Abstract
Background
Studies have linked migraine with aura to an increased risk of ischemic stroke, particularly among women. Data on the relationship of migraine and functional outcome from ischemic cerebral events are sparse.
Methods and Results
Prospective cohort study among 27,852 women enrolled in the Women’s Health Study for whom we had information on migraine and measured cholesterol values and who had no prior stroke or transient ischemic attack (TIA). Migraine was classified into no history of migraine, active migraine with aura, active migraine without aura, and past history of migraine. Possible functional outcomes were no stroke or TIA, TIA, and stroke with modified Rankin Scale (mRS) score 0–1, mRS 2–3, and mRS 4–6. We used multinomial logistic regression to evaluate the relationship of migraine with functional outcomes after ischemic stroke. During a mean of 13.5 years of follow-up, 398 TIAs and 345 ischemic strokes occurred. Compared with women without history of migraine and who did not experience a TIA or stroke, women who reported migraine with aura had adjusted relative risk (95% confidence interval) of 1.56 (1.03–2.36) for TIA, 2.33 (1.37–3.97) for stroke with mRS 0–1, 0.82 (0.30–2.24) for mRS 2–3, and 1.18 (0.28–4.97) for mRS 4–6. The risk of any outcome was not significantly elevated for women who experienced migraine without aura or who had a past history of migraine.
Conclusion
Results of this large prospective cohort suggest that women with migraine with aura are at increased risk of experiencing TIA or ischemic stroke with good functional outcome.
Keywords: Migraine, stroke, epidemiology, women
Migraine is a chronic-intermittent primary headache disorder that affects about 18% of the female population.1 Up to one third of migraineurs have transient neurologic symptoms that usually manifest as visual disturbances known as migraine aura.2 Migraine, in particular migraine with aura, has been shown to be a risk factor for ischemic stroke, a leading cause of morbidity and mortality in the US and worldwide. A recent meta-analysis found an increased risk of ischemic stroke among subjects with any migraine (relative risk, 1.73; 95% CI, 1.31 to 2.29).3 This risk was further increased for women (relative risk, 2.08; 95% CI, 1.13 to 3.84) and especially among those with migraine with aura (relative risk, 2.16; 95% CI, 1.53 to 3.03).
Population-based research has shown evidence of an increased risk of lacunar stroke among women who experience migraine with aura.4 Since lacunar strokes are usually less severe than other ischemic stroke subtypes,5 one can hypothesize that migraine with aura may be associated with milder levels of disability after stroke. However, little research has been done on the association between migraine with aura and functional outcome after stroke. Such an association would be of importance, particularly for women, who are three to four times more likely to suffer from migraine and who are at increased likelihood of being more disabled after stroke than men.6,7 Thus, we aimed to analyze the association between migraine and functional outcome from incident ischemic cerebral events in the Women’s Health Study (WHS), a large prospective cohort of female health professionals.
Methods
Study Population
The WHS was a randomized, placebo-controlled trial of the effects of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. The design of the WHS has been published in detail previously.8 Briefly, at baseline (1993–1996), 39,876 US female health professionals age 45 or older without a history of cardiovascular disease, cancer, or other major illnesses were randomized to receive active aspirin and placebo vitamin E, active vitamin E and placebo aspirin, both active agents or both placebos. The clinical trial of the WHS ended in March 20049, 10 and the women have been followed on an observational basis since the end of the trial. At baseline, participants were sent a questionnaire asking about personal characteristics, medical history (including migraine) and health habits. Participants were sent a questionnaire twice within the first year and yearly thereafter asking about study compliance and study outcomes (including stroke and transient ischemic attacks [TIAs]).
In the baseline questionnaire, women were asked whether they would be willing to provide a venous blood sample by mail. A total of 28,345 women (71.1%) provided samples, 27,939 of which could be assayed for total cholesterol, high-density-lipoprotein cholesterol (HDL-C) and directly obtained low-density-lipoprotein cholesterol (LDL-C). For this analysis, we used information collected until March 2, 2009.
Informed consent was obtained from all participants and the institutional review board at Brigham and Women’s Hospital approved the WHS.
Exposure Assessment
On the baseline questionnaire, women were asked, “Have you ever had migraine headaches?” and “In the past year, have you had migraine headaches?” Women who experienced migraine headaches within the past year were asked further about the frequency of the attacks and characteristics of their migraine, including the presence of aura or any other indication that a migraine is coming. From this information, we divided the women into four possible categories: (1) “no migraine history”, (2) “active migraine with aura,” which included those who reported migraine within the past year and who reported presence of aura or any indication that a migraine is coming, (3) “active migraine without aura,” and (4) “history of migraine,” which included those who reported ever having migraines but did not experience any migraines within the past year.
