Barker NW. Some different types of hypertension: their course and prognosis. Am J Med Sci. 1939;197:332–343. doi: 10.1097/00000441-197412000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Nanayakkara PW, Gaillard CA. Vascular disease and chronic renal failure: new insights. Neth J Med. 2010;68(1):5–14. [PubMed] [Google Scholar]
- 47.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86(4):363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olson JA, Whitelaw CM, McHardy KC, Pearson DW, Forrester JV. Soluble leucocyte adhesion molecules in diabetic retinopathy stimulate retinal capillary endothelial cell migration. Diabetologia. 1997;40(10):1166–1171. doi: 10.1007/s001250050802. [DOI] [PubMed] [Google Scholar]
- 49.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 50.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124(1):87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 51.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44(6):603–607. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- 52.Alibrahim E, Donaghue KC, Rogers S, et al. Retinal vascular caliber and risk of retinopathy in young patients with type 1 diabetes. Ophthalmology. 2006;113(9):1499–1503. doi: 10.1016/j.ophtha.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114(10):1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–243. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 55.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(2):244–249. doi: 10.1001/archopht.1989.01070010250031. [DOI] [PubMed] [Google Scholar]
- 56.Klein R. The epidemiology of diabetic retinopathy. In: Duh E, editor. Contemporary diabetes: diabetic retinopathy. Totowa, NJ: Humana Press; 2008. pp. 67–107. [Google Scholar]
- 57.Friedman AH. The retinal lesions of the acquired immune deficiency syndrome. Trans Am Ophthalmol Soc. 1984;82:447–491. [PMC free article] [PubMed] [Google Scholar]