Abstract
The G-protein coupled receptor (GPCR), Neurokinin-1 Receptor (NK1R), and its preferred ligand, substance P (SP), are reviewed in relationship to the immune system and selected infections. NK1R and substance P are ubiquitous throughout the animal kingdom. This important pathway has unique functions in numerous cells and tissues. The interaction of SP with its preferred receptor, NK1R, leads to the activation of nuclear factor-kappa-b (NF-κb) and proinflammatory cytokines. NK1R has two isoforms, both a full-length and a truncated form. These isoforms have different functional significances and differ in cell signaling capability. The proinflammatory signals modulated by substance P are important in bacterial, viral, fungal, and parasitic diseases, as well as in immune system function. The SP-NK1R system is a major Class 1, rhodopsin-like GPCR ligand-receptor interaction.
Keywords: substance P, Neurokinin-1 receptor, immunology
Overview
This review addresses several major aspects of the G-protein coupled receptor (GPCR), neurokinin-1 receptor (NK1R), and its preferred ligand, substance P (SP). Special emphasis will be placed on the relationship of neurokinin-1 receptor (NK1R) and substance P in clinical disease states, namely, these relationships to inflammation, infection, and immune disorders. Select infectious diseases that have altered neurokinin-1 receptor and/or substance P will be considered briefly as examples of the importance of this pathway in disease immunopathogenesis.1 Bremer and Leeman have published a recent overview of the biochemistry and pharmacology of substance P and neurokinin-1 in the Encyclopedia of Life Sciences.2 The interaction of the neuropeptides with their receptors are central in inflammation. In this review, a number of interactions will be highlighted, in particular, the role these interactions in the exacerbation and maintenance of acute and chronic inflammatory responses. Wherever possible, the role of neurokinin-1 and substance P in disease pathogenesis will be reviewed. In order to understand the role of substance P as a proinflammatory modulator of the immune response that acts in either an autocrine or paracrine fashion, the biology of substance P and its receptor, NK1R, will be reviewed.
Tachykinins
Substance P, an undecapeptide tachykinin, is encoded by the TAC1 gene. Transcription of TAC1 produces pre-pro tachykinin-A (PPTA), which is converted into one of four PPTA mRNA-splice variants. Four mRNA variants code for a pro-tachykinin polypeptide, which contains substance P with β and γ transcripts, and also encode neurokinin-A.3 Substance P is secreted by cells and then either binds to its preferred receptor, neurokinin-1, or is degraded by metalloproteases. The organization of the tachykinin receptor genes supports the possibility that splice variants of the tachykinins are generated.4
The mammalian tachykinins are a family of related peptides with important biologic functions.5 The tachykinin peptides include substance P (SP), neurokinin A and neurokinin B (NK-A, NK-B; both decapeptides), neuropeptide K (NPK), and neuropeptide Y (NPY). The primary structure of SP, neurokinin A (NK-A) and neurokinin B (NK-B) are very similar in all mammalian species.6 The nomenclature for tachykinins and their genes has been modified: preprotachykinin-1 (PPT-A) (TAC1; encodes SP and NKA), PPT-B (TAC3; encodes NKB), and PPT-C (TAC4; encodes hemokinin-1 (HK-1) and its shorter derivative hemokinin(4-11), as well as four related peptides, the endokinins).7, 8 Hemokinin-1 is related to B-lymphocyte hematopoiesis, and is expressed in murine dendritic cells.9, 10 Hemokinin-1 has substance P-like function in murine models of inflammation.11
Substance P
Substance P (SP) was initially detected in a crude alcoholic extract of equine intestine and brain. Von Euler and Gaddum found that the compound had a potent stimulant action in rabbit jejunum and produced hypotension.12 Subsequently, substance P was termed a “tachykinin” because it produced a rapid contractile response in smooth muscle. The first isolation of SP was achieved by Chang and Leeman in 1970, who isolated a sialagogic peptide from extracts of bovine central nervous system tissue that they characterized as substance P.13 In 1971, Chang, Leeman, and Niall reported the amino acid sequence of isolated substance P.14 SP is an undecapeptide with the amino acid sequence Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2.
Substance P has been found in multiple cell systems which also bear neurokinin-1 receptors. These receptors have been detected histochemically using an array of neurokinin-1 antibodies, as well as detection at the messenger RNA level. In particular, the cells which express substance P include human immune cells, monocytes, macrophages, lymphocytes, microglia, dendritic cells, bone marrow stem cells, and others. In the central nervous system, substance P receptors are expressed on neurons, astrocytes, microglia, and cerebral endothelial cells. We have recently performed an extensive study of neurokinin-1 and substance P in the Rhesus macaque, and in SIV-infected Rhesus macaques.15 Following SIV infection, there is enhancement of microglial substance P expression.6, 15
Substance P tissue localization
Substance P is present in the central and peripheral nervous system, as well as in the immune system.16-18 There are several important physiologic and immunologic consequences of the release of substance P.19, 20 These diverse biologic effects include: immune stimulation;20 secretion stimulation (gastrointestinal, pulmonary); smooth muscle contraction (pulmonary airways, urinary, gastrointestinal tract and vascular system); and unique effects on the central nervous system. SP has numerous functions, including a role as a neuronal sensory transmitter associated with pain and central responses to stress and anxiety.16, 18, 21 In the immune system, SP has a role in augmenting inflammatory responses.16-18 SP interaction with NK1R leads to activation of NF-κB and enhanced production of proinflammatory cytokines, including IL-1, IL-6, TNF-α, MIP-1β and IFN-γ.16, 21 Neurokinin-1 receptors are expressed in a number of important sites in the nervous system.22-24 The tachykinins have been studied extensively in neurons and play a major role in unmyelinated sensory somatic and visceral nerve fibers, enteric sensory neurons and several nervous system sites.25, 26 The release of the agonist (substance P) from peripheral ends of neurons has a major role in neurogenic inflammatory responses.27-29 SP is involved in neurogenic inflammation; within the dura mater this may be a major source of migraine headache pain.30 Substance P is produced at central terminals of primary sensory neurons in the brainstem, cranial nerve nuclei and spinal cord dorsal horn. At these sites, substance P is a sensory neurotransmitter and neuromodulator related to nocioceptive pain pathways. NK1R and NK-3R receptors are expressed throughout the CNS.