In a previous study, we have shown that among WHS participants who reported active migraine, 83.5% fulfilled all but one International Classification of Headache Disorders-I (ICHD-I) criteria for migraine (code 1.7, migrainous disorder) and 46.6% fulfilled all ICHD-I criteria for “migraine without aura” (code 1.1 migraine without aura).11 In addition, we have shown that in a subsample of the WHS, 87.7% of women with self-reported active migraine could be diagnosed as migraine without aura (71.5%) or probable migraine without aura (16.2%) according to ICHD-II criteria.12
Outcome Assessment
After a woman reported a stroke or a TIA in her yearly questionnaire, she was asked for permission to review her medical records. An Endpoints Committee of physicians (including a board certified vascular neurologist) reviewed the medical records and confirmed cases of stroke and TIA. A nonfatal stroke was defined as a focal neurologic deficit of sudden or rapid onset and vascular mechanism that lasted >24 hours. A TIA was defined as a focal neurologic deficit of sudden or rapid onset and vascular mechanism that resolved within 24 hours. To confirm cases of fatal stroke, all available sources, including death certificates and hospital records, were used to find evidence of a cerebrovascular mechanism. We classified the stroke according to major subtypes (ischemic, hemorrhagic, or unknown). Interobserver agreement for major stroke subtype classification was excellent (Cohen’s κ statistics=0.96).13 Additionally, all ischemic strokes were further classified into subtypes based on their mechanism (atherothrombotic, atheroembolic, cardiogenic embolism, lacunar, infarct unknown mechanism or cerebral infarct related to a procedure). For all confirmed strokes, the Endpoint Committee also assigned a mRS score based on the degree of impairment experienced by the participant at hospital discharge. The mRS is a seven-point scale (0 = no symptoms at all; 1 = no significant disability despite symptoms; 2 = slight disability; 3 = moderate disability; 4 = moderately severe disability; 5 = severe disability; 6 = death)14, 15 that has strong test-retest reliability, interrater reliability, and validity.16, 17 We categorized the mRS score into three levels (0 to 1, 2 to 3, 4 to 6) to avoid possible problems with model convergence due to sparse data.18,19 When examining the association between migraine status and incident ischemic cerebral events, our possible functional outcomes were “no stroke or TIA”, TIA, and ischemic stroke with the three possible categories of the mRS.
Analysis
Of the 27,939 women from who plasma cholesterol, HDL-C and LDL-C could be assessed, we excluded 79 with missing information on migraine status and 8 who reported having had a TIA prior to receiving the baseline questionnaire leaving 27,852 women for this analysis. Because of differences in the age distribution according to migraine status, we calculated age standardized distributions and proportions of baseline characteristics.
We used Cox proportional hazards models to determine the relative risk of TIA and ischemic stroke, using women without a history of migraine as the reference group. To test the assumption of proportional hazards, we included an interaction term between the log transformation of time in the study and migraine status. No significant violation was found.
We used multinomial logistic regression to calculate odds ratios and 95% confidence intervals as a measure of the relative risk of the relationship between migraine status and functional outcome from incident ischemic cerebral events. Multinomial logistic regression is an extension of binary logistic regression where the dependent variable is allowed to have more than two categories and each category is simultaneously compared to two reference categories, one for the exposure and one for the outcome. Our reference categories were women with no migraine history (the reference category for the exposure) and who had not experienced a stroke or TIA (the reference category for the outcome). Women who experienced a hemorrhagic stroke (N=82) or a stroke of unknown type (N=2) were excluded from this analysis.
For our assessment of risk of TIA and ischemic stroke and of functional outcome from ischemic stroke models, we ran age- and multivariable-adjusted models. In our multivariable-adjusted models, we adjusted for the following possible confounders as obtained at baseline: age (continuous), history of hypertension ≥140/90 (yes/no), baseline treatment with medication for high blood pressure (yes/no), midpoint of systolic blood pressure category (categories were defined by 10 mmHg increments), history of high cholesterol ≥240 (yes/no), baseline treatment with cholesterol lowering medication (yes/no), alcohol consumption (rarely/never, 1 to 3 drinks/month, 1 to 6 drinks/week, ≥ 1 drink/day), postmenopausal status (premenopausal, postmenopausal, uncertain), ever used oral contraceptives ≥2 months (yes/no), smoking status (never, past, smoke < 15 cig/day, smoke ≥ 15 cig/day), ever used postmenopausal hormones of any type (never, past, current), body mass index (continuous), exercise (rarely/never, <1 time/week, 1 to 3 times/week, ≥ 4 times/week), and total cholesterol levels in quartiles. In addition, we adjusted for randomized aspirin and vitamin E assignment. We decided not to adjust for HDL-C and LDL-C levels because the results were very similar to the results when we only adjusted for total cholesterol.
