Abstract
Mild cognitive impairment (MCI) is often associated with the preclinical phase of Alzheimer's disease (AD). Special scoring of word-list recall data for serial position has been suggested to improve discrimination of normal aging from dementia. We examined serial position effects in word-list recall for MCI participants compared to Alzheimer patients and controls. Individuals with MCI, like Alzheimer patients, had a diminished primacy effect in recalling words from a list. No alternative scoring system was better than standard scoring of word list recall in distinguishing MCI patients from controls. Retention weighted scoring improved the discrimination of MCI and AD groups.
Serial position effects are observed when a series of items, such as words, that exceeds attention span are learned. In cognitively normal individuals words at the beginning and end of the list are more frequently recalled than words in the middle (Deese & Kaufman, 1957). These preferential recalls are called primacy and recency effects. The usual interpretation of this robust finding is that the primacy effect occurs because there is more opportunity for rehearsal of items in long-term (episodic) memory. When using a one-trial learning paradigm, there also is no proactive interference from previous items. The recency effect usually is attributed to information being temporarily stored in short-term memory, which lasts for approximately 20 seconds and has limited capacity. Other interpretations of the recency effect have been proposed. Baddeley and Hitch (1993) review data in which the last few words on a list are preferentially retained after a distracter activity or a prolonged delay, which would argue against a simple short-term memory explanation. However, Glanzer and Cunitz (1966) found the recency effect was removed when a delay of 10 seconds or longer filled with a distracter activity was imposed before recall. Craik (1970) found that words in the terminal serial position were retrieved best in immediate recall but least well after a delay.
Patients with amnesia from a variety of conditions show a recency effect but they poorly retain primacy items (Carlesimo, Marfia, Loasses, & Caltagirone, 1996). Patients with anterior temporal lobe resections show significant declines in recall from primary and middle portions of a word list, but not the recency portion, compared with preoperative performance (Hermann, et al., 1996). The lack of a primacy effect also is a defining feature of word-list learning in patients with Alzheimer disease (Carlesimo, Fadda, Sabbadini, & Caltagirone, 1996). Foldi and her colleagues (Foldi, Brickman, Schaefer, & Knutelska, 2003) used the 16-word California Verbal Learning Test (CVLT) and compared recall total across five trials of primacy and middle regions of the list with items in the recency region. The controls recalled the primacy and recency regions equally, while the AD group recalled recency > middle > primacy. When performance on the first trial of the CVLT for a mild AD group (MMSE < 23.5) and a very mild AD group (MMSE > 23.5) was compared with controls, the mild and very mild AD patients had a recency effect but no primacy effect (Bayley, et al., 2000). There was no difference in recall on trial one between the mild and very mild groups. As expected normal primacy and recency effects were observed for the controls. Gainotti and colleagues (Gainotti, Marra, Villa, Parlato, & Chiarotti, 1998) used the 15 unrelated words from the Rey Auditory Verbal Learning Test and compared mild to moderately demented Alzheimer patients with controls on the recency and the primacy for totals across 5 learning trials. In the AD group primacy was impaired but not recency.
The diagnosis of MCI can be difficult because memory impairment associated with MCI is often in a transition stage between normal age-related decline and the more serious deficit associated with Alzheimer's disease. The limited serial position data available from MCI patients have been mixed. Bennett's group (Bennett, Golob, Parker, & Starr, A. 2006) found a normal serial position effect for MCI participants on a 15-word list presented in a different order on each of three trials. However, Shankle et al. (2005) found a difference between MCI participants and controls in the pattern of words recalled on the CERAD Word List, which is a 10-word list presented in a different order on each of three trials (Welsh, et al., 1994). The findings suggest the possibility that serial position effects can be used to identify people who are developing MCI.
Special scoring of word-list recall data for serial position has been suggested to improve discrimination of normal aging from dementia of varying etiologies but mostly Alzheimer's disease (Buschke, et al., 2006; Shankle, Mangrola, Chan, & Hara, 2009; Shankle, et al., 2005). Buschke and colleagues administered a single presentation of a 10-word list over the telephone and devised a retention weighted scoring (RWS) to measure serial position effects in which items are weighted inversely to recency of presentation, with the first word weighted 10, the second 9, and so forth. They found that the RWS improved the discrimination of the mild AD and control groups compared to conventional scoring. Shankle and colleagues used correspondence analysis (CA) to quantify serial position effects on the CERAD Word-List task of groups of participants with mild cognitive impairment (MCI), mild dementia, and controls. Correspondence analysis, while not specific for serial position effects, takes into account the words recalled and not recalled and produces a weighted combination of values that optimally distinguishes the groups. They found this scoring increased sensitivity in classifying MCI and controls compared to both the total recall score summed over all four trials and the delayed-recall score alone.
The present study examined serial position effects in MCI participants compared to Alzheimer patients and controls. Like AD patients, MCI patients have impaired episodic memory, although less severe. We predicted that MCI patients would outperform Alzheimer patients on a word-list learning test but resemble Alzheimer patients for serial position effects. We also predicted that an enhanced scoring position that encompassed the serial position of words recalled might detect MCI better than standard scoring. Previous studies of enhanced scoring for serial position have used widely different word-list tasks. We aimed to learn whether several scoring systems applied to the same word-list test might be preferable to standard scoring for identifying MCI. We introduced a novel method for scoring serial position that is easy to score and takes into account the order in which words from a list are recalled. This novel scoring method was based on the assumption that any of the last three words in the list that are recalled first, second, or third are recalled from short-term memory. In all, four scoring methods were compared: standard raw score method, Buschke's RWS, Shankle's CA method, and a short-term memory (STM) method.
Method
Participants
Two sets of participants participated, an initial study and a replication study. All were community dwelling seniors who were recruited through advertisement and presentations at local retirement communities.
Study 1
The groups consisted of 21 participants who had diagnoses of mild cognitive impairment (MCI-1), 27 with mild Alzheimer's disease (AD), and 41 with intact cognition (Intact-1). Except for the Alzheimer patients, participants were cognitively intact when they enrolled in the Oregon Brain Aging Study (OBAS), a longitudinal study of aging in community-dwelling seniors (Howieson, et al., 1997). At entry they ranged in age from 65 to 104 years. All had Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) scores ≥ 24 and had no neurological diseases or brain trauma, depression, use of medicines that might impair cognition, or risk factors for vascular disease such as hypertension. The participants and their collateral informants reported that the participants were functioning normally in the community and none had sought professional attention for memory complaints. Participants who developed medical problems during the course of the study were not dropped, but those who developed a neurological diagnosis other than possible or probable AD were not included in these analyses. Neurological and neuropsychological examinations were conducted annually. Serial position scoring was initiated during the course of the longitudinal study. The first examination in which serial position was recorded was used for these analyses, by which time some participants cognitively intact at entry had developed MCI.
Participants with a diagnosis of MCI at this examination were rated as questionable dementia based on a Clinical Dementia Rating (CDR; Morris, 1993) score of 0.5 on one (n = 12) or two consecutive (n = 9) annual visits and no reversion to CDR = 0 in subsequent visits at the time of the data analysis. Ratings were based on an interview with the patient and a collateral source and a cognitive screening examination as part of the neurological examination or an MMSE < 24. The healthy controls also were enrolled in OBAS and had no CDRs > 0 or MMSEs < 24, even at visits subsequent to these data analyses. Participants with mild AD were from the Layton Aging & Alzheimer's Disease Center and met the National Institute of Neurological and Communicative Disorders and Stroke and AD and Related Disorders Association criteria for probable (n =20) or possible AD (n =7) (McKhann, et al., 1984) based on a consensus conference and had CDRs = 1 and MMSEs ≥ 20.
Study 2
Data were available from participants newly enrolled in a second, independent study of MCI and were used to test for replication of the serial position effects found in the first study. No Alzheimer patients participated in this study. All participants were age 70 or older, in average health, had a CDR <1 and a MMSE ≥ 22, a Geriatric Depression Score (Sheikh & Yesavage, 1986) ≤ 7/15, and were independently mobile. They were either cognitively intact or had a diagnosis of MCI based on a review of neurological and neuropsychological evaluations, interviews with collateral sources, and assessment of functional activities. Final diagnosis was based on a consensus conference using the Petersen MCI criteria (Petersen, 2004) that reviewed participant's performance on cognitive tests that included five cognitive domains: memory, executive function, attention/speed, language, and visuospatial constructions. Scores were judged to be impaired if they were at least 1.5 standard deviations below age-appropriate normative data from the Alzheimer's Disease Centers (Weintraub, et al., 2009) or in the case of WAIS-R Block Design, from our own Oregon Brain Aging data base. The memory criterion was performance on WMS-R Logical Memory II Story A (not word list recall). Functional independence was based on the Functional Activity Questionnaire (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982). Subjective memory complaint was not a criterion for MCI because it is less reliable in community samples than other criteria (Purser, Fillenbaum, & Wallace, 2006). None met diagnostic criteria for dementia. The final groups consisted of 31 participants with a MCI diagnosis (MCI-2) and 186 who were judged to be cognitively intact (Intact-2).
Procedure
All participants were given a neurological examination and the same battery of neuropsychological tests. Serial position was examined with the CERAD Word-List task (Morris et al, 1989), which consists of a list of 10 unrelated words read aloud by subjects on each of three trials with immediate recall after each trial. The words are in a different order on each trial, which allows for a comparison of serial position recall dissociated from learning from previous trials. We defined the primacy section of the list as the first 3 words and the recency as the last 3 words. After a short delay with an intervening cognitive activity, delayed recall of the list is elicited. There also is a yes/no recognition task, the data of which are not presented here. Two lists of equal level of difficulty were alternated for the MCI and Intact groups to minimize practice effects during the longitudinal study. No practice effects were observed with the Alzheimer group using only one list. Performance on the three acquisition trials and the delayed recall trial was recorded according to which words were recalled and in what order.
Data Analyses
Scoring Methods
(1) The standard (CERAD) scoring consists of totaling the number of words recalled on each of the acquisition trials. (2) The RWS method uses a gradient of scores in which the weighting is inversely related to the recency of presentation (Buschke, et al., 2006). Scores range from 10 for recall of the first word to 1 for recall of the last word. (3) The CA method also uses weighted scores, but uses a statistical program that maximizes the correlation between groups and recall (Shankle, et al., 2005). (4) The STM penalty scoring gives a reduced weight (1/2 point) to any of the last 3 words in the list recalled first, second, or third. The last three words were considered the recency portion (Capitani, Della Sala, Logie, & Spinnler, 1992). A full point is given to any of the other 7 words in the list recalled or any of the last 3 words recalled after at least 3 earlier words were recalled. This scoring is based on the assumption that recall is easiest for the last three words provided they are recalled before any other words (Tulving and Colota, 1970; Waugh and Norman, 1965).
Statistical methods
Demographic characteristics and conventional global and memory test scores for the subjects in Study 1 and 2 were evaluated using summary statistics (mean, SD, percentages) and compared between groups using two sample t-testing, non-parametric Wilcoxon rank sum testing, analysis of variance, analysis of covariance, and chi-square tests as appropriate. Group differences for Study 1 were examined using one-way analyses of covariance (ANCOVA), with age as the covariate. Significant omnibus effects were followed up with LSD posthoc tests for planned comparisons. ROC curves provided measures of a model's ability to discriminate between groups. We statistically compared the area under the ROC curves among each scoring technique using the nonparameteric approach of DeLong and colleagues (DeLong, DeLong, & Clarke-Pearson, 1988). All analyses were performed by using SAS version 9.2 software (SAS Institute, Inc, Cary, NC).
Results
Study 1
Group characteristics
The group characteristics are presented in Table 1a. The MCI-1 group is significantly older than the Intact-1 and AD groups. The groups do not differ in education, percent female, or socioeconomic status (SES) (Hollingshead, 1975). Corrected for age differences, the MCI-1 group's MMSE is intermediate between the Intact-1 group and the AD group.
Table 1a.
Characteristics of the groups expressed as means and standard deviations for Study 1.
| Intact-1 | MCI-1 | AD | p | Post hoc | |
|---|---|---|---|---|---|
| n = 41 | n =21 | n = 27 | |||
| Age, years | 79.9 ± 10.9 | 91.6 ± 6.9 | 79.7 ± 12.9 | < .0001 | MCI-1 vs. Intact-1, p < .0001 MCI-1 vs. AD, p < .0001 |
| Education, years | 16.1 ± 2.2 | 15.4 ± 2.1 | 15.7 ± 2.8 | 0.56 | N/A |
| Sex, % female | 56% | 43% | 59% | 0.49 | N/A |
| Race, % white | 100% | 100% | 100% | 1.0 | N/A |
| SES | 52.5 ± 8.5 | 49.8 ± 11.1 | 52.0 ± 10.7 | 0.58 | N/A |
| MMSE* | 29.2 ± 1.0 | 26.6 ± 2.7 | 23.3 ± 2.3 | < .0001 | MCI-1 vs. Intact-1, p < .0001 MCI-1 vs. AD, p < .0001 |
| CERAD Word List acquisition* | 22.5 ± 3.6 | 15.1 ± 3.3 | 12.8 ± 3.1 | < .0001 | MCI-1 vs. Intact-1, p < .0001 MCI-1 vs. AD, p = .0071 |
| CERAD Word List delayed recall* | 7.8 ± 1.6 | 3.9 ± 2.0 | 1.6 ± 1.7 | < .0001 | MCI-1 vs. Intact-1, p < .0001 MCI-1 vs. AD, p < .0001 |
Age-adjusted
Note. SES = Socioeconomic status; MMSE = Mini-Mental State Examination; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; Intact-1 = controls for Study 1; MCI-1 = mild cognitive impairment participants for Study 1; AD = Alzheimer's disease; N/A = Not applicable
Word-list learning
When corrected for age, the MCI-1 group recalled significantly fewer words during the three learning trials than the Intact-1 group and more words than Alzheimer group (F(3, 85) = 52.70, MSE = 589.76, p <.0001, Table 1a). These group differences were the same for the delayed recall scores (F(3, 85) = 80.34, MSE = 224.73, p < 0.0001).
Serial position curves
The serial position curves for each of the three learning trials are shown in Figure 1a. The first trial shows the typical serial position effect for the Intact-1 group and a comparatively reduced primacy effect for the MCI-1 and AD groups. This pattern also is seen on Trial 2. By Trial 3, all groups are recalling words throughout the list and the serial position effect is diminished.
Figure 1.
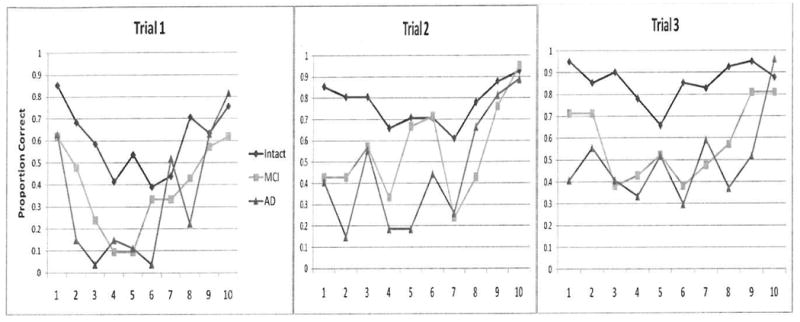
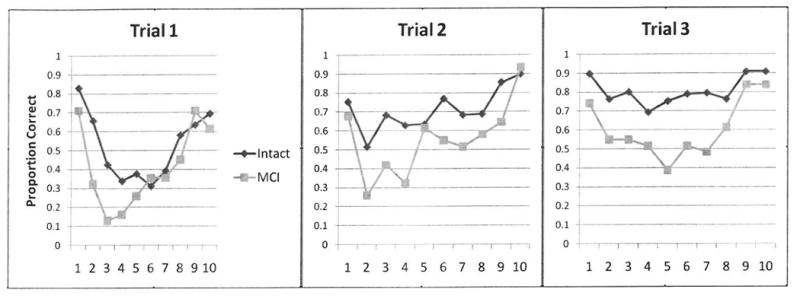
Figure 1a. Study 1 serial position curves for each trial.
Figure 1b. Study 2 Serial position curves for each trial.
The groups were compared for the total number of words recalled over three trials in the primacy position, defined as the first three words in the list, and the number of words recall in the recency position, the last three words. The Intact-1 group recalled the most words in the primacy position followed by the MCI-1 group (see Table 2a). As predicted the MCI-1 and AD groups recalled more words in the recency position than the primacy position and their recency scores did not differ from each other. Relative to the primacy effect, the recency effect (recency to primacy ratio) of the groups were AD > MCI-1 > Intact-1.
Table 2a.
Mean number of total words recalled in the primacy and recency positions over three trials and the Recency/Primacy ratio by groups in Study 1. Group comparisons are age adjusted.
| Intact-1 | MCI-1 | AD | p | Post hoc | |
|---|---|---|---|---|---|
| Primacy | 7.3 | 4.6 | 3.3 | <0.0001 | MCI-1 vs. Intact-1, p < .0001 MCI-1 vs. AD, p < .0077 |
| Recency | 7.4 | 6.0 | 5.9 | <0.0001 | MCI-1 vs. Intact-1, p = .0003 MCI-1 vs. AD, p = .85 |
| Recency/Primacy Ratio | 0.01 | 0.25 | 0.31 | <0.0001 | MCI-1 vs. Intact-1, p < .0258 MCI-1 vs. AD, p < .0144 |
Note. Recency/Primacy Ratio = Recency-Primacy/ Recency+Primacy
Study 2
Group characteristics
The group characteristics are presented in Table 1b. The Intact-2 group and the MCI-2 group were similar in terms of age, education, and socioeconomic status. The MCI-2 group had a larger percentage of males and non-white subjects. The Intact-2 group scored significantly higher on the MMSE.
Table 1b.
Group characteristics, means and standard deviations for Study 2.
| Intact-2 | MCI-2 | p | |||
|---|---|---|---|---|---|
| n = 186 | n = 31 | ||||
| Age, yrs | 83.5 ± 5.3 | 83.4 ± 5.6 | 0.89 | ||
| Education, yrs | 15.6 ± 2.5 | 14.8 ± 3.4 | 0.31 | ||
| Sex, % female | 77.4% | 48.4% | <0.001 | ||
| Race, % white, black, Asian | 84.4%, 13.4%, 2.2% | 64.5%, 22.6%, 12.9% | <0.01 | ||
| SES | 51.2 ± 10.8 | 46.4 ± 15.2 | 0.17 | ||
| MMSE | 28.6 ± 1.5 | 27.0 ± 2.0 | <0.0001 | ||
| CERAD Word List acquisition | 20.6 ± 3.8 | 16.4 ± 3.9 | <0.0001 | ||
| CERAD Word List delayed recall | 6.8 ± 1.9 | 4.9 ± 2.2 | <0.0001 |
Note. SES = Socioeconomic status; MMSE = Mini-Mental State Examination; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; Intact-2 = controls for Study 2; MCI-2 = mild cognitive impairment participants for Study 2
Word-list learning
Similar to Study 1, the MCI-2 group recalled significantly fewer words during the three learning trials and on delayed recall than the Intact-2 group.
Serial position curves
The Trial 1 serial position profile in Study 2 is similar to the profile in Study 1 in that both the Intact-2 and MCI-2 group differences are greatest in the primacy position (Figure 1b). Recall of the first word is similar, but the groups diverge on the other two primacy words. This difference is also seen on Trial 3 but less so Trial 2. The recency to primacy ratio is significantly greater for the MCI-2 group (Table 2b).
Table 2b.
Mean number of total words recalled in the primacy and recency positions over three trials and the Recency/Primacy ratio by groups in Study 2.
| Intact-2 | MCI-2 | p | |||
|---|---|---|---|---|---|
| Primacy | 6.3 | 4.4 | <0.0001 | ||
| Recency | 6.9 | 6.2 | 0.02 | ||
| Recency/Primacy Ratio | 0.05 | 0.19 | <0.001 |
Note. Recency/Primacy Ratio = Recency-Primacy/ Recency+Primacy
Recency/primacy ratios comparison
The percentages of subjects in each group that had reduced primacy as defined as a recency/primacy ratio of ≥ .10 are: Study 1, Intact 12%, MCI 62%, AD 74%: Study 2, Intact 33%, MCI 61%. Follow-up visits occurred for 53 of 62 Intact and MCI subjects in Study 1. Recency/primacy ratios below the cut-off number were obtained by 38 of the 53 (72%) and the other 15 (28%) had ratios above that. Of the 38, only 2 (5%) progressed to dementia whereas of the 15, 8 (53%) progressed. The dementia diagnoses were probable or possible Alzheimer disease except for two cases of mixed Alzheimer/vascular dementia. The groups that did and did not progress to dementia did not differ in gender or education but the group that progressed was older. Using a Cox Proportion Hazard model, the hazard ratio of those above the recency/primary ratio cut-off on progression to dementia was 12.4, p = 0.002. After adjusting for age, the hazard ratio was 5.0, p = 0.05. The survival curves showed that the group with ratios above the cut-off had a higher likelihood to progress to AD; log-rank test p < 0.0001.”
Scoring Methods Comparison
In Study 1, all scoring methods were good at distinguishing the MCI-1 and Intact-1 groups (see Table 3 for areas under the ROC curves) and no alternative scoring was statistically better than the standard scoring for total number of words recalled during Trials 1-3. In Study 2, again all scoring methods were equivalent in distinguishing the MCI-2 versus Intact-2 groups. However, the areas under the ROC curves (AUC) were less than obtained in Study 1, indicating more similarity in the performance of the groups.
Table 3.
Areas under the ROC Curve by scoring technique and p-value comparisons with standard scoring
| Intact vs. MCI: Study 1 | Intact vs. MCI: Study 2 | MCI vs. AD: Study 1 | |
|---|---|---|---|
| Acquisition | 0.94 reference curve |
0.78 reference curve |
0.73 reference curve |
| RWS | 0.92, p =0.24 | 0.80, p = 0.36 | 0.81, p = 0.01 |
| STM | 0.93, p = 0.11 | 0.79, p = 0.37 | 0.72, p = 0.42 |
| CA | 0.92, p = 0.29 | 0.81, p = 0.12 | 0.75, p = 0.65 |
Note. Acquisition = standard score trials 1 -3; RWS = retention weighted scoring; STM = short term memory penalty scoring; CA = correspondence analysis
Only the RWS scoring method was superior to the standard scoring of Trials 1-3 for distinguishing the MCI-1 and AD groups. Often in clinical practice the Delayed Recall score is the main measure used for diagnosis of MCI or AD. We also compared the three scoring methods (RWS, STM, CA) discriminative abilities to Delayed Recall score. None of the AUCs were significantly different than the Delayed Recall AUCs.
Discussion
In both studies, the MCI groups scored lower than their respective control groups for Acquisition and Delayed Recall on the CERAD Word List. As predicted, the MCI groups' serial positions compared to controls showed diminished primacy effects relative to recency effects. In Study 1, which included a group with mild AD, the reduced primacy effect of the MCI group was intermediate between the intact and AD groups.
Although the MCI groups had a depressed primacy effect, none of the alternative scoring methods was better than standard scoring for distinguishing MCI participants from controls, which was unexpected. STM scoring according to order of words recalled was not beneficial for differentiating the MCI groups from the intact groups. The two studies did not find a benefit of CA weighted scoring over standard scoring on the CERAD Word List. However, our methods were different from that of Shankle and colleagues ((Shankle, et al., 2009; Shankle, et al., 2005) who found a benefit when they totaled performance on the CERAD three acquisition trials plus the delayed recall trial. The delayed recall trial was not included in the present analyses because none of the words upon delay are recalled from immediate memory (recency). The primacy and recency position of words would be ambiguous as the word order was different on each of the three acquisition trials.
RWS was not superior to standard scoring for differentiating MCI from age-normal recall performance, but it was the best scoring method for distinguishing MCI and AD groups. The superiority of RWS over STM scoring is due to its increasing weighting of recall from items earlier in the list where the group differences are greatest. Compared to CA scoring, RWS is based on a simple theoretical assumption and is simple and fast to score, which may increase its accessibility and use in clinical settings. The CA method requires computer-based weighting, which may limit its use (Buschke, et al., 2006).
Our findings appear to differ from a study by Bennett e al. who studied MCI patients using the University of California-Repeatable Episodic Memory Test (USC-REMT), a 15-word list with words presented in a different order for each of three learning trials (Bennett, Golob, Parker, & Starr, 2006). Both neurologically intact and amnestic MCI groups recalled more items from the end of the University of California-Repeatable Episodic Memory Test 15-word list than the beginning or middle and there was no group by serial position interaction. The difference is probably best explained by the difference in level of difficulty between the studies. List length affects primacy but not recency (Carlesimo, Marfia, et al., 1996). They used a longer the list, which likely depressed the primacy recall of their intact comparison group.
Assuming that many if not most patients in our MCI groups had preclinical AD, the findings are consistent with data from Pepin and Eslinger in showing that the reduced primacy effect is a function of the level of dementia (Pepin & Eslinger, 1989). In their study mildly demented patients had the usual U-shaped curve, but with increasing severity of dementia the shape of the curve flattened with a reduced primacy effect and, eventually, a decreased recency effect as well. By contrast, Bayley et al found no difference between serial position curves between mild and very mild AD groups as both groups lacked a significant primacy effect but had a robust recency effect (Bayley, et al., 2000). Bayley and colleagues used one trial with a 16-word list while Pepin and Eslinger used three learning trials with a 9-word list. Carlesimo et al. showed that with enough repetitions of a list, in this case five trials with a 15-word list, Alzheimer patients also show a primacy effect (Carlesimo, Marfia, et al., 1996). Similarly, Gianotti et al found that only one-third of AD patients lacked a primacy effect when using five trials with a 15-word list (Gainotti, et al., 1998).
Serial position analyses appear to be useful in identifying persons with MCI. Although the Recency/Primacy ratio differed between MCI and Intact groups in both studies, comparison of the two studies showed that Study 1 data more closely matched predictions. In comparison with Study 1, the Study 2 MCI group's curve was more similar to that of the Intact group. Study 1 likely benefited from the longitudinal design that allowed for correction for age-related fluctuations in functioning. Participants who were labeled “intact” at the visit but subsequently converted to MCI were omitted from the Intact-1 group. In a similar vein, participants were eliminated from the MCI-1 group who were “questionable dementia” at the visit but subsequently converted to “intact.” By contrast, Study 2 used a cross-sectional design with inherent limitations. Large intraindividual variability in performance across tests is not uncommon in older adults (Binder, Iverson, & Brooks, 2009); (Salthouse, 2007), which makes group assignments based on cross-sectional neuropsychological test scores less than precise. The comparison of the serial position curves of Studies 1 and 2 suggests the possibility that the MCI group's performance in Study 2 likely contained low performing normals classified as MCI and who may revert to “intact” during follow-up.
The possibility that serial position curves may be useful in identifying persons at risk for AD is bolstered by the report by La Rue et al. that a group of middle aged asymptomatic persons at risk for AD by virtue of family history showed a slight, but statistically significant reduced primary effect compared to control subjects (La Rue, et al., 2008).
The shape of serial position curves is influenced by a number of factors and choosing the best word list task for serial position studies depends on matching characteristics of the individuals studied, such as age, with the task. In our study the high recall of the first words on the list on Trial 1 is likely due to the shortness of the list we used. If the list is over learned, such as on Trial 3 of our study, the serial position curves begin to flatten. Primacy also is affected by presentation rate, word frequency, and semantic similarity of items in the list (Capitani, et al., 1992).
It is possible that the serial position effect is most marked when word order is preserved from trial to trial. It is assumed that words in the primary position are preferentially stored in long term memory. If word order is the same from trial to trial, the opportunity for consolidating these words increases and the primacy effect should become stronger. When word order is randomized from trial to trial and a serial position curve is still retained, the strength of the serial position effect is demonstrated.
In summary, individuals with MCI, like AD patients, have a diminished primacy effect in recalling words from a list. The depressed primacy effect in seniors with MCI compared with controls likely stems from a diminished ability to consolidate new items into long term memory, a feature characteristic of Alzheimer disease. Assuming that a higher level of accuracy of diagnosing MCI may be achieved by considering the presence of multiple cognitive markers, the addition of serial position data from word-list learning tests might improve diagnosis. However, none of the alternative scoring systems that were examined with the CERAD Word List was better than standard scoring in distinguishing MCI patients from controls. Retention weighted scoring of this test improved the discrimination of MCI and AD groups and is indicative of the more severe episodic memory impairment in AD patients compared to those with MCI.
Acknowledgments
We wish to thank William R. Shankle for assistance in understanding and computing Correspondence Analysis. Supported by Department of Veterans Affairs, NIH P30 AG008017, M01 RR000334, UL1 RR024140, NIH P50 AT00066, NIH R01 AG024059, P30 AG024978, NIA K01 AG023014.
References
- Baddeley AD, Hitch G. The recency effect: implicit learning with explicit retrieval? Memory and Cognition. 1993;21:146–155. doi: 10.3758/bf03202726. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Salmon DP, Bondi MW, Bui BK, Olichney J, Delis DC, et al. Comparison of the serial position effect in very mild Alzheimer's disease, mild Alzheimer's disease, and amnesia associated with electroconvulsive therapy. Journal of the International Neuropsychological Society. 2000;6:290–298. doi: 10.1017/s1355617700633040. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Golob EJ, Parker ES, Starr A. Memory evaluation in mild cognitive impairment using recall and recognition tests. Journal of Clinical and Experimental Neuropsychology. 2006;28:1408–1422. doi: 10.1080/13803390500409583. [DOI] [PubMed] [Google Scholar]
- Binder LM, Iverson GL, Brooks BL. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Archive of Clinical Neuropsychology. 2009;24:31–46. doi: 10.1093/arclin/acn001. [DOI] [PubMed] [Google Scholar]
- Buschke H, Sliwinski MJ, Kuslansky G, Katz M, Verghese J, Lipton RB. Retention weighted recall improves discrimination of Alzheimer's disease. Journal of the International Neuropsychological Society. 2006;12:436–440. doi: 10.1017/s135561770606053x. [DOI] [PubMed] [Google Scholar]
- Capitani E, Della Sala S, Logie RH, Spinnler H. Recency, primacy, and memory: reappraising and standardising the serial position curve. Cortex. 1992;28:315–342. doi: 10.1016/s0010-9452(13)80143-8. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Fadda L, Sabbadini M, Caltagirone C. Recency effect in Alzheimer's disease: a reappraisal. Quarterly Journal of Experimental Psychology A, Human Experimental Psychology. 1996;49:315–325. doi: 10.1080/713755622. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Marfia GA, Loasses A, Caltagirone C. Recency effect in anterograde amnesia: evidence for distinct memory stores underlying enhanced retrieval of terminal items in immediate and delayed recall paradigms. Neuropsychologia. 1996;34:177–184. doi: 10.1016/0028-3932(95)00100-x. [DOI] [PubMed] [Google Scholar]
- Deese J, Kaufman RA. Serial effects in recall of unorganized and sequentially organized verbal material. Journal of Experimental Psychology. 1957;54:180–187. doi: 10.1037/h0040536. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Foldi NS, Brickman AM, Schaefer LA, Knutelska ME. Distinct serial position profiles and neuropsychological measures differentiate late life depression from normal aging and Alzheimer's disease. Psychiatry Research. 2003;120:71–84. doi: 10.1016/s0165-1781(03)00163-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Marra C, Villa G, Parlato V, Chiarotti F. Sensitivity and specificity of some neuropsychological markers of Alzheimer dementia. Alzheimer Disease and Associated Disorders. 1998;12:152–162. doi: 10.1097/00002093-199809000-00006. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Wyler A, Davies K, Christeson J, Moran M, et al. The effects of human hippocampal resection on the serial position curve. Cortex. 1996;32:323–334. doi: 10.1016/s0010-9452(96)80054-2. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Position. New Haven, CT: Author; 1975. [Google Scholar]
- Howieson DB, Dame A, Camicioli R, Sexton G, Payami H, Kaye JA. Cognitive markers preceding Alzheimer's dementia in the healthy oldest old. Journal of the American Geriatrics Society. 1997;45:584–589. doi: 10.1111/j.1532-5415.1997.tb03091.x. [DOI] [PubMed] [Google Scholar]
- La Rue A, Hermann B, Jones JE, Johnson S, Asthana S, Sager MA. Effect of parental family history of Alzheimer's disease on serial position profiles. Alzheimers Dementia. 2008;4:285–290. doi: 10.1016/j.jalz.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris J. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Pepin EP, Eslinger PJ. Verbal memory decline in Alzheimer's disease: a multiple-processes deficit. Neurology. 1989;39:1477–1482. doi: 10.1212/wnl.39.11.1477. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. Journal of the American Geriatrics Society. 2006;54:335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Implications of within-person variability in cognitive and neuropsychological functioning for the interpretation of change. Neuropsychology. 2007;21:401–411. doi: 10.1037/0894-4105.21.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankle WR, Mangrola T, Chan T, Hara J. Development and validation of the Memory Performance Index: reducing measurement error in recall tests. Alzheimers Dementia. 2009;5:295–306. doi: 10.1016/j.jalz.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Shankle WR, Romney AK, Hara J, Fortier D, Dick MB, Chen JM, et al. Methods to improve the detection of mild cognitive impairment. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4919–4924. doi: 10.1073/pnas.0501157102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JL, Yesavage J. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York, NY: Haworth Press; 1986. pp. 165–173. [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheiimer's Disease Centers' Uniform Data Set (UDS): The Neuropsychologic Test Battery. Alzheimer Disease and Associated Disorders. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]


