Abstract
Angiotensin II (AngII) and AngII type-1 receptors (AT1r) have been implicated in the pathogenesis of hypertension and ischemic stroke. The objectives of this study was to determine if/how chronic AngII administration affects blood-brain barrier (BBB) function and blood cell adhesion in the cerebral microvasculature. AngII-loaded osmotic pumps were implanted in wild type (WT) and mutant mice. Leukocyte and platelet adhesion were monitored in cerebral venules by intravital microscopy and BBB permeability detected by Evans blue leakage. AngII(2 wk) infusion increased blood pressure in WT mice. This was accompanied by an increased BBB permeability and a high density of adherent leukocytes and platelets. AT1r (on the vessel wall, but not on blood cells) was largely responsible for the microvascular responses to AngII. Immunodeficient(Rag-1−/−) mice exhibited blunted blood cell recruitment responses without a change in BBB permeability. A similar protection pattern was noted in RANTES−/− and P-selectin−/− mice, with bone marrow chimeras (blood cell deficiency only) yielding responses comparable to the respective knockouts. These findings implicate AT1r in the microvascular dysfunction associated with AngII-induced hypertension and suggest that immune cells and blood cell-associated RANTES and P-selectin contribute to the blood cell recruitment, but not the BBB failure, elicited by AngII.
Keywords: hypertension, stroke, inflammation, leukocyte-endothelial cell adhesion
Introduction
Hypertension is a major risk factor for ischemic stroke and other cardiovascular diseases. Animal studies and clinical trials have revealed a role for angiotensin II and the AT1 receptor (AT1r) in the pathogenesis of ischemic stroke.1–4 Some studies suggest that the deleterious effects of AngII-mediated activation of AT1r extend beyond vasoconstriction and blood pressure elevation to include oxidative stress, activation of the immune system, and altered hemostasis.5,6 It has been recently demonstrated that T-lymphocytes, cytokines (TNF-α), and chemokines (RANTES) contribute to the hypertension and vasomotor dysfunction that results from chronic AngII infusion in mice.5 A similar involvement of T-cells and cytokines has been demonstrated in murine models of ischemic stroke.7 The involvement of AngII and AT1r in ischemic stroke is supported by studies describing exaggerated brain injury and inflammatory responses to ischemia/reperfusion (I/R)in mutant mice that overexpress renin and angiotensinogen1,8, and in studies that demonstrate an attenuated ischemic brain injury response in WT mice treated with an AT1r antagonist and in AT1r-deficient mice2,4,6,8. Collectively, the literature suggests that elevated AngII levels render the brain susceptible to ischemic tissue injury via a mechanism that is dependent on AT1r and immune system activation.
While the large body of evidence implicating AngII and AT1r in stroke pathogenesis suggests that the cerebral microvasculature may be particularly vulnerable to the hypertensive and immune consequences of AngII/AT1r system activation, relatively little is known about the direct actions of AngII on the brain microcirculation and whether immune system activation contributes to the cerebral microvascular dysfunction that accompanies the hypertension elicited by chronic infusion of AngII. Previous studies on the brain and other vascular beds have shown that acute exposure (≤ 24 hrs) to elevated AngII levels promotes the adhesion of leukocytes and platelets in postcapillary venules via an AT1r-dependent, P-selectin mediated mechanism8, 9, 10, 14. Rapid increases in blood pressure induced by AngII administration are also associated with blood brain barrier failure and brain edema, which can be prevented by prior treatment with an AT1r antagonist12, 13. While the AngII-induced barrier failure is often attributed to mechanical stresses on the endothelial cells resulting from the increased pressure, studies on monolayers of cultured cerebral microvascular endothelial cells suggest that acute (≤ 6 hrs) AngII exposure increases BBB permeability via a direct, AT1r-dependent action on endothelial cells.11 Whether a slower, more prolonged elevation in blood pressure induced by chronic AngII administration produces similar changes in BBB permeability and if the resultant barrier failure is dependent on immune system activation remain unclear. Hence, the overall objectives of this study were to determine if/how chronic AngII administration affects blood-brain barrier (BBB) function and the recruitment leukocytes and platelets in cerebral microvasculature, and to assess the contribution of immune cells (T-lymphocytes) and different inflammatory mechanisms to the AngII-induced cerebral microvascular dysfunction. The relative contributions of blood cell- vs endothelial cell-associated AT1r, RANTES, and P-selectin to the AngII responses were assessed using mutant mice and bone marrow chimeras generated from these mutants. Our findings implicate blood cell-associated AT1r in the leukocyte and platelet recruitment and vessel wall-associated AT1r in the BBB failure elicited by chronic AngII administration. T-lymphocytes, RANTES, and P-selectin are also implicated in the blood cell recruitment, but not the BBB dysfunction, associated with prolonged AngII infusion.