In secondary analysis, we tested for effect modification of the association between migraine and functional outcome from ischemic cerebral events. To avoid problems with model convergence, we decided to further collapse our functional outcomes from ischemic stroke into three categories: no stroke or TIA, TIA or stroke with mRS 0 to 1, and stroke with mRS 2 to 6. An interaction term between migraine status and the possible effect modifiers was included in separate age-adjusted models. The possible effect modifiers were age (< 55 years or ≥ 55 years), history of hypertension (yes/no), smoking status (never, past, current), randomized assignment to aspirin (yes/no), total serum cholesterol (quartiles), and Framingham risk score20 (≤12 points/>12 points).21,22
For all models, if a woman had missing information on body mass index (N=344) or midpoint of systolic blood pressure category (N=291), she was assigned to the median value of that variable. Less than 100 women were missing information on all other covariates. They were assigned to the reference category (ie, lowest exposure category) or to the past user category (smoking) or unclear exposure category (postmenopausal hormones).
To explore whether our method of handling missing data had an impact on our results, we performed sensitivity analyses where we deleted all women with missing baseline covariate information. Results of the two methods of handling missing information were similar in age- and multivariable-adjusted models (data not shown).
All statistical analyses were performed using SAS 9.1. All probability values are two-tailed and P <0.05 was considered statistically significant.
Results
Of the 27,852 women, 5,129 (18.4%) reported any history of migraine and 3,612 had active migraine of whom 1,435 (39.7%) reported migraine with aura. The age-adjusted baseline characteristics of the participants according to their migraine status are summarized in Table 1. Women who experience migraine were younger than those with no history or a past history of migraine and were more likely to be never smokers. Those with a past history of migraine were more likely to have a history of hypertension. Women with no migraine history were the least likely to have a history of high cholesterol.
Table 1.
Age-adjusted baseline characteristics of women according to migraine status in the Women’s Health Study (n=27,852).
| Migraine |
||||
|---|---|---|---|---|
| Characteristic | No migraine history, n = 22,723 |
Migraine with aura, n = 1,435 |
Migraine without aura, n = 2,177 |
History of migraine, n = 1,517 |
| Mean (SE) age, y | 54.9 (0.05) | 53.2 (0.19) | 52.6 (0.15) | 55.5 (0.18) |
| Body mass index (SE), kg/m2 | 25.9 (0.03) | 25.8 (0.13) | 26.2 (0.11) | 26.1 (0.13) |
| History of hypertension, % | 24.6 | 25.5 | 26.1 | 30.4 |
| Antihypertensive medication use, % |
13.0 | 13.9 | 14.2 | 17.2 |
| History of high cholesterol, % | 28.9 | 33.3 | 33.6 | 33.2 |
| Cholesterol lowering medication use, % |
3.1 | 3.5 | 3.7 | 3.1 |
| Postmenopausal, % | 54.4 | 53.3 | 54.3 | 55.1 |
| Oral contraceptive use, % | 69.1 | 72.3 | 71.3 | 71.8 |
| Postmenopausal hormone use, % |
||||
| Never | 47.4 | 37.2 | 42.8 | 43.6 |
| Past | 9.9 | 11.4 | 9.7 | 11.8 |
| Current | 42.7 | 51.4 | 47.4 | 44.6 |
| Alcohol consumption, % | ||||
| Rarely/never | 43.5 | 48.3 | 47.3 | 45.2 |
| 1–3 drinks/month | 13.1 | 13.1 | 15.4 | 14.2 |
| 1–6 drinks/week | 32.7 | 30.3 | 30.1 | 30.3 |
| ≥1 drinks per day | 10.8 | 8.3 | 7.1 | 10.3 |
| Randomized aspirin assignment, % |
50.2 | 49.1 | 50.3 | 47.8 |
| Randomized vitamin E assignment, % |
50.1 | 51.5 | 47.8 | 50.0 |
| Vigorous physical activity, % | ||||
| Rarely/never | 37.0 | 38.2 | 39.5 | 39.2 |
| <1/week | 19.2 | 20.5 | 21.6 | 20.1 |
| 1–3 times/week | 32.3 | 30.7 | 29.1 | 29.4 |
| ≥4 times/week | 11.6 | 10.7 | 9.6 | 11.3 |
| Smoking status, % | ||||
| Never | 51.3 | 52.6 | 56.2 | 50.7 |
| Past | 37.0 | 37.4 | 34.5 | 35.3 |
| Current <15 cig/day | 4.4 | 3.9 | 3.5 | 5.2 |
| Current ≥15 cig/day | 7.3 | 6.2 | 5.7 | 8.9 |
| Diabetes, % | 2.5 | 1.8 | 1.6 | 2.7 |
| Mean total cholesterol (SE), mg/dL |
211.4 (0.27) | 213.4 (1.08) | 212.7 (0.88) | 214.8 (1.05) |
Numbers may not add up to 100% because of rounding or missing data.