Substance P in the nervous and immune systems
SP-containing afferent nerve fibers innervate the brainstem in the region of the nucleus tractus solitarius, a region that is involved in the control of nausea and vomiting (emesis).31-33 SP preferring receptors (NK1R) also are located at several major sites in the brain. SP is commonly co-localized with other neurotransmitters such as serotonin in the nuclei raphes, with dopamine in the midbrain and striatum, with GABA and acetylcholine in the cortex, and with corticotropin-releasing hormone in the hypothalamus.34 SP is involved in complex brain functions, including neuronal sensory transmission associated with pain, depression, anxiety and central responses to stress.16, 18, 21
In the immune system SP generally has proinflammatory effects or augments inflammatory responses in the respiratory, gastrointestinal, and musculoskeletal systems.10, 17, 18, 35 In the brain, high levels of SP have been identified in areas known to be implicated in the modulation of stress and anxiety such as the cingulate cortex, caudate, putamen, nucleus accumbens, septum, hippocampus, amygdala, various hypothalamic areas as well as periaqueductal gray matter, dorsal raphe nucleus, locus coeruleus, parabrachial nuclei and in the nucleus of the tractus solitaries.36 In most of these brain regions there is a positive correlation between the presence of SP and NK1R expression.36 The anatomic distribution of NK1R in the human nervous system differs among different anatomic sites.23 In particular, NK1R mRNA has the highest concentration in the locus coeruleus and ventral striatum. Moderate hybridization signals are found in the cerebral cortex, hippocampus, and different amygdaloid nuclei and low levels were detected in the cerebellum and the thalamus.23
Our investigations 17, 21, 37-41 and others42 have demonstrated the presence of NK1 receptors at the mRNA and protein levels, and we have investigated SP mRNA and protein production in cells of the human immune system and the nervous system in an autocrine manner. In addition to the functional effects of SP on monocyte-macrophages, a number of other cell systems are relevant in examining these interactions. These cells include microglia,39 stem cells,40 human peripheral blood mononuclear phagocytes (PBMCs),17 and lymphocytes.38 Neurokinin-1 antagonists inhibit HIV infectivity in both MDMs24 and PBMCs.43 The NK1R antagonist (CP-96,345) inhibited the SP effect in NT2 neurons.44
We have recently quantitated both full-length and truncated NK1R mRNA in human autopsy brain tissue.45 Lower levels of expression of full-length NK1R were found in the cingulate cortex from HIV-positive individuals, whereas the expression of the truncated form was similar in HIV-positive and HIV-negative individuals. In contrast, in the cerebellum, there were no differences observed between HIV-positive and HIV-negative subjects in this small sample.46
Substance P (SP) and its preferring receptor, NK1R, are central mediators in the interaction between the immune system and the nervous system.5, 20, 47 SP and SP preferring receptors (neurokinin-1R) have important associations with depression, anxiety and psychologic and physiologic stress, in general, and particularly in HIV-infected individuals.48-53 We have demonstrated that HIV infected men and women have elevated levels of circulating plasma SP in comparison to uninfected subjects.49-51 The addition of SP in vitro enhances HIV replication in blood-isolated mononuclear phagocytes.50 We have demonstrated that the non-peptide SP antagonist (CP-96,345) inhibits HIV replication in human mononuclear phagocytes, at least in part, through down-regulation of CCR5 chemokine receptor, which is the principal co-receptor for HIV entry into macrophages and also through inhibition of endogenous SP production.22, 24 Further, aprepitant is more potent ex vivo than CP96,345 in HIV antiviral activity.52, 53 The SP autocrine loop has a major role in regulating cytokine and inflammatory responses.22, 24 HIV reciprocally enhances SP expression in human immune cells, eliciting a “feed-forward cycle.” Substance P, through binding to NK1R, facilitates HIV entry into macrophages and NK1R antagonists inhibit HIV entry through down-regulation of CCR5.24 We have demonstrated that the nonpeptide SP antagonist, CP96,345, blocks HIV infection of macrophages by antagonizing SP receptors and SP interactions with the chemokine receptor, CCR5.24
Neurokinin receptors
There are three main classes of neurokinin receptors: NK1R (the substance P preferring receptor), NK2R, and NK3R. These tachykinin receptors belong to the class I (rhodopsin-like) G-protein coupled receptor (GPCR) family.6, 54-65 The various tachykinins have different binding affinities to the neurokinin receptors: NK1R, NK2R, and NK3R.49 These neurokinin receptors are in the superfamily of transmembrane G-protein coupled receptors (GPCR) and contain seven transmembrane loops. Neurokinin-1 receptor interacts with the Gαq-protein and induces activation of phospholipase C followed by production of inositol triphosphate (IP3) leading to elevation of intracellular calcium as a second messenger.60-64 Further, cyclic AMP (cAMP) is stimulated by NK1R coupled to the Gαs-protein.59, 66-71 The neurokinin receptors are expressed on many cell types and tissues.19 The endogenous agonists are peptides which share the common C-terminal sequence Phe-X-G1y-Leu-Met-NH2 (X is Phe for SP and Val for NKA and NK-B).
Neurokinin 1 receptor
Neurokinin 1 receptor (NK1R) is the substance P–preferring receptor (Fig. 1). NK1R is a highly conserved receptor which binds high affinity ligands, including SP, and the SP-like hemokinin and endokinin peptides. NK1R is a G-protein coupled receptor which couples to a subgroup of G-proteins, Gq/11. Interaction with SP, the preferred ligand, leads to activation of phospholipase CB (PLCB), and results in a transient increase in intracellular inositol 1,4,5 triphosphate (IP3) diacyl-glycerol and increased cytosolic calcium concentration.72,73 This interaction occurs in several cell systems and is involved in exocrine gland secretion (e.g., sialogogue), endocrine secretion, pain transmission, vasodilatation, connective-tissue cell proliferation, and neuroimmune modulation.16, 18
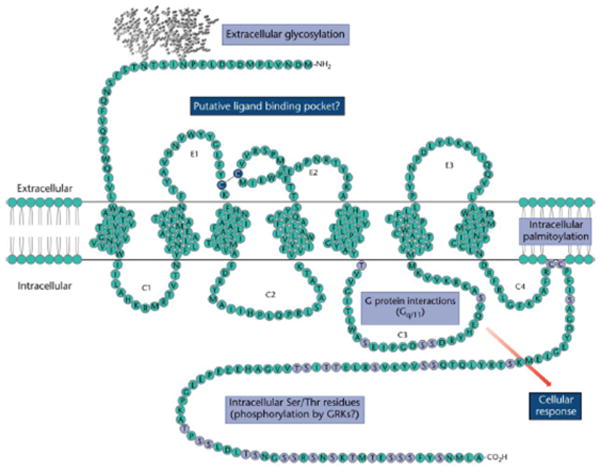
Figure 1.
Schematic model of the NK-1 receptor membrane topography. The rat NK-1 receptor contains an extracellular N-terminus, seven putative hydrophobic membrane-spanning domains, intervening extracellular loops (E1, E2, and E3) and intracellular loops (C1, C2, C3 and a possible C4 due to Cys residue palmitoylation) and an intracellular C-terminus. Asn14 and Asn18 are indicated as putative sites of Asn-glycosylation. A disulfide bond exists between Cys105 and Cys180, connecting the first and second extracellular loops. [Orignally published in Bremer & Leeman (2010). Reproduced with permission.]
The human neurokinin-1 receptor consists of a 1221 nucleotide open reading frame, which is divided in five exons. A single polymorphism in the human neurokinin-1 receptor gene has been identified;74 this is a Y192H variant, which results in a tyrosine-to-histidine substitution. The Y192H variant was expressed using site-directed mutagenesis and displayed physiologic properties similar to that of the wildtype variant; there were no differences observed in calcium responses following treatment with substance P.
Neurokinin-1 receptor isoforms
The truncated (“tail-less”) NK1R occurs in various human tissues.4, 23, 42, 45 Caberlotto et al. reported differences in the expression of the truncated and full-length NK1R mRNA in human autopsy tissues, including brain.23 In particular, the full-length form is expressed in several brain regions, with the exception of the cerebellum. In contrast, the truncated form was predominant in peripheral tissues, including heart, lung, prostate, spleen, monocytes and gastrointestinal mucosal tissues.23 The truncated C-terminus form is present in studies of human peripheral tissues, namely, monocytes present in gastrointestinal mucosal tissues.42 Page also has shown that 311 truncated NK1R exists in various human tissues.4
The primary structure, tissue distribution and functions of tachykinins and their receptors have been reviewed.18 Human NK1 genes produce two spliced isoforms:75 the truncated form, which lacks 96 residues at the C-terminus, and the full-length form.75-77 The truncated form has a 10-fold less binding affinity to SP than the full-length NK1R and elicits a diminished calcium response. A population of NK1R mRNA is not spliced at the donor splice site of exon 4 or at the acceptor splice site of exon 5. This failure of splicing is a possible mechanism whereby a premature stop codon is encountered before the start of exon 5. This splice failure may lead to the loss of the 96 amino acid residue C-terminal tail,78 and results in truncated NK1R. Isolation and sequence of the cDNA which encodes the truncated NK1R obtained from guinea pig celiac ganglion and brain by two-step RT-PCR, revealed a truncated NK1R sequence which corresponded to a splice variant missing the final exon 5, which encoded a 311 amino acid protein truncated just after transmembrane domain 7. This position is identical to the 311 truncated variant of the human NK1R.78 There is widespread occurrence of mRNA encoding truncated NK1R (311 aa) in the guinea pig nervous system, including sympathetic ganglia, spinal cord, midbrain and brain stem.78
The structure and sequence of NK1R have been partially investigated Fong et al. 75 concluded that there were two NK1R isoforms: a truncated isoform (311 aa) and a full-length form (407 aa) of NK1R. An oscillating signal in xenopus oocytes expressing the full-length form was observed, whereas the truncated form bound substance P with a 10-fold lower affinity than the full-length form, and elicited a weaker electrophysiologic response in comparison to the full-length form.75 The two naturally-occurring forms differ in the length of the carboxyl-terminus: a full-length receptor consisting of 407 amino acids (aa) and a truncated receptor consisting of 311 aa. Two clones were obtained: an 800-base pair and a 400-base pair cDNA fragment; both clones encode the carboxyl terminal region of NK1R. The 800-base pair clone contained a stop codon after amino acid 407, whereas the 400-base pair contained a stop codon after the sequence encoding the amino acid 311 (Fig. 1).
Additional forms of NK1R have been identified and involve a variety of mutations with truncated C-terminal tails. KNRK cells and CHO cells have truncated NK1R coupled to calcium mobilization.79, 80 The truncated NK1R is capable of calcium mobilization when it is 324, 342 and 354 amino acids in length. These mutants have been further studied in interaction systems with the β-arrestins and ERK 1/2. This interaction is contingent on the phosphorylation of serine and threonine residues in the carboxyl terminal domain of NK1R. The truncated NK1R, which lacks these residues, fails to interact with β-arrestins.81 The major and naturally-occurring alternatively spliced NK1R truncated form is a 311 amino acid receptor.75 Both the full-length and the truncated forms of NK1R proteins have been detected in rat salivary glands 82 and paratid.66, 67, 76, 83
Truncated (“tail-less”) and long isoforms of NK1R may have direct and distinct functions.18 We have recently studied whether there are differential signaling properties attributable to the C-terminus of NK1R using stably transfected Human Embryonic Kidney (HEK293) cell lines which express either full-length or truncated NK1R.77 Substance P (SP) specifically triggered intracellular calcium increase in HEK293 cells expressing full-length NK1R, but had no effect in the cells expressing the truncated NK1R. In addition, in cells expressing full-length NK1R, SP activated NF-κB and IL-8 mRNA expression, but in cells expressing the truncated NK1R, SP did not activate NF-κB and had decreased IL-8 mRNA expression. In cells expressing full-length NK1R, SP stimulated phosphorylation of PKCδ, but inhibited phosphorylation of PKCδ in cells expressing truncated NK1R.77 There are also differences in the timing of SP-induced ERK activation in cells expressing the two different forms of the receptor. Full-length NK1R activation of ERK was rapid (peak within 1-2 min), while truncated NK1R-mediated activation was slower (peak at 20-30 min). Thus, the C-terminus of NK1R is the structural basis for differences in the functional properties of the full-length and truncated NK1R. These differences are summarized in Table 1, which outlines our current understanding of these processes, and provide an approach toward the design of new, novel NK1R receptor antagonists.77, 84, 85
Table 1. Differences in the length of the carboxyl terminus mediate functional properties of NK1R.
| Full-length NK1R | Truncated NK1R | |
|---|---|---|
| Structure | ||
| Length of NK1R | 407 aa | 311 aa, no C-terminus |
| Biological Effects | ||
| Calcium mobilization | Yes | No |
| PKCδ phosphorylation | Increased | Decreased |
| Activation of ERK | fast (< 1 min) and sustained | slow (peaked at 20-30 min) |
| Activation of NF-κB | Yes | No |
| IL-8 mRNA expression | Increased | Decreased |
The neurokinin-1 receptor is a cell membrane receptor, which has been cloned from several species. Neurokinin-1 receptor is a glycosylated receptor.86. This receptor exists in two naturally occurring forms that differ in the length the carboxyl terminus, full length receptor consisting of 407 amino acids, and a truncated receptor consisting of 311 amino acids. In our recent studies, we have demonstrated that treatment with SP triggers intracellular calcium in cells which express the full length NK1R receptor, but not in cells which express the truncated receptor NK1-R. These observations suggest that truncated form of the receptor does not couple to GQ1.77, 84 Furthermore, we have demonstrated that mRNA expression of IL-8 and nuclear factor, κB, requires the full length receptor. The full length NK1R receptor also stimulates phosphorylation of phosphokinase-C when stimulated with substance P.45, 75, 85 Our studies show differences in the timing of signaling of the truncated receptor in contrast to full length receptor. The neurokinin-1 receptor is highly conserved and binds high infinity ligands including substance P, neurokinin A and B, and endokinin peptides. The receptor is a G-protein coupled receptor, which couples to a subgroup of G-proteins. This receptor interacts with diacylglycerol, leading to increased intracellular calcium. These interactions are detectable several cells and tissues. Of note, NK1-R is widely distributed throughout the immune and nervous system.
Modulation of NK1R function by TGF-β
Beinborn et al. recently observed 87 that the function of NK1R is modulated by TGF-β. The finding demonstrates that a G-coupled protein receptor is modulated by a serine-threonine-kinase receptor. In this study, mucosal T cells, isolated from a mouse model of inflammatory bowel disease and a granuloma model of T cells in murine schistosomiasis were investigated. Incubation of T lymphocytes with TGF-β delayed substance P induced neurokinin-1 internalization. In this system, NK1R stimulation activated the NF-κβ of activated T cells, and activator-protein 1 dependent signaling pathways. These pathways are known triggers of effector T cell cytokine production.87 TGF-β markedly increased SP induced activation of these signaling cascades, this observation suggests that delayed NK1R internalization results in enhanced cell signaling. These findings suggest a possible direct mechanism for NK1R amplification of immune function. Whether TGF-β influences a specific form of the neurokinin-1 receptor has yet to be investigated. This unique observation is related to NK1R prototypic of many of the endosomal pathways.88 G-protein coupled receptors initiate a series of complex endosomal molecular pathways. The downstream physiologic consequences of this signaling leads to numerous physiologic and pharmacologic consequences. Murphy et al. 88 have reviewed the endosomal signaling for NK1R as well as other protein coupled receptors, in detail through a variety of beta-arrestins and scaffolds. They have designated these pathways as “signalosomes.”88
NK1R glycosylation and consequences
The demonstrations by Tansky, Pothoulakis, and Leeman86 that the amino terminus of the neurokinin-1 receptor has two positive Asn (N)-linked glycosylation sites is an important functional observation. This study demonstrated that the binding affinity for substance P and neurokinin A are comparable between the non-glycosylated, single-site or both-site (wild-type) glycosylation receptors. The non-glycosylated receptor, however, showed half of the maximal binding capacity of the wild-type receptor. Similar to our observations comparing the truncated and full-length NK1R, the interaction with non-glycosylated NK1 receptor led to activation of MAP kinase, however, SP-induced IL-8 secretion was attenuated.86 The non-glycosylated NK1 receptor was more rapidly internalized than the wild-type receptor, suggesting the possibility that NK1R glycosylation may stabilize NK1R in the cell membrane.86 These consequences have possible implications toward the development of NK1R antagonists for pharmacologic use in clinical conditions.