Methods
Animals
Wild type mice (WT), lymphocyte-deficient Rag-1−/− mice, angiotensin II type 1a-deficient (AT1r−/−) mice, P-selectin deficient (P-sel−/−) mice, and RANTES (CCL5)-deficient (RANTES−/−) mice, all on a C57Bl/6 background, were obtained from Jackson Laboratories (Bar Harbor, Me). A breeding colony was established for production of AT1r−/− and RANTES−/− mice in the animal resource facility of the Louisiana State University Health Sciences Center, Shreveport. The experimental procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), and were in compliance with the guidelines of the National Institutes of Health.
Bone marrow chimeras
Bone marrow (BM) cells, collected from the femurs and tibias of donor mice (WT, P-sel−/−, RANTES−/−, or AT1r−/−), were injected (2 × 106 BM cells) via the femoral vein into recipient mice (congenic WT with same phenotype as C57BL/6J mice; B6.SJLPtprcaPep3b/BoyJ), following total-body irradiation sufficient to eliminate the recipient’s blood cells. The BM chimeras were housed in autoclaved cages, with 0.2% neomycin added to drinking water for the first 2 weeks. After 6~8weeks, reconstitution of BM cells was verified using flow cytometry by testing for the % blood leukocytes positive for CD45.1 (recipient isoform of CD45) versus CD45.2 (donor isoform of CD45). Successful BM reconstitution was set as >90% of the CD45.2-positive population in total leukocytes as previously described. Three BM chimeras were produced, i.e., WT recipients receiving BM from either RANTES−/− (RANTES−/− → WT), P-sel−/− (P-sel−/− → WT) or AT1r−/− (AT1r−/− → WT) donor mice, as previously described.15–17 These chimeras yielded mice with circulating blood cells that are deficient in the targeted protein (either RANTES, AT1r, or P-sel) and vascular and extravascular cells with a wild type phenotype, i.e., that express normal levels of the same proteins.
Angiotensin II infusion
Saline or AngII was infused over 14 days using micro-osmotic pumps (Alzet, Cupertino, CA, model 1002) which were implanted subcutaneously in the intrascapular region of isofluorane anesthetized mice. Sterile procedures were used and a topical antibiotic (Neosporin) was applied to prevent post-operative infection at the site of implantation. The pumps in the control group were loaded with only the saline vehicle, while the experimental groups received AngII loaded pumps that delivered the peptide at a rate of 2 ug/kg/min. While most mice were placed on normal chow, a limited number of animals were placed on a high salt diet (4.0% NaCl, Harlan Teklad) to produce a larger increase in blood pressure. Blood pressure determinations were obtained in conscious mice via a tapered femoral artery catheter implanted (under isofluorane anesthesia) 3 hrs prior to data collection via a pressure transducer coupled to a computer system.
Intravital video microscopy
After obtaining blood pressure measurements in conscience mice implanted with either an AngII- or saline-loaded pump, the mice were prepared for intravital microscopic examination of the cerebral microvasculature. The mice were anesthetized with intraperitonealketamine (100mg/kg) and xylazine (10mg/kg), a tracheotomy was performed, and the animals were placed on a ventilator. The femoral vein was cannulated for administration of platelets and rhodamine-6G. Core temperature was maintained at 36°C using a thermistor-controlled heating pad and lamp. As previously described, a craniectomy (1 mm posterior, 4 mm lateral from the bregma) was performed, the exposed brain tissue was soaked with artificial cerebrospinal fluid, and a glass coverslip placed over the cranial window. An upright fluorescent microscope with a 3CCD video camera system was used to randomly select 200 μm segments of pialvenules (20–70 μm diameter) for study. 100 × 106 platelets from donor mice were labeled ex vivo with carboxyfluoresceindiacetatesuccinimudylester.6 Thegreen fluorescent platelets were administered to recipient mice followed by continuous infusion of 0.02% rhodamine 6G, which labeled leukocytes red. Adherent leukocytes and platelets were defined as cells remaining stationary in venules for 30 and 2 seconds, respectively. Cell adhesion data was expressed as number of cells per mm2 of venular surface, calculated from venular diameter and length, assuming cylindrical geometry.