During a mean of 13.5 years (377,329 person-years) of follow-up, 398 TIAs and 345 ischemic strokes occurred. Women who reported migraine with aura had a statistically significant increased risk of TIA (relative risk, 1.55; 95% CI, 1.03 to 2.34; P=0.04) and ischemic stroke (relative risk, 1.63; 95% CI, 1.05 to 2.52; P=0.03) compared with those women without a history of migraine in multivariable-adjusted analysis. Women who experienced migraine without aura or who had a past history of migraine did not have a significant increase or decrease in their risk of TIA or ischemic stroke.
The association between migraine status and functional outcome from incident ischemic cerebral events is summarized in Table 2 and Figure 1. Compared with women who had no history of migraine and who did not experience a stroke or TIA, women with migraine with aura had multivariable-adjusted relative risk (95% CI) of 1.56 (1.03 to 2.36) for TIA, 2.33 (1.37 to 3.97) for functional outcome from stroke with mRS 0 to 1, 0.82 (0.30 to 2.24) for mRS 2 to 3, and 1.18 (0.28 to 4.97) for mRS 4 to 6. No significant increase or decrease in the risk of any of our outcomes was observed among women who experienced migraine without aura or who had a past history of migraine compared to those without a history of migraine who did not experience an ischemic stroke or TIA. Results only adjusting for age were very similar to our multivariable-adjusted results (Table 2).
Table 2.
Age- and multivariable-adjusted* relative risks of functional outcomes after ischemic stroke according to migraine status in the Women’s Health Study (N=27,768).
| No TIA or Stroke (n=27,025) |
TIA (n=398) |
mRS 0–1 (n=171) |
mRS 2–3 (n=126) |
mRS 4–6 (n=48) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | RR | RR | RR | |||||||||||
| n | % | n | % | (95% CI) | n | % | (95% CI) | n | % | (95% CI) | n | % | (95% CI) | |
| Age-adjusted | ||||||||||||||
| No history | 22049 | 81.6 | 324 | 81.4 | 1.00 | 135 | 79.0 | 1.00 | 106 | 84.1 | 1.00 | 40 | 83.3 | 1.00 |
| MA | 1379 | 5.1 | 25 | 6.3 | 1.56 (1.03, 2.36) |
16 | 9.4 | 2.41 (1.42, 4.07) |
4 | 3.2 | 0.77 (0.28, 2.09) |
2 | 4.2 | 1.15 (0.28, 4.79) |
| MO | 2137 | 7.9 | 17 | 4.3 | 0.73 (0.45, 1.20) |
11 | 6.4 | 1.15 (0.62, 2.14) |
8 | 6.4 | 1.07 (0.52, 2.20) |
1 | 2.1 | 0.42 (0.06, 3.10) |
| Past history | 1460 | 5.4 | 32 | 8.0 | 1.42 (0.98, 2.05) |
9 | 5.3 | 0.96 (0.48, 1.88) |
8 | 6.4 | 1.08 (0.53, 2.23) |
5 | 10.4 | 1.76 (0.69, 4.48) |
|
Multivariable -adjusted* |
||||||||||||||
| No history | 22049 | 81.6 | 324 | 81.4 | 1.00 | 135 | 79.0 | 1.00 | 106 | 84.1 | 1.00 | 40 | 83.3 | 1.00 |
| MA | 1379 | 5.1 | 25 | 6.3 | 1.56 (1.03, 2.36) |
16 | 9.4 | 2.33 (1.37, 3.97) |
4 | 3.2 | 0.82 (0.30, 2.24) |
2 | 4.2 | 1.18 (0.28, 4.97) |
| MO | 2137 | 7.9 | 17 | 4.3 | 0.73 (0.44, 1.19) |
11 | 6.4 | 1.11 (0.60, 2.08) |
8 | 6.4 | 1.12 (0.54, 2.31) |
1 | 2.1 | 0.42 (0.06, 3.09) |
| Past history | 1460 | 5.4 | 32 | 8.0 | 1.34 (0.92, 1.94) |
9 | 5.3 | 0.88 (0.45, 1.75) |
8 | 6.4 | 1.02 (0.49, 2.11) |
5 | 10.4 | 1.57 (0.61, 4.03) |
TIA = transient ischemic attack; mRS = modified Rankin scale; RR = relative risk; CI = confidence interval; MA = migraine with aura, MO = migraine without aura.
Relative risks are odds ratios calculated from a multinominal logistic regression model using women without history of migraine and without TIA or ischemic stroke as reference group.
Adjusted for age, history of hypertension ≥140/90, baseline treatment with medication for high blood pressure, midpoint of systolic blood pressure category, history of high cholesterol ≥240, baseline treatment with cholesterol lowering medication, randomized treatment assignment to aspirin and vitamin E, alcohol consumption, postmenopausal status, ever used oral contraceptives ≥2 months, smoking status, ever used postmenopausal hormones of any type, body mass index, exercise, and total cholesterol in quartiles.
Figure 1.
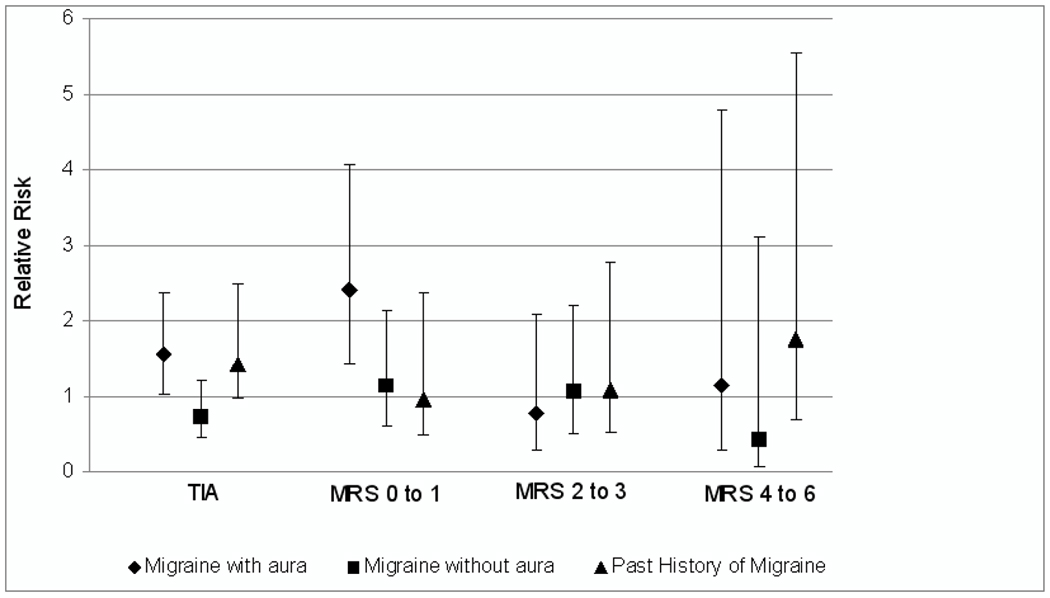
Multivariable-adjusted relative risks of functional outcomes after cerebral ischemic events according to migraine status in the Women’s Health Study (N=27,768). TIA indicates transient ischemic attack; mRS indicates modified Rankin scale
We found no significant evidence of effect modification by age (Pinteraction=0.46), history of hypertension (Pinteraction=0.45), smoking status (Pinteraction=0.54), randomized aspirin assignment (Pinteraction=0.69), total serum cholesterol (Pinteraction=0.87), or Framingham risk score (Pinteraction=0.99) on the association between migraine status and functional outcome from stroke.
Table 3 presents the number of each of the ischemic stroke subtypes and the proportions of ischemic stroke subtypes with “good” outcome (mRS 0–1) by migraine status. Due to low case counts, it was not possible to use multinomial logistic regression to evaluate the association between migraine status and functional outcome for each of the ischemic stroke subtypes separately. For many of the ischemic stroke cases in our cohort, a mechanism could not be determined. None of the strokes among migraineurs were due to atherothrombotic or atheroembolic mechanism. A high proportion of “good outcomes” was observed for ischemic strokes due to cardiogenic embolism, lacunar infarcts or infarcts of unknown mechanism among migraineurs with aura.
Table 3.
Numbers of each of the ischemic stroke subtypes and the percentage of ischemic stroke subtypes with “good” outcome (mRS 0–1) by migraine status.
| No history of migraine (%) |
Migraine with aura (%) |
Migraine without aura (%) |
Past history of migraine (%) |
|
|---|---|---|---|---|
| Atherothrombotic | 10 (30.0) | 0 | 0 | 0 |
| Atheroembolic | 7 (29.0) | 0 | 0 | 0 |
| Cardiogenic embolism | 51 (37.0) | 5 (80.0) | 2 (100.0) | 3 (33.0) |
| Lacunar | 46 (59.0) | 2 (100.0) | 3 (67.0) | 6 (66.0) |
| Infarct unknown mechanism | 157 (51.0) | 11 (73.0) | 13 (54.0) | 11 (36.0) |
| Cerebral infarct/procedure | 10 (40.0) | 4 (50.0) | 2 (0.0) | 2 (0.0) |
Discussion
Data from this large, prospective cohort study of women aged 45 or older at baseline show that women who report migraine with aura are at increased risk of TIA or ischemic stroke with good functional outcome (mRS 0 to 1), but do not have an increased risk of a more unfavorable functional outcome after ischemic stroke.
While, to the best of our knowledge, no other studies have directly reported on the association between migraine and functional outcomes from symptomatic ischemic cerebral events, two studies reported association between migraine and subclinical infarct-like brain lesions, as detected by magnetic resonance imaging. The first is a longitudinal study from Iceland showing that migraine with aura is associated with an increased risk of subclinical life infarct-like lesions later in life among women.23 The second is a population-based cross-sectional study from the Netherlands and found an increase in posterior circulation infarct-like lesions among migraineurs.24 The authors hypothesize that these lesions are most likely ischemic in origin and may be caused by hypoperfusion and/or embolism and not by atherosclerosis or small vessel disease based on the characteristics of the lesions.
In a recent publication from the Reykjavik Study, migraine was associated with increased risk of mortality from total stroke, a finding only apparent among male migraineurs with aura.25 Data from the WHS suggest that migraine with aura may increase the risk of fatal hemorrhagic stroke26 but not fatal ischemic stroke, as suggested from the present analysis. However, the number of fatal strokes in these two studies was small and future studies are needed to evaluate whether migraine with aura differentially affects the functional outcome of the two major stroke subtypes.
Current theories postulate that local vascular phenomena may underlie the association between migraine and ischemic stroke. For example, a previous study from the WHS showed that the association between migraine with aura and risk of stroke was strongest among women who had a rather healthy vascular health status as estimated by the Framingham risk score.22 This may support the idea that the biological mechanisms underlying the association between migraine with aura and stroke are different from atherosclerosis but may instead involve microvascular phenomena.22 Potential scenarios include local vascular processes leading to thrombosis or microemboli obliterating small vessels. There is further evidence of a complex interrelationship between migraine and the vascular system from an animal study demonstrating that cortical spreading depression, the likely physiological correlate of migraine aura, can be triggered by microemboli-induced hypoperfusion.27 Reduced cerebral blood flow activates local inflammatory mechanisms, which may cause cortical spreading depression and ultimately increase the risk of ischemic stroke.28
The results of the present study further support the idea that the association between migraine with aura and ischemic stroke may be due to microvascular phenomena instead of other mechanisms. First, no ischemic stroke with an atherothrombolic or atheroembolic mechanism occurred among women with migraine with aura, suggesting that large vessel disease does not play an important role in the association between migraine with aura and stroke. Additionally, we observed a low frequency of cardioembolic induced strokes, a group in which patent foramen ovale rather than atrial fibrillation would be likely to play a role in our relatively young cohort. Finally, we observed a high proportion (about 56%) of cases labeled as “infarct of unknown mechanism” which corresponds to the “undetermined” ischemic stroke type of the TOAST classification. “Infarcts of unknown mechanism” are non-lacunar type of infarcts that have no detectable cardiac/aortic source of embolism or evidence of “large vessel” disease as the stroke mechanism.
Although our data support the idea that the association between migraine with aura and ischemic cerebrovascular events is not due to large vessel disease or a detectable cardiac/aortic source of embolism, the precise vascular mechanism underlying the association between migraine with aura and stroke remains unclear. Additionally, it is plausible that the reason for the observed low level of disability from an ischemic cerebral event observed among migraineurs with aura may be a function of the small size of the infarcts rather than of their specific underlying mechanism.
To assess functional outcome from stroke, we used the mRS. Due to its strong emphasis on mobility, the mRS is primarily a measure of disability or “global health index”29, 30 even though it claims to be a measure of handicap. While there are limitations to the mRS,16, 17 it is widely accepted for functional stroke outcome assessment in clinical trials31–34 and has several advantages in population-based studies. First, the mRS can be ascertained from medical records and takes pre-stroke disability into account when determining the post-stroke mRS score.15 Second, it is simple to administer and has strong test-retest reliability, interrater reliability and validity.16 Finally, unlike the Barthel Index, the mRS does not have a “ceiling effect”.35
Strengths to our study include its prospective nature, the large number of participants and outcome events, the confirmation of reported TIAs and strokes by medical record review, and high interobserver agreement on the classification of major stroke subtype.
Some limitations to our study should be noted. First, migraine status was self-reported. While our cohort enrolled only female health professionals who likely report health information more accurately than the general population36 and validation studies have shown good agreement of our migraine classification with International Headache Society criteria,11, 12, 28 some degree of misclassification may still be possible. However, such misclassification would likely be non-differential and is thus an unlikely explanation of the observed associations. In addition, misclassification of migraine status could also occur if women who experience migraines only mention headache but no migraine features. However, as reports of non-migraine headache was not associated with increased risk of ischemic stroke in the WHS,37 we believe that such potential misclassification is an unlikely reason for our findings. Second, ascertainment of the mRS was not done in a pre-specified time frame after stroke and we had no information on the length of hospital stay. However, as patients with the most severe non-fatal stroke have likely the longest hospital stay, mRS score and hospital stay are expected to be correlated. Third, we did not have information on migraine-specific drug use, such as the use of triptans and ergot alkaloids, during follow-up. However, available evidence does not support the notion that migraine-specific drug use would confound the relationship between migraine status and risk of stroke or functional outcomes from stroke. Previous studies have shown associations only between migraine with aura and stroke risk and migraine-specific drugs are used by all migraineurs not just those patients who have migraine with aura. Moreover, other studies have not found a relationship between migraine-specific drug use and risk of stroke.38–41 Thus, it seems unlikely that migraine-specific drug use confounds the relationship between migraine status and stroke risk. Lastly, since all WHS participants are primarily white females 45 years of age or older, the generalizability of our results to other populations with different age, sex and racial distributions may be limited.
In conclusion, data of this large, prospective study of initially apparently healthy women suggest that the association between migraine with aura and ischemic cerebrovascular events generally favors events with good functional outcomes. Future studies are warranted to further elucidate the biological mechanisms underlying this association and to determine if treatment of migraine may help to reduce the risk of stroke.
Acknowledgments
Funding Sources: The Women’s Health Study is supported by grants from the National Heart, Lung, and Blood Institute (HL-043851, HL-080467, and HL-099355) and the National Cancer Institute (CA-047988). The research for this work was supported by grants from the Donald W. Reynolds Foundation, the Leducq Foundation, and the Doris Duke Charitable Foundation. The sponsors of the study played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Journal Subject Codes: [8] Epidemiology; [44] Acute Cerebral Infarction
Disclosures: P. Rist has nothing to disclose.
Dr. Buring has received investigator-initiated research funding and support from the US National Institutes of Health and Dow Corning Corporation; research support for pills and/or packaging from Bayer Heath Care and the Natural Source Vitamin E Association.
Dr. Kase serves on scientific advisory boards for Sanofi-Aventis, Lundbeck A/S, and Ferrer International, and receives research support from the US National Institutes of Health.
Dr. Schürks has received an investigator-initiated research grant from the Migraine Research Foundation and honoraria from L.E.K. Consulting for telephone surveys.
Dr. Kurth has received investigator-initiated research funding from the French National Research Agency, the US National Institutes of Health, Merck, the Parkinson’s Disease Foundation, and the Migraine Research Foundation. He is a consultant to i3 Drug Safety and World Health Information Science Consultants, LLC, and has received honoraria from Genzyme, Merck, and Pfizer for educational lectures.
References
- 1.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321–334. doi: 10.1016/j.ncl.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 3.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systemic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacClellan LR, Giles W, Cole J, Wozniak M, Stern B, Mitchell BD, Kittner SJ. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438–2445. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- 5.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 7.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 10.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 12.Schurks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women's Health Study. Cephalalgia. 2009;29:1086–1090. doi: 10.1111/j.1468-2982.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atiya M, Kurth T, Berger K, Buring JE, Kase CS. Interobserver agreement in the classification of stroke in the Women's Health Study. Stroke. 2003;34:565–567. doi: 10.1161/01.str.0000054159.21017.7c. [DOI] [PubMed] [Google Scholar]
- 14.Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke. 2005;36:1984–1987. doi: 10.1161/01.STR.0000177872.87960.61. [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 16.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 17.New PW, Buchbinder R. Critical appraisal and review of the Rankin scale and its derivatives. Neuroepidemiology. 2006;26:4–15. doi: 10.1159/000089536. [DOI] [PubMed] [Google Scholar]
- 18.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 19.Rist PM, Berger K, Buring JE, Kase CS, Gaziano JM, Kurth T. Alcohol Consumption and Functional Outcome After Stroke in Men. Stroke. 2010;41:141–146. doi: 10.1161/STROKEAHA.109.562173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 22.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. doi: 10.1136/bmj.a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–2570. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruit M, van Buchem M, Launer L, Terwindt G, Ferrari M. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia. 2010;30:129–136. doi: 10.1111/j.1468-2982.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudmundsson LS, Scher AI, Aspelund T, Eliasson JH, Johannsson M, Thorgeirsson G, Launer L, Gudnason V. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ. 2010;341:c3966. doi: 10.1136/bmj.c3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurth T, Kase CS, Schurks M, Tzourio C, Buring JE. Migraine and risk of haemorrhagic stroke in women: prospective cohort study. BMJ. 2010;341:c3659. doi: 10.1136/bmj.c3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, Wang Y, Frosch MP, Waeber C, Ayata C, Moskowitz MA. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. 2010;67:221–229. doi: 10.1002/ana.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864–1871. doi: 10.1212/WNL.0b013e3181a71220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Haan R, Limburg M, Bossuyt P, van der Meulen J, Aaronson N. The clinical meaning of Rankin 'handicap' grades after stroke. Stroke. 1995;26:2027–2030. doi: 10.1161/01.str.26.11.2027. [DOI] [PubMed] [Google Scholar]
- 30.Wade DT. Measurement in Neurological Rehabilitation. New York: Oxford University Press; 1992. [PubMed] [Google Scholar]
- 31.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 33.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH ECASS Study Group. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 34.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 35.Weimar C, Kurth T, Kraywinkel K, Wagner, Busse O, Haberl RL, Diener HC. Assessment of functioning and disability after ischemic stroke. Stroke. 2002;33:2053–2059. doi: 10.1161/01.str.0000022808.21776.bf. [DOI] [PubMed] [Google Scholar]
- 36.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 37.Kurth T, Slomke MA, Kase CS, Cook NR, Lee IM, Gaziano JM, Diener HC, Buring JE. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64:1020–1026. doi: 10.1212/01.WNL.0000154528.21485.3A. [DOI] [PubMed] [Google Scholar]
- 38.Dodick DW, Martin VT, Smith T, Silberstein S. Cardiovascular tolerability and safety of triptans: a review of clinical data. Headache. 2004;44 Suppl 1:S20–S30. doi: 10.1111/j.1526-4610.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- 39.Hall GC, Brown MM, Mo J, MacRae KD. Triptans in migraine: the risks of stroke, cardiovascular disease, and death in practice. Neurology. 2004;62:563–568. doi: 10.1212/01.wnl.0000110312.36809.7f. [DOI] [PubMed] [Google Scholar]
- 40.Martin VT, Goldstein JA. Evaluating the safety and tolerability profile of acute treatments for migraine. Am J Med. 2005;118 Suppl 1:36S–44S. doi: 10.1016/j.amjmed.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Velentgas P, Cole JA, Mo J, Sikes CR, Walker AM. Severe vascular events in migraine patients. Headache. 2004;44:642–651. doi: 10.1111/j.1526-4610.2004.04122.x. [DOI] [PubMed] [Google Scholar]