NK1R and inflammation and malignancy
Recent results by Gillespie and Leeman (unpublished; 89) suggest a role for the truncated form in the transition of colonic epithelial cells from quiescent colitis to high-grade dysplasia to carcinoma in colitis associated colorectal cancer. 89 Further, Palma 90 have suggested that in certain tumors, e.g., neuroblastoma, breast cancer, and prostate cancer, tachykinins may have a role in facilitating metastatic infiltration and cell motility. It is further speculated that specific NK1R isoforms in neoplasms may affect tumor characteristics and responses to therapy (see 2).
Neurokinin-1R and substance P in viral responses
There are unique relationships between tachykinins and antiviral responses.16 Their effects have been recently reviewed, in particular, in relationship to murine gamma herpes viruses, HIV infection, and airway hyper-responsiveness following paramyxovirus infection.16 In each of these systems, there is specific interaction between the tachykinin and the organism or target cells.
HIV / AIDS
HIV has been investigated in our laboratory over several years, demonstrating a bidirectional relationship between substance P and HIV.37 We have observed that the serum levels of substance P are elevated in the sera of men, women, and in SIV-infected Rhesus macaques.24, 49, 51, 76, 77, 84, 91 Further, there is an association between viral input and levels of substance P produced by monocyte derived macrophages in vitro.37 Substance P enhances HIV replication, and reciprocally, substance P antagonists block HIV in part through CCR5,24 as well as through down-regulation of other pathways.
The effects of SP on HIV infection are most likely mediated through several interrelated pathways: by enhancing HIV entry into immune cells; by facilitating HIV replication directly within immune cells; and/or by indirectly affecting virus proliferation through replication through effects on HIV co-receptors.24, 37 Our measurement of circulating SP level demonstrates higher plasma levels in HIV-infected men 49 and women 51 than in healthy individuals. Furthermore, we have demonstrated that plasma SP levels have diurnal variation (AM higher that PM) and gender differences (men higher than women).49, 51 NK1R antagonists down regulate SP production by macrophages.22 Men with HIV/AIDS have increased life-event stress 24, 92, 93 and women have increased incidence of depression.94 Impairment in innate immunity, in particular, natural killer cell function, is associated with stress/depression in HIV.93, 94
Murine gamma herpes virus 68
Elsawa et al. 95 demonstrated that following intragastric inoculation with murine gamma herpes virus 68, the expression of substance P and its receptor NK1R was increased in mucosal and peripheral lymphoid organs in wild type strains of mice. Mutant mice genetically deficient in substance P receptor expression had an increased viral burden when compared with syngeneic C57/Black 6 mice. The substance P receptor deficient mice had reduced cytotoxic lymphocyte response against herpes virus 68, suggesting a mechanism which explains the increased viral burden in these mice. The observation is further supported by the finding of increased neurokinin-1 mRNA expression following virus infection in the NK-1 deficient mice. Further, these mice had decreased IL-12 levels, suggesting that IL-12 is an important component of host responses against herpes virus 68.95
Paramyxoviruses
Johnson and Graham 96 have reviewed respiratory syncytial virus (RSV). RSV is a pleiomorphic enveloped virus of the pneumovirus paramyxoviridae family. In a rat model of respiratory syncitial virus there is increased expression of substance P. It is well known that the binding of substance P to its receptor results in increased vascular permeability. This leads to lymphocyte infiltration in various tissues, including the lung. Substance P is an important vasoconstrictor in smooth muscle hyperresponsiveness during RSV disease may be in part related to substance P. A number of studies have shown that treatment with an NK-1 antagonist may limit bronchoconstriction in a guinea pig model of RSV.97 Other studies have shown rats infected RSV challenged with capsaicin upregulate the proinflammatory effects of substance P.98, 99 Capsaicin releases substance P from the bronchial airways. Furthermore, in Bovine Syncytial Virus (BSV), Virokinin is part of the viral fusion protein and may represent molecular mimicry which modulate the inflammatory and immune responses.100
Encephalomyocarditis virus
An experimental model of murine myocarditis, which is caused by infection with encephalomyocarditis virus (EMCV) has been investigated. EMCV in mice is a fatal disease, accompanied by increased cardiac inflammation, necrosis, cardiomyocyte apoptosis and hypertrophy with resultant heart failure. Robinson et al. demonstrate that as wild-type mice develop fatal EMCV, and have elevated substance P levels.101 Substance P precursor knockout mice were protected from EMCV mortality, cardiomegaly, cardiac inflammation, and cardiomyocyte apoptosis. This finding suggests NK1R and SP may have a role in the pathogenesis of EMCV.
Neurokinin-1 and substance P in bacterial and fungal responses
Borrelia burgdorferi, Neisseria meningitides, and Trypanosoma brucei
Several results have been demonstrated with Neisseria meningitides and Trypanosoma brucei infection. Substance P is the most abundant tachykinin in the brain. We have recently demonstrated NK1R and substance P expression in the Rhesus macaques.15 Studies in rodents by Chauhan et al. 102 demonstrate that treatment with substance P synergistically augments Borrelia burgdorferi induced expression of cyclo-oxygenase 2 in microglia and secretion of this prostanoid PGE2 is an important feedback loop.
Histoplasma capsulatum
Cooper et al. 103 demonstrated that Histoplasma capsulatum encodes a peptide that cleaves substance P. This peptidase may modulate interferon by altering proinflammatory signaling. In fungal disease, substance P has a major role in the granulomatous response.
Cryptosporidium parvum
Robinson et al. studied murine cryptosporidiosis.104 SP levels are elevated in the intestinal fluid of Cryptosporidium parvum infected mice. Treatment with the neurokinin-1 antagonist, aprepitant, reduced the substance P levels in these fluids. The finding has been extended to the Rhesus macaque. In this system, basal ion secretion and glucose metabolism C. parvum infected macaques. C. parvum infection was accompanied by increased basal ion secretion and glucose malabsorption. Treatment ex vivo with aprepitant reversed the increase in basal ion secretion and corrected the glucose malabsorption.
Neurokinin-1 and substance P in parasite responses
Cysticercosis
Infection with larval cysts of the cestode Taenia solium. Garza et al.105 have demonstrated that substance P contributes to granuloma formation. Granuloma size and levels in wild-type mice were larger than in mice deficient in substance P precursor or in NK1R.
Trypanosoma brusei
Studies of a mouse model of treatment reactive encephalopathy demonstrated that substance P has an important role.27-29 These mice treated with a substance P antagonist RP67,580 have a reduction in the severity of the inflammatory response and the degree of astrocyte activation in the brain.29 Further, an inactive enantiomer of this NK1R antagonist had no effect. Kennedy also showed surprisingly that NK1R knockout mouse had an increase rather than a decrease in the neuroinflammatory response in comparison to wild-type mice.28 These findings suggested that there is a dissociation between clinical and neuroinflammatory responses in the model. Furthermore, and surprisingly, treatment with NK2 and NK3 antagonists reduced neuroinflammatory scores in the knockout mice. (The dissociation suggests that the interaction between various neurokinin receptors in neuroinflammation, neuropathogenesis is mediated through complex receptor interactions.) In human trypanosomiasis, these pathways are not yet worked out, although various chemokines are involved.27-29
Kennedy27-29 demonstrated that mice lacking NK1R, namely the knockout mice followed their course of inflammation in the central nervous system during the chronic phase of autoimmune encephalitis (EAE). These findings further substantiate the role of substance P and NK1R in neuroinflammation.106
Schistosoma models
SP was found in granuloma response to schistosoma during the course of natural infection in neurokinin receptor knockout mice. Further, Weinstock et al. demonstrated a major role for substance P in somatostatin granuloma formation in a murine model.107
Neurokinin-1 receptor antagonists
NK1R antagonists have been studied as a novel therapeutic approach to stress and mood therapy and control of emesis.31-33, 108-113 The SP pathway offers the potential for dual benefit in HIV infection based on its ability to modulate HIV replication.
Several investigators have linked neurokinin-1 receptor (substance P preferring) antagonists to beneficial effects on stress, mood and sleep.8 As reviewed by Baby,8 the evolution of a connection between depression or antidepressant pharmacotherapy and substance P or other neurokinins involved findings that: (i) substance P interacts with serotonergic neuronal systems and that substance P is co-localized with 5-HT in some neurons;114-117 (ii) the administration of a TCA or the selective 5-HT neuronal re-uptake inhibitors zimeldine or alaproclate for 14 days alters neurokinin levels in the brain and spinal cord of rats;118, 119 (iii) withdrawal from subchronic administration of the antidepressants desipramine, chlorimipramine, trimipramime or zimeldine increase the sensitivity of rat cingulate-cortex neurons to substance P;117 and, finally, (iv) repeated electroconvulsive shock, equivalent to ECT, increases substance P levels in the cingulate cortex of rats.120
Neurokinin-1R antagonists have been developed that have activity directed against each of the three mammalian tachykinin receptors: NK1R, NK2R, and NK3R.8, 32, 121, 122 All three human neurokinin receptors have been cloned.55, 63, 123 The expression of these receptors in cell lines has facilitated the ability to rapidly screen agents for receptor selectivity.122 The human NK-1 receptor has been cloned and its gene localized to chromosome 2.56, 123, 124 The NK-2R receptor gene has also been cloned and is localized to chromosome 10.55, 125 NK-3R has been cloned.63, 126, 127 and has more extended C and N termini in comparison to NK1R and NK-2R. NK1R and NK-2R are receptors of the seven transmembrane G-protein coupled type receptor (GPCR).
Chemically, NK1R receptor antagonists fall into peptide and non-peptide classes.121, 122 Among the selective non-peptide antagonists, there are NK1R, dual NK1R, NK2R, NK-2R, NK-3R and some with pan NK-R activity. The NK-R antagonists are classified as: diamines, amino ethers, perhydroisoindoles, 4-amino-2-benzylpiperidine amides, quinoline and 1,7-naphthyridine amides and tryptophan analogues.122 The first non-peptide NK1R antagonists were discovered by Pfizer in 1991 128 and have been extensively investigated.
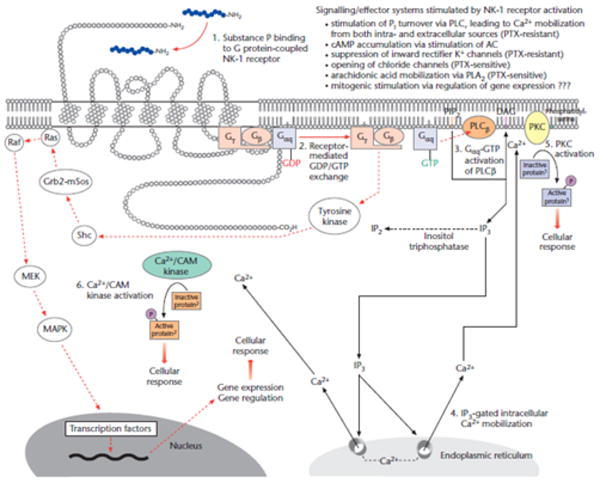
Figure 2.
Proposed signal transduction pathways following NK-1 receptor activation. Functional interaction of substance P with the NK-1 receptor stimulates NK-1 receptor-mediated CDP/CTP exchange on the Gaq subunit and the subsequent activation of PLC β (phospholipase C β). The two intracellular second messengers, IP3 (inositol 1,4,6-triphosphate) and DAG (diacylgylcerol), then stimulate the mobilization of intracellular Ca2+ and the activation of PKC (protein kinase C), respectively. Current interest is focusing on the possible gene regulatory effects of G protein-coupled receptor activation mediated by the Gργ subunits. [Orignally published in Bremer & Leeman (2010). Reproduced with permission.]
Acknowledgments
We thank the faculty, staff, and students of the Douglas and Leeman laboratories for their experiments, insights, and dedication to the investigation of Neurokinin-1 Receptor; the National Institutes of Health – National Institutes of Mental Health extramural program officials, in particular Drs. Jeymohan Joseph, Kathy L. Kopinsky and Dianne M. Rausch for their interest and encouragement in the investigations. We are grateful to Joshua A. Taton for his help in preparing this manuscript. The studies in the Douglas laboratory are currently supported by NIH-NIMH P01-MH-076388, NIMH-U01-MH-090325, and NIMH-R01-MH-49981 to S.D.D.
References
- 1.Pascual DW. The role of tachykinins on bacterial infections. Front Biosci. 2004;9:3209–3217. doi: 10.2741/1473. [DOI] [PubMed] [Google Scholar]
- 2.Bremer AA, Leeman SE. Encyclopedia of Life Sciences (ELS) John Wiley & Sons, Ltd.; Chichester: 2010. Substance P. [Google Scholar]
- 3.Lai JP, et al. Identification of a delta isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J Neuroimmunol. 1998;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- 4.Page NM. Characterization of the gene structures, precursor processing and pharmacology of the endokinin peptides. Vascul Pharmacol. 2006;45:200–208. doi: 10.1016/j.vph.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Severini C, et al. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 6.Pennefather JN, et al. Tachykinins and tachykinin receptors: a growing family. Life Sciences. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Patacchini R, et al. Newly discovered tachykinins raise new questions about their peripheral roles and the tachykinin nomenclature. Trends Pharmacol Sci. 2004;25:1–3. doi: 10.1016/j.tips.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Baby S, et al. Substance P antagonists: the next breakthrough in treating depression? Journal of Clinical Pharmacy and Therapeutics. 1999;24:461–469. doi: 10.1046/j.1365-2710.1999.00257.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson DA, Bost KL. Non-neuronal mammalian tachykinin expression. Front Biosci. 2004;9:2166–2176. doi: 10.2741/1372. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DA, Marriott I, Bost KL. Expression of hemokinin 1 mRNA by murine dendritic cells. J Neuroimmunol. 2004;155:94–102. doi: 10.1016/j.jneuroim.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Metwali A, et al. Cutting edge: hemokinin has substance p-like function and expression in inflammation. J Immunol. 2004;172:6528–6532. doi: 10.4049/jimmunol.172.11.6528. [DOI] [PubMed] [Google Scholar]
- 12.von Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol (Lond) 1932;72:577–583. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. Journal of Biological Chemistry. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 14.Chang MM, Leeman SE, Niall HD. Amino-acid sequence of substance P. Nature - New Biology. 1971;232:86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 15.Vinet-Oliphant H, et al. Neurokinin-1 Receptor (NK1-R) Expression in the Brains of SIV-Infected Rhesus Macaques: Implications for Substance P in NK1-R Immune Cell Trafficking into the CNS. American Journal of Pathology. 2010 doi: 10.2353/ajpath.2010.091109. ePub 2010 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bost KL. Tachykinin-mediated modulation of the immune response. Frontiers in Bioscience. 2004;9:3331–3332. doi: 10.2741/1484. [DOI] [PubMed] [Google Scholar]
- 17.Ho WZ, et al. Human monocytes and macrophages express substance P and neurokinin-1 receptor. Journal of Immunology. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 18.Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Current Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- 19.Greco SJ, et al. Tachykinins in the emerging immune system: relevance to bone marrow homeostasis and maintenance of hematopoietic stem cells. Frontiers in Bioscience. 2004;9:1782–1793. doi: 10.2741/1373. [DOI] [PubMed] [Google Scholar]
- 20.Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regulatory Peptides. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 21.Ho WZ, Douglas SD. Substance P and neurokinin-1 receptor modulation of HIV. Journal of Neuroimmunology. 2004;157:48–55. doi: 10.1016/j.jneuroim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Lai JP, et al. A non-peptide substance P antagonist down-regulates SP mRNA expression in human mononuclear phagocytes. J Neuroimmunol. 2002;128:101–108. doi: 10.1016/s0165-5728(02)00164-9. [DOI] [PubMed] [Google Scholar]
- 23.Caberlotto L, et al. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. European Journal of Neuroscience. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- 24.Lai JP, et al. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantyh CR, et al. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantyh PW, et al. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci U S A. 1989;86:5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy KJ, et al. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. Journal of Neuroimmunology. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy PG, et al. Clinical and neuroinflammatory responses to meningoencephalitis in substance P receptor knockout mice. Brain. 2003;126:1683–1690. doi: 10.1093/brain/awg160. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy PG, et al. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrettini WH, et al. CSF substance P immunoreactivity in affective disorders. Biol Psychiatry. 1985;20:965–970. doi: 10.1016/0006-3223(85)90193-3. [DOI] [PubMed] [Google Scholar]
- 31.Rupniak NM. Animal models of depression challenges from a drug development perspective. Behav Pharmacol. 2003;14:385–390. doi: 10.1097/01.fbp.0000087738.21047.91. [DOI] [PubMed] [Google Scholar]
- 32.Rupniak NM. Elucidating the antidepressant actions of substance P (NK1 receptor) antagonists. Curr Opin Investig Drugs. 2002;3:257–261. [PubMed] [Google Scholar]
- 33.Rupniak NM, Kramer MS. Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends Pharmacol Sci. 1999;20:485–490. doi: 10.1016/s0165-6147(99)01396-6. [DOI] [PubMed] [Google Scholar]
- 34.Goodman A. Neurobiology of addiction. An integrative review. Biochemical pharmacology. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor TM, et al. The role of substance P in inflammatory disease. Journal of Cellular Physiology. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 36.Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- 37.Ho WZ, et al. HIV enhances substance P expression in human immune cells. FASEB Journal. 2002;16:616–618. doi: 10.1096/fj.01-0655fje. [DOI] [PubMed] [Google Scholar]
- 38.Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. Journal of Neuroimmunology. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 39.Lai JP, et al. Detection of substance P and its receptor in human fetal microglia. Neuroscience. 2000;101:1137–1144. doi: 10.1016/s0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Douglas SD, Ho W. Human stem cells express substance P gene and its receptor. Journal of Hematotherapy and Stem Cell Research. 2000;9:445–452. doi: 10.1089/152581600419107. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, et al. Human neuronal cells (NT2-N) express functional substance P and neurokinin-1 receptor coupled to MIP-1 beta expression. Journal of Neuroscience Research. 2003;71:559–566. doi: 10.1002/jnr.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goode T, et al. Differential expression of neurokinin-1 receptor by human mucosal and peripheral lymphoid cells. Clinical and Diagnostic Laboratory Immunology. 2000;7:371–376. doi: 10.1128/cdli.7.3.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manak M, et al. Anti-HIV Activity of Aprepitant and Synergistic interactions with other Antiretrovirals. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 44.Wang X, et al. A non-peptide substance P antagonist (CP-96,345) inhibits morphine-induced NF-kappaB promoter activation in human NT2-N neurons. Journal of Neuroscience Research. 2004;75:544–553. doi: 10.1002/jnr.10873. [DOI] [PubMed] [Google Scholar]
- 45.Lai JP, et al. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. Journal of Neuroscience Methods. 2008;168:127–133. doi: 10.1016/j.jneumeth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douglas SD, Lynch KG, Lai JP. Neurokinin-1 receptor mRNA expression differences in brains of HIV-infected individuals. J Neurol Sci. 2008;272:174–177. doi: 10.1016/j.jns.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartung HP, Wolters K, Toyka KV. Substance P binding properties and studies on cellular responses in guinea pig macrophages. J Immunol. 1986;136:3856–3863. [PubMed] [Google Scholar]
- 48.Michaels LA, et al. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92:3148–3151. [PubMed] [Google Scholar]
- 49.Douglas SD, et al. Elevated substance P levels in HIV-infected men. Aids. 2001;15:2043–2045. doi: 10.1097/00002030-200110190-00019. [DOI] [PubMed] [Google Scholar]
- 50.Ho WZ, et al. Substance P modulates human immunodeficiency virus replication in human peripheral blood monocyte-derived macrophages. AIDS Research and Human Retroviruses. 1996;12:195–198. doi: 10.1089/aid.1996.12.195. [DOI] [PubMed] [Google Scholar]
- 51.Douglas SD, et al. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res Hum Retroviruses. 2008;24:375–378. doi: 10.1089/aid.2007.0207. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, et al. Neurokinin-1 receptor antagonist (aprepitant) inhibits drug-resistant HIV-1 infection of macrophages in vitro. J Neuroimmune Pharmacol. 2007;2:42–48. doi: 10.1007/s11481-006-9059-6. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. Neurokinin-1 receptor antagonist (Aprepitant) suppresses HIV-1 infection of microglia/macrophages. Journal of Neuroimmune Pharmacology. 2008;3:257–264. doi: 10.1007/s11481-008-9117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almeida TA, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11:2045–2081. 2014. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- 55.Gerard NP, et al. The human neurokinin A (substance K) receptor. Molecular cloning of the gene, chromosome localization, and isolation of cDNA from tracheal and gastric tissues. Journal of Biological Chemistry. 1990;265:20455–20462. [PubMed] [Google Scholar]
- 56.Gerard NP, et al. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry (Mosc) 1991;30:10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- 57.Krause JE, et al. Structure, expression and second messenger-mediated regulation of the human and rat substance P receptors and their genes. Regul Pept. 1993;46:59–66. doi: 10.1016/0167-0115(93)90012-w. [DOI] [PubMed] [Google Scholar]
- 58.Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 59.Maggi CA, Schwartz TW. The dual nature of the tachykinin NK1 receptor. Trends in Pharmacological Sciences. 1997;18:351–355. doi: 10.1016/s0165-6147(97)01107-3. [DOI] [PubMed] [Google Scholar]
- 60.Masu Y, et al. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature. 1987;329:836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi S, et al. Molecular characterization of mammalian tachykinin receptors and a possible epithelial potassium channel. Recent Prog Horm Res. 1990;46:59–83. doi: 10.1016/b978-0-12-571146-3.50007-9. discussion 83-54. [DOI] [PubMed] [Google Scholar]
- 62.Shigemoto R, et al. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990;265:623–628. [PubMed] [Google Scholar]
- 63.Takahashi K, et al. The primary structure and gene organization of human substance P and neuromedin K receptors. European Journal of Biochemistry. 1992;204:1025–1033. doi: 10.1111/j.1432-1033.1992.tb16724.x. [DOI] [PubMed] [Google Scholar]
- 64.Torrens Y, et al. Tachykinin receptors of the NK1 type (substance P) coupled positively to phospholipase C on cortical astrocytes from the newborn mouse in primary culture. J Neurochem. 1989;52:1913–1918. doi: 10.1111/j.1471-4159.1989.tb07276.x. [DOI] [PubMed] [Google Scholar]
- 65.Yokota Y, et al. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989;264:17649–17652. [PubMed] [Google Scholar]
- 66.Blount P, Krause JE. Functional nonequivalence of structurally homologous domains of neurokinin-1 and neurokinin-2 type tachykinin receptors. Journal of Biological Chemistry. 1993;268:16388–16395. [PubMed] [Google Scholar]
- 67.Holst B, et al. Two active molecular phenotypes of the tachykinin NK1 receptor revealed by G-protein fusions and mutagenesis. Journal of Biological Chemistry. 2001;276:19793–19799. doi: 10.1074/jbc.M100621200. [DOI] [PubMed] [Google Scholar]
- 68.Labrou NE, et al. Interaction of Met297 in the seventh transmembrane segment of the tachykinin NK2 receptor with neurokinin A. J Biol Chem. 2001;276:37944–37949. doi: 10.1074/jbc.M106330200. [DOI] [PubMed] [Google Scholar]
- 69.Lecat S, et al. Mutations in the extracellular amino-terminal domain of the NK2 neurokinin receptor abolish cAMP signaling but preserve intracellular calcium responses. J Biol Chem. 2002;277:42034–42048. doi: 10.1074/jbc.M203606200. [DOI] [PubMed] [Google Scholar]
- 70.Nakajima Y, et al. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J Biol Chem. 1992;267:2437–2442. [PubMed] [Google Scholar]
- 71.Palanche T, et al. The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J Biol Chem. 2001;276:34853–34861. doi: 10.1074/jbc.M104363200. [DOI] [PubMed] [Google Scholar]
- 72.Hunter JC, Goedert M, Pinnock RD. Mammalian tachykinin-induced hydrolysis of inositol phospholipids in rat brain slices. Biochem Biophys Res Commun. 1985;127:616–622. doi: 10.1016/s0006-291x(85)80205-9. [DOI] [PubMed] [Google Scholar]
- 73.Ma HT, et al. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- 74.Randolph GP, et al. Identification of single-nucleotide polymorphisms of the human neurokinin 1 receptor gene and pharmacological characterization of a Y192H variant. Pharmacogenomics J. 2004;4:394–402. doi: 10.1038/sj.tpj.6500276. [DOI] [PubMed] [Google Scholar]
- 75.Fong TM, et al. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Molecular Pharmacology. 1992;41:24–30. [PubMed] [Google Scholar]
- 76.Lai JP, et al. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci U S A. 2006;103:7771–7776. doi: 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai JP, et al. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci U S A. 2008;105:12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baker SJ, Morris JL, Gibbins IL. Cloning of a C-terminally truncated NK-1 receptor from guinea-pig nervous system. Brain Res Mol Brain Res. 2003;111:136–147. doi: 10.1016/s0169-328x(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 79.Bohm SK, et al. Identification of potential tyrosine-containing endocytic motifs in the carboxyl-tail and seventh transmembrane domain of the neurokinin 1 receptor. J Biol Chem. 1997;272:2363–2372. doi: 10.1074/jbc.272.4.2363. [DOI] [PubMed] [Google Scholar]
- 80.Li H, et al. A substance P (neurokinin-1) receptor mutant carboxylterminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci U S A. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kage R, Leeman SE, Boyd ND. Biochemical characterization of two different forms of the substance P receptor in rat submaxillary gland. J Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 83.Mantyh PW, et al. Differential expression of two isoforms of the neurokinin-1 (substance P) receptor in vivo. Brain Res. 1996;719:8–13. doi: 10.1016/0006-8993(96)00050-9. [DOI] [PubMed] [Google Scholar]
- 84.Tuluc F, et al. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Chernova I, et al. Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R) J Leukoc Biol. 2009;85:154–164. doi: 10.1189/jlb.0408260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tansky MF, Pothoulakis C, Leeman SE. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc Natl Acad Sci U S A. 2007;104:10691–10696. doi: 10.1073/pnas.0703394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beinborn M, et al. TGF-beta regulates T-cell neurokinin-1 receptor internalization and function. Proc Natl Acad Sci U S A. 2010;107:4293–4298. doi: 10.1073/pnas.0905877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy JE, et al. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gillespie E, et al. Gene expression of the truncated neurokinin-1 receptor as well as two NF-kB subunits is altered in the transition of colonic epithelial cells from quiescent colitis to high grade displasia to carcinoma in colitis associated colorectal cancer. Presented at Digestive Disease Week; New Orleans, LA. May 1-6, 2010.2010. [Google Scholar]
- 90.Palma C. Tachykinins and their receptors in human malignancies. Curr Drug Targets. 2006;7:1043–1052. doi: 10.2174/138945006778019282. [DOI] [PubMed] [Google Scholar]
- 91.Douglas SD, et al. Neurokinin-1 receptor expression and function in human macrophages and brain: perspective on the role in HIV neuropathogenesis. Ann N Y Acad Sci. 2008;1144:90–96. doi: 10.1196/annals.1418.007. [DOI] [PubMed] [Google Scholar]
- 92.Evans DL, et al. Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am J Psychiatry. 1995;152:543–550. doi: 10.1176/ajp.152.4.543. [DOI] [PubMed] [Google Scholar]
- 93.Evans DL, et al. Severe life stress as a predictor of early disease progression in HIV infection. American Journal of Psychiatry. 1997;154:630–634. doi: 10.1176/ajp.154.5.630. [DOI] [PubMed] [Google Scholar]
- 94.Evans DL, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. American Journal of Psychiatry. 2002;159:1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- 95.Elsawa SF, et al. Reduced CTL response and increased viral burden in substance P receptor-deficient mice infected with murine gamma-herpesvirus 68. J Immunol. 2003;170:2605–2612. doi: 10.4049/jimmunol.170.5.2605. [DOI] [PubMed] [Google Scholar]
- 96.Johnson TR, Graham BS. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:S46–57. doi: 10.1097/01.inf.0000108192.94692.d2. [DOI] [PubMed] [Google Scholar]
- 97.Bost KL. Tachykinin-modulated anti-viral responses. Front Biosci. 2004;9:1994–1998. doi: 10.2741/1376. [DOI] [PubMed] [Google Scholar]
- 98.King KA, et al. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am J Respir Cell Mol Biol. 2001;24:101–107. doi: 10.1165/ajrcmb.24.2.4264. [DOI] [PubMed] [Google Scholar]
- 99.Auais A, et al. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L105–113. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- 100.Zimmer G, et al. Virokinin, a bioactive peptide of the tachykinin family, is released from the fusion protein of bovine respiratory syncytial virus. J Biol Chem. 2003;278:46854–46861. doi: 10.1074/jbc.M306949200. [DOI] [PubMed] [Google Scholar]
- 101.Robinson P, et al. Substance P is required for the pathogenesis of EMCV infection in mice. Int J Clin Exp Med. 2009;2:76–86. [PMC free article] [PubMed] [Google Scholar]
- 102.Chauhan VS, et al. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J Immunol. 2008;180:8241–8249. doi: 10.4049/jimmunol.180.12.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cooper KG, Zarnowski R, Woods JP. Histoplasma capsulatum encodes a dipeptidyl peptidase active against the mammalian immunoregulatory peptide, substance P. PLoS One. 2009;4:e5281. doi: 10.1371/journal.pone.0005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robinson P, et al. Substance P receptor antagonism for treatment of cryptosporidiosis in immunosuppressed mice. J Parasitol. 2008;94:1150–1154. doi: 10.1645/GE-1458.1. [DOI] [PubMed] [Google Scholar]
- 105.Garza A, et al. Substance P signaling contributes to granuloma formation in Taenia crassiceps infection, a murine model of cysticercosis. J Biomed Biotechnol. 2010;2010:597086. doi: 10.1155/2010/597086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reinke EK, et al. Substance P receptor mediated maintenance of chronic inflammation in EAE. J Neuroimmunol. 2006;180:117–125. doi: 10.1016/j.jneuroim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Weinstock JV, et al. Immunoregulation within the granulomas of murine schistosomiasis mansoni. Microbes Infect. 1999;1:491–498. doi: 10.1016/s1286-4579(99)80087-2. [DOI] [PubMed] [Google Scholar]
- 108.Kramer MS. Update on Substance P (NK-1 receptor) antagonists in clinical trials for depression. Neuropeptides. 2000;34:255. doi: 10.1054/npep.2000.0830. [DOI] [PubMed] [Google Scholar]
- 109.Kramer MS, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 110.Kramer MS, et al. Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology. 2004;29:385–392. doi: 10.1038/sj.npp.1300260. [DOI] [PubMed] [Google Scholar]
- 111.Rupniak NM. New insights into the antidepressant actions of substance P (NK1 receptor) antagonists. Can J Physiol Pharmacol. 2002;80:489–494. doi: 10.1139/y02-048. [DOI] [PubMed] [Google Scholar]
- 112.Alvaro G, Di Fabio R. Neurokinin 1 receptor antagonists--current prospects. Curr Opin Drug Discov Devel. 2007;10:613–621. [PubMed] [Google Scholar]
- 113.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 114.Barden N, et al. Perturbation of rat brain serotonergic systems results in an inverse relation between substance P and serotonin concentrations measured in discrete nuclei. J Neurochem. 1983;41:834–840. doi: 10.1111/j.1471-4159.1983.tb04816.x. [DOI] [PubMed] [Google Scholar]
- 115.Chan-Palay V, Jonsson G, Palay SL. Serotonin and substance P coexist in neurons of the rat's central nervous system. Proc Natl Acad Sci U S A. 1978;75:1582–1586. doi: 10.1073/pnas.75.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hokfelt T, et al. Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience. 1978;3:517–538. doi: 10.1016/0306-4522(78)90017-9. [DOI] [PubMed] [Google Scholar]
- 117.Jones RS, Olpe HR. Monoaminergic modulation of the sensitivity of neurones in the cingulate cortex to iontophoretically applied substance P. Brain Research. 1984;311:297–305. doi: 10.1016/0006-8993(84)90092-1. [DOI] [PubMed] [Google Scholar]
- 118.Brodin E, et al. Chronic treatment with the serotonin uptake inhibitor zimelidine elevates substance P levels in rat spinal cord. Acta Physiol Scand. 1984;122:209–211. doi: 10.1111/j.1748-1716.1984.tb07501.x. [DOI] [PubMed] [Google Scholar]
- 119.Brodin E, Ogren SO, Theodorsson-Norheim E. Effects of subchronic treatment with imipramine, zimelidine and alaproclate on regional tissue levels of substance P- and neurokinin A/neurokinin B-like immunoreactivity in the brain and spinal cord of the rat. Neuropharmacology. 1987;26:581–590. doi: 10.1016/0028-3908(87)90151-1. [DOI] [PubMed] [Google Scholar]
- 120.Brodin K, et al. Increased levels of substance P and cholecystokinin in rat cerebral cortex following repeated electroconvulsive shock and subchronic treatment with a serotonin uptake inhibitor. Acta Physiol Scand. 1989;136:613–614. doi: 10.1111/j.1748-1716.1989.tb08710.x. [DOI] [PubMed] [Google Scholar]
- 121.Giardina GA, Gagliardi S, Martinelli M. Antagonists at the neurokinin receptors--recent patent literature. IDrugs. 2003;6:758–772. [PubMed] [Google Scholar]
- 122.Swain CJ. Neurokinin receptor antagonists. Prog Med Chem. 1998;35:57–81. doi: 10.1016/s0079-6468(08)70034-1. [DOI] [PubMed] [Google Scholar]
- 123.Takeda Y, et al. Molecular cloning, structural characterization and functional expression of the human substance P receptor. Biochem Biophys Res Commun. 1991;179:1232–1240. doi: 10.1016/0006-291x(91)91704-g. [DOI] [PubMed] [Google Scholar]
- 124.Hopkins B, et al. Isolation and characterisation of the human lung NK-1 receptor cDNA. Biochem Biophys Res Commun. 1991;180:1110–1117. doi: 10.1016/s0006-291x(05)81181-7. [DOI] [PubMed] [Google Scholar]
- 125.Kris RM, et al. Cloning and expression of the human substance K receptor and analysis of its role in mitogenesis. Cell Growth Differ. 1991;2:15–22. [PubMed] [Google Scholar]
- 126.Buell G, et al. Molecular characterization, expression and localisation of human neurokinin-3 receptor. FEBS Lett. 1992;299:90–95. doi: 10.1016/0014-5793(92)80107-r. [DOI] [PubMed] [Google Scholar]
- 127.Huang RR, et al. cDNA sequence and heterologous expression of the human neurokinin-3 receptor. Biochem Biophys Res Commun. 1992;184:966–972. doi: 10.1016/0006-291x(92)90685-e. [DOI] [PubMed] [Google Scholar]
- 128.Snider RM, et al. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991;251:435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]