Blood-brain barrier (BBB) dysfunction
A 2% solution of Evans blue (EB; Sigma-Aldrich, MO) was injected (4ml/kg) intravenously. 24 hours later, the blood was obtained for plasma collection and the brain was sampled after transcardial perfusion with 0.9% normal saline (PBS; 100mmHg, 5 minutes). The brain and plasma samples were homogenized, sonicated and centrifuged in 50% trichloroacetic acid (Sigma-Aldrich, MO). The brain supernatant was diluted with ethanol and the concentrations of EB in brain tissue and plasma were measured using a fluorescence spectrophotometer (FLUOstar Optima, BMG LABTECH, Inc., NC). BBB permeability was determined by dividing tissue EB concentration (mg/g brain weight) by the plasma concentration (mg/g).16
The cerebral microvascular responses to chronic AngII infusion were evaluated using the following experimental groups placed on a normal diet: 1) WT mice implanted with a saline-loaded pump (n=43), 2) WT mice with an AngII-loaded pump (n=32), 3) WT mice with AngII pump treated with the AT1r antagonist losartan (25 mg/kg/day) in drinking water for 7 days prior to the experiment (n=13), 4) AT1r−/− mice with an AngII pump (n=10), 5) AT1r−/−→ WT chimeras with an AngII pump (n=22), 6) Rag-1−/− mice with an AngII pump (n=23), 7) RANTES−/− mice with an AngII pump (n=12), 8) RANTES−/− → WT chimeras with an AngII pump (n=15), 9) P-sel−/− mice with an AngII pump (n=17), and 10) P-sel−/− → WT chimeras with an AngII pump (n=16). An additional series of experiments was performed on mice placed on a high salt diet beginning 4 days prior to pump implantation and continued for the entire 2 wk pump implantation period. These include: 11) WT mice with a saline pump (n=18), 12) WT mice with an AngII pump (n=32), 13) losartan-treated WT mice with an AngII pump (n=14), 14) AT1r−/− with an AngII pump (n=12), and 15) AT1r−/− → WT chimeras with an AngII pump (n=26). The number of mice indicated for each group reflects the animals used to collect blood pressure and blood cell adhesion data, which was typically collected in the same animals, and BBB permeability measurements, which were performed in a separate set of animals.
Statistical analyses
All data are expressed as mean ± SE. Statistical difference between groups was determined by ANOVA, using the Fisher’s post-hoc test. Statistical significance was set as p<0.05.
Results
Cerebral microvascular responses to chronic AngII infusion
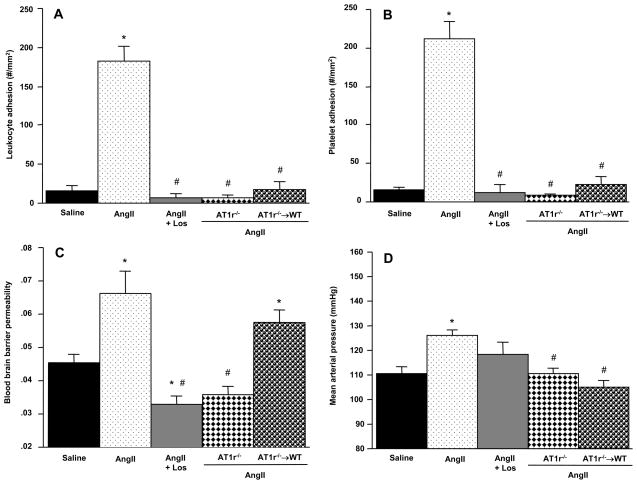
Chronic implantation of an AngII-loaded pump yielded values for blood cell adhesion, BBB permeability, and mean arterial blood pressure that were significantly different than those detected in mice with saline-loaded pumps (Figure 1). In mice with saline-loaded pumps, 16.5 ± 5.3 adherent leukocytes per mm2 were detected in cerebral venules. This value increased (p<.0001) to 182.5 ± 19.2 in mice with AngII-loaded pumps (panel A). A similar highly significant (p<.0001) difference was noted for the platelet adhesion response (panel B), with saline-loaded pumps yielding 14.9 ± 3.8 cells/mm2, compared to 211.8 ± 21.7 cells/mm2 in mice receiving AngII. Of the total number of platelets adhering in cerebral venules of mice with AngII pumps, only 14.1 ± 2.3% were directly bound to endothelial cells and the remaining 85.9% were attached to adherent leukocytes (data not shown). Neither leukocytes nor platelets were observed to adhere in cerebral arterioles of mice with a saline-or AngII-loaded pump. BBB permeability (panel C) in control (saline pump) mice was 0.045 ± 0.002 and this was also significantly (p<.001) increased by Ang II (0.066 ± 0.007). Mean arterial pressure in the mice with saline-loaded pumps was 110.7 ± 2.6 mmHg, and this was increased (p<.001) to 126.0 ± 2.3 mmHg by chronic AngII infusion (panel D).
Figure 1.
Effects of chronic angiotensin II administration (2.0ug/kg/min, 14 days) on the adhesion of leukocytes (panel A) and platelets (panel B) in cerebral venules, blood brain barrier (BBB) permeability, and mean arterial pressure in wild type (WT) and AT1r-deficient mice, and AT1r−/− bone marrow chimeras (AT1R−/− → WT). Saline = WT mice with implanted saline pump;AngII + Los = losartan-treated WT mice with AngII pump. * denotes significance relative to WT mice with saline pumps, # denotes significance relative to WT mice with AngII pumps.
Role of AT1-receptors in AngII-mediated cerebral microvascular responses
The contribution of AT1r to the AngII-mediated responses was assessed using WT mice treated with losartan (AT1r antagonist), AT1r−/− mice, and AT1r−/− → WT chimeras (Figure 1). Although WT mice treated with losartan did not exhibit a significantly reduced (118.1 ± 5.2 mmHg, p=0.12) blood pressure response (compared to WT-AngII mice), the AngII response was significantly reduced in AT1r−/− mice (110.8 ± 1.8 mmHg, p< 0.001). The AT1r−/− → WT chimeras exhibited reductions in blood cell adhesion and arterial pressure (104.8 ± 2.9 mmHg, p<0.001) during AngII infusion that were comparable to the responses seen in AT1r−/− mice, suggesting that blood cell-associated AT1r contribute to these responses. However, the AT1r−/− → WT chimeras did not show a protective response for the AngII-induced BBB failure, suggesting that vessel wall-, rather than blood cell-, associated AT1r activation mediates this response.
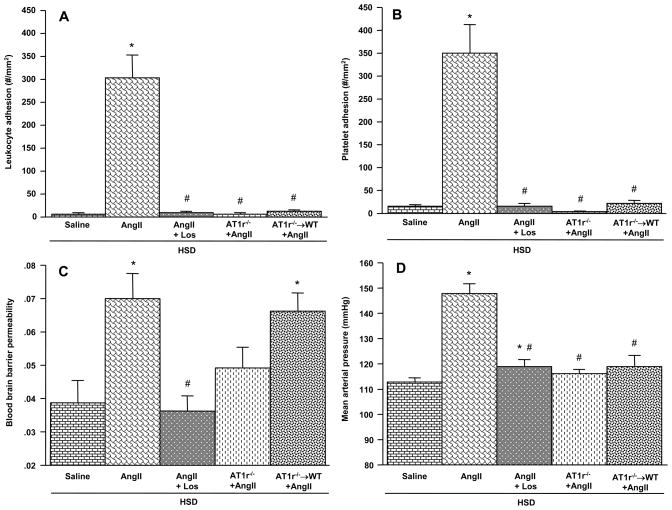
We also evaluated the cerebral microvascular responses to chronic AngII infusion in mice that were placed on a high salt diet (HSD) in order to produce a larger elevation in arterial blood pressure (Figure 2). Compared to the responses noted in WT mice placed on a normal diet (Figure 1), AngII produced a significantly larger increase in arterial blood pressure (148.0 ± 3.9 mmHg, p<0.001), leukocyte adhesion (304.2 ± 50.1 cells/mm2, p=0.026), and platelet adhesion (350.6 ± 61.8 cells/mm2, p=0.035), while not altering the BBB permeability response (0.07 ± .007, p=0.72) in WT mice on HSD. The contributions of AT1r to the AngII-induced responses in HSD mice were similar to those noted in mice on a normal diet with some exceptions. Most notably, the protection against BBB barrier failure (seen in mice on a normal diet) was not detected in AT1r−/− mice on HSD. Furthermore, losartan-treated WT mice, AT1r−/− mice, and AT1r−/− → WT chimeras on HSD all exhibited more dramatic protection against AngII hypertension than their normal diet counterparts.
Figure 2.
Responses to chronic AngII infusion in mice placed on a high salt diet (HSD). The effects of AngII on the adhesion of leukocytes (panel A) and platelets (panel B) in cerebral venules, blood brain barrier (BBB) permeability, and mean arterial pressure are shown for wild type (WT) and AT1r-deficient mice, and AT1r−/− bone marrow chimeras (AT1R−/− → WT) implanted with an AngII-loaded pump. Saline = WT mice with implanted saline pump;AngII + Los = losartan-treated WT mice with AngII pump. * denotes significance relative to WT mice with saline pumps, # denotes significance relative to WT mice with AngII pumps.
Contribution of lymphocytes to AngII-induced microvascular dysfunction
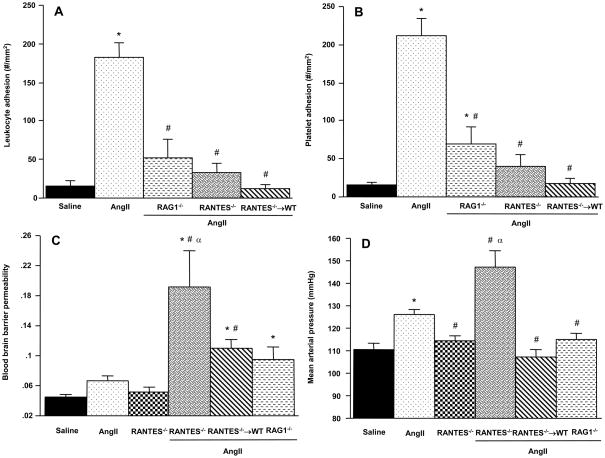
T-lymphocytes have been recently implicated in the genesis of AngII-induced hypertension.5 Our findings on the blood pressure responses of lymphocyte-deficient Rag-1−/− mice (on a normal diet) to chronic AngII infusion support a role for T-cells (Figure 3, panel D) since blood pressure was reduced to 114.9 ± 2.7 mmHg (p<0.01). The Rag-1−/− mice with AngII pumps also exhibited significantly reduced numbers of adherent leukocytes (panel A) and platelets (panel B) in cerebral venules, compared to WT mice with AngII pumps. The BBB response to AngII in Rag-1−/− was not reduced. Instead, a higher BBB permeability (0.096 ± 0.02) was detected in Rag-1−/−, but this did not achieve statistical significance (p=0.07).
Figure 3.
Role of lymphocytes and RANTES in the cerebral microvascular responses to chronic AngII infusion. The effects of AngII on the adhesion of leukocytes (panel A) and platelets (panel B) in cerebral venules, blood brain barrier (BBB) permeability, and mean arterial pressure are shown for wild type (WT), lymphocyte-deficient (Rag-1−/−) and RANTES-deficient mice, and RANTES−/− bone marrow chimeras (RANTES−/− → WT) implanted with an AngII-loaded pump. Data are also shown for BBB permeability and arterial pressure in RANTES−/− mice not exposed to AngII. Saline = WT mice with implanted saline pump. * denotes significance relative to WT mice with saline pumps, # denotes significance relative to WT mice with AngII pumps. α indicates significance relative to RANTES−/− (control)
Role of RANTES in the AngII mediated microvascular responses
RANTES-deficient and RANTES−/− → WT were used to assess the contribution of this chemokine to the AngII-induced responses (Figure 3). RANTES−/− mice were protected against the leukocyte (panel A) and platelet (panel B) recruitment responses normally elicited by chronic AngII infusion. However, these mice also exhibited highly exaggerated BBB permeability (191 ± 0.05, p<0.01) and arterial blood pressure (147.4 ± 7.0 mmHg, p=0.002) responses to AngII. RANTES−/− mice not exposed to AngII exhibited a basal BBB permeability and arterial pressure that was no different than WT-saline mice. However, RANTES−/− mice do exhibit significant alterations in blood leukocyte and platelet counts. Blood lymphocyte counts were increased in RANTES−/− from 3158 ± 181 (WT) to 6141 ± 389 per uL blood (RANTES−/−). Similar increases were noted for neutrophils (741 ± 66 vs 4167 ± 270 per uL) and platelets (545,000 ± 17,842 vs 959,167 ± 46,177).
While the blood cell adhesion responses to AngII in RANTES−/− → WT chimeras were comparable to those noted in RANTES−/− mice, the BBB permeability and blood pressure changes in RANTES−/− → WT mice did not parallel those of RANTES−/− mice. BBB permeability in RANTES−/− → WT mice was significantly higher (0.111 ± 0.011, p<0.01) than the response detected in WT mice with an AngII pump, but lower than that measured in RANTES−/−. Blood pressure in RANTES−/− → WT mice with an AngII pump (107.3 ± 2.9 mmHg, p=0.42) did not differ from the pressure detected in WT mice with a saline pump.
P-selectin mediated responses during chronic AngII infusion
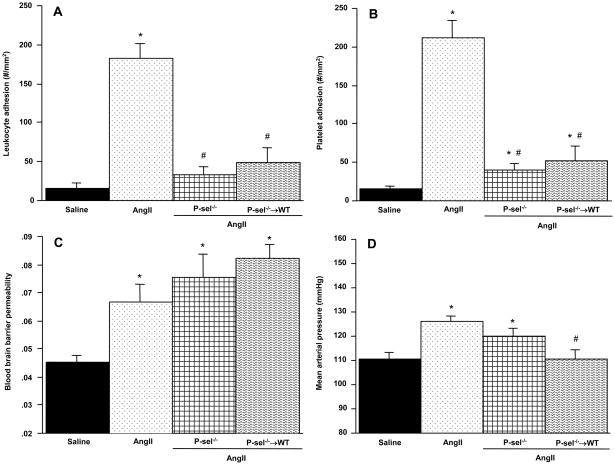
Figure 4 summarizes the responses to chronic AngII infusion in P-selectin deficient and P-sel−/− → WT chimeras. Compared to WT mice with an AngII pump, both P-sel−/− and P-sel−/− → WT mice exhibited attenuated leukocyte and platelet adhesion responses. The BBB permeability response to AngII was not altered in either P-sel−/− or P-sel−/− → WT mice. Although the blood pressure response to AngII was not significantly attenuated in P-sel−/− mice, a significant reduction (110.3 ± 3.9 mmHg, p<0.002) was noted in P-sel−/− → WT chimeras.
Figure 4.
Role of P-selectin in AngII-mediated cerebral microvascular responses. The effects of AngII on the adhesion of leukocytes (panel A) and platelets (panel B) in cerebral venules, blood brain barrier (BBB) permeability, and mean arterial pressure are shown for wild type (WT), and P-selectin-deficient (P-sel−/−) mice, and P-sel−/− bone marrow chimeras (P-sel−/− → WT) implanted with an AngII-loaded pump. Saline = WT mice with implanted saline pump. * denotes significance relative to WT mice with saline pumps, # denotes significance relative to WT mice with AngII pumps.
Discussion
Although the renin-angiotensin system (RAS) has been implicated in the inflammatory and thrombogenic responses associated with cardiovascular diseases and risk factors, relatively little is known about the direct actions and consequences of chronically elevated AngII levels in different regional vascular beds. Given the well-established role of the RAS in ischemic stroke1–4, we examined the actions of chronic AngII infusion on the cerebral microvasculature. The results of our study demonstrate that AngII induces an inflammatory and prothrombogenic phenotype in the cerebral microcirculation that is accompanied by impaired BBB function. Our findings support the involvement of AT1r receptors in the AngII-mediated microvascular responses and implicate a role for immune cells, RANTES and P-selectin in the blood cell recruitment responses. These findings may explain why angiotensin II mediated hypertension appears to be associated with an increased incidence and severity of ischemic stroke3,20.
As expected from the literature, chronic AngII infusion in WT mice produced significant hypertension that is mediated by AT1r and this AT1r-dependent blood pressure response was exacerbated by placing the animals on a high salt diet21. Our observation that lymphocyte deficient Rag-1−/− mice exhibit a blunted blood pressure response to AngII is consistent with a recent report by Guzik et al that described a similar attenuation in AngII-induced hypertension in Rag-1−/− mice5. They also demonstrated, in adoptive transfer experiments, that AT1r on T-cells plays a critical role in mediating the blood pressure response. Our observation that AT1r−/− → WT chimeras also exhibit a blunted AngII-induced hypertension response is consistent with a role for lymphocyte-associated AT1r. However, our results with the AT1r−/− chimeras do not agree with the recent results of Crowley et al22 who observed a slightly augmented hypertensive response to AngII infusion over 21 days in similar bone marrow chimeras. An explanation for the discrepancy between the two studies is not readily apparent but it may relate to the differences in AngII dose administered and/or the uninephrectomy that accompanied the AngII infusion in the Crowley study. A novel finding in our study is that AngII-induced hypertension is greatly exaggerated in RANTES−/− mice while RANTES−/− → WT chimeras are somewhat protected against the AngII-induced blood pressure response. Several studies have implicated RANTES in AngII-mediated actions on the vasculature.5, 18, 19 Acute administration of AngII has been shown to produce a significant elevation in plasma RANTES concentration.23 Angiotensin II has also been shown to increase the expression of RANTES (CCL5) in rat glomerular endothelial cells and the renal cortex.18 Furthermore, it has been recently proposed that RANTES downregulates Ang II-induced hypertensive activity via an action on vascular smooth muscle.19
Acute administration (either i.p. or topical) of AngII elicits the adhesion of leukocytes in rat mesenteric venules.10, 23 A combination of AT1- (losartan) and AT2-(PD123, 319) receptor antagonists, while neither agent alone, completely blocked this acute response to AngII, as did a P-selectin blocking antibody.10 In our study, WT mice treated with losartan as well as AT1r-deficient mice were completely protected against the leukocyte adhesion response elicited by AngII. Our observation that AT1r−/− → WT chimeras exhibited the same level of protection as AT1r−/− mice suggests that engagement of AngII with AT1r expressed on circulating cells, rather than on the vessel wall, underlies the leukocyte response in cerebral venules during chronic AngII administration. This is consistent with the involvement of blood cell-associated AT1r in mediating the enhanced recruitment of leukocytes in postcapillary venules of hypercholesterolemic mice.15 As reported for acute AngII exposure10, we also observed that P-selectin deficiency largely abolished the recruitment of adherent leukocytes induced by AngII. However, we noted a similar level of protection in P-sel−/− → WT mice, suggesting that platelet-associated P-selectin contributes to the leukocyte recruitment. An involvement of platelet P-selectinin AngII-induced leukocyte adhesion is consistent with human studies demonstrating that infusion of AngII results in an increased expression of P-selectin on platelets.24 Platelet are also a major source of RANTES, a chemokine that is known to promote leukocyte-endothelial cell adhesion.16, 23, 25 Our study implicates RANTES in the AngII-induced leukocyte recruitment response. The observation that RANTES−/− → WT chimeras offer similar protection against leukocyte adhesion as RANTES−/− mice supports the view that platelets are the likely source of the RANTES in our model.
The pattern of platelet adhesion responses to chronic AngII infusion closely parallels the changes noted for leukocyte adhesion in all experimental groups. This likely reflects the fact that most (>85%) of the platelets that accumulated on the walls of cerebral microvessels were attached to adherent leukocytes. The pathophysiological relevance of the platelet-leukocyte adhesion in inflamed microvessels remains unclear. However, there is evidence indicating that the attachment of platelets to leukocytes can enhance the inflammatory potential of the latter. For example, arachidonic acid released by platelets can be used by neutrophils via transcellular metabolic reactions to produce increased quantities of inflammatory leukotrienes.26 The attachment of activated platelets to neutrophils also enables the latter to produce larger quantities of superoxide and PAF than either cell is capable of producing alone., 27, 28 The dependency of both leukocyte and platelet adhesion on platelet-associated P-selectin during chronic AngII administration may reflect the fact that platelet-leukocyte adhesion often involves an interaction between platelet P-selectin with PSGL-1 on leukocytes. Alternatively, it may suggest that the small percentage of platelets that directly attach to cerebral venules create a P-selectin-rich platform onto which leukocytes can bind via constitutively expressed PSGL-1.29, 30
Acute changes in blood pressure can lead to blood-brain barrier dysfunction and a condition known as hypertensive encephalopathy.11–13 The mechanisms that underlie the BBB failure that accompanies hypertension remain undefined and controversial, with some studies supporting a role for mechanical stresses related to the increased pressure while others attribute the response to receptor-mediated signaling pathways11,31. Our study reveals that chronic AngII infusion leads to a significant increase in BBB permeability that is abolished in AT1r−/− mice, but not in AT1r−/− → WT chimeras, suggesting that engagement of AngII with endothelial cell AT1r largely accounts for the BBB failure. This observation is consistent with a recent in vitro study that examined the responses of cerebral microvascular endothelial cell (MEC) monolayers to AngII.11 It was noted that AngII elicits increases in both paracellular and transcellular permeability, and that the increased monolayer permeability was prevented by AT1r, but not AT2r, blockade. Our in vivo studies also tend to rule out a major role for leukocyte and platelet adhesion in the AngII-induced BBB dysfunction, since inhibition of P-selectin mediated blood cell recruitment did not alter the BBB permeability response to AngII. Furthermore, we did not detect a clear correlation between the blood pressure response to AngII and BBB permeability in the different experimental groups. For example, placing the AngII pump mice on a high salt diet produced a more profound increase in blood pressure but this was not associated with a larger increase in BBB permeability. Moreover, while the AT1r−/− → WT chimeras had a lower blood pressure during AngII infusion, BBB permeability remained at the level detected in WT mice with an AngII pump.
In conclusion, the results of this study indicate that the chronic AngII infusion affects the cerebral microcirculation by promoting the recruitment of leukocytes and platelets in postcapillary venules, and by increasing blood-brain barrier permeability. The blood cell recruitment response and increased blood pressure elicited by AngII involves blood cell-associated AT1r, while vessel wall-associated AT1r accounts for the BBB failure. A clear link between the BBB permeability response and either blood pressure or blood cell recruitment could not be demonstrated. These findings may bear on the higher incidence and greater severity of ischemic stroke in human hypertension.
Acknowledgments
Supported by a grant from the National Heart Lung and Blood Institute (R01 HL26441-29)
References
- 1.Chen S, Li G, Zhang W, Wang J, Sigmund CD, Olson JE, Chen Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1526–31. doi: 10.1152/ajpregu.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhorn T, Doerfler A, Heusch G, Schulz R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoAreductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neuro sci Lett. 2006;406:92–96. doi: 10.1016/j.neulet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen SE, Lyle PA, Kizer JR, Dahlof B, Devereux RB, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn SM, Harris KE, Wedel H. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J Clin Hypertens (Greenwich) 2005;7:152–158. doi: 10.1111/j.1524-6175.2005.04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- 5.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–15. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–12. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 8.Walther T, Olah L, Harms C, Maul B, Bader M, Hörtnagl H, Schultheiss HP, Mies G. Ischemic injury in experimental stroke depends on angiotensin II. FASEB J. 2002;16:169–76. doi: 10.1096/fj.01-0601com. [DOI] [PubMed] [Google Scholar]
- 9.Rojas M, Zhang W, Lee DL, Romero MJ, Nguyen DT, Al-Shabrawey M, Tsai NT, Liou GI, Brands MW, Caldwell RW, Caldwell RB. Role of IL-6 in angiotensin II induced retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2010;51:1709–18. doi: 10.1167/iovs.09-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piqueras L, Kubes P, Alvarez A, O’Connor E, Issekutz AC, Esplugues JV, Sanz MJ. Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT(1) and AT(2) receptor-mediated P-selectinupregulation. Circulation. 2000;102:2118–23. doi: 10.1161/01.cir.102.17.2118. [DOI] [PubMed] [Google Scholar]
- 11.Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–7. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- 12.Kitiyakara C, Guzman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol. 1998;9:133–142. doi: 10.1681/ASN.V91133. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Takemori K, Kawai J, Suzuki T. AT1 receptor antagonist prevents brain edema without lowering blood pressure. Acta Neurochir Suppl. 2000;76:141–145. doi: 10.1007/978-3-7091-6346-7_29. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez A, Cerdá-Nicolás M, Naim Abu Nabah Y, Mata M, Issekutz AC, Panés J, Lobb RR, Sanz MJ. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood. 2004;104:402–8. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- 15.Petnehazy T, Stokes KY, Wood KC, Russell J, Granger DN. Role of blood cell-associated AT1 receptors in the microvascular responses to hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:313–8. doi: 10.1161/01.ATV.0000193625.32499.71. [DOI] [PubMed] [Google Scholar]
- 16.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–70. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori M, Salter JW, Vowinkel T, Krieglstein CF, Stokes KY, Granger DN. Molecular determinants of the prothrombogenic phenotype assumed by inflamed colonic venules. Am J Physiol Gastrointest Liver Physiol. 2005;288:G920–6. doi: 10.1152/ajpgi.00371.2004. [DOI] [PubMed] [Google Scholar]
- 18.Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U, Zahner G, Helmchen U, Stahl RA. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J Clin Invest. 1997;100:1047–58. doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Kim HS. Downregulation of angiotensin II-induced 12-lipoxygenase expression and cell proliferation in vascular smooth muscle cells from spontaneously hypertensive rats by CCL5. Korean J Physiol Pharmacol. 2009;13:385–92. doi: 10.4196/kjpp.2009.13.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papademetriou V. Inhibition of the renin-angiotensin-aldosterone system to prevent ischemic and atherothrombotic events. Am Heart J. 2009;157(6 Suppl):S24–30. doi: 10.1016/j.ahj.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Crowley SD, Gurley SB, Coffman TM. AT(1) receptors and control of blood pressure: the kidney and more. Trends Cardiovasc Med. 2007;17:30–4. doi: 10.1016/j.tcm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateo T, Abu Nabah YN, Abu Taha M, Mata M, Cerdá-Nicolás M, Proudfoot AE, Stahl RA, Issekutz AC, Cortijo J, Morcillo EJ, Jose PJ, Sanz MJ. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J Immunol. 2006;176:5577–86. doi: 10.4049/jimmunol.176.9.5577. [DOI] [PubMed] [Google Scholar]
- 24.Larsson PT, Schwieler JH, Wallén NH. Platelet activation during angiotensin II infusion in healthy volunteers. Blood Coagul Fibrinolysis. 2000;11:61–9. [PubMed] [Google Scholar]
- 25.Vilela MC, Mansur DS, Lacerda-Queiroz N, Rodrigues DH, Lima GK, Arantes RM, Kroon EG, da Silva Campos MA, Teixeira MM, Teixeira AL. The chemokine CCL5 is essential for leukocyte recruitment in a model of severe Herpes simplex encephalitis. Ann N Y Acad Sci. 2009;1153:256–63. doi: 10.1111/j.1749-6632.2008.03959.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Sugimura K, Hasegawa K, et al. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301–1306. doi: 10.1080/003655201317097164. [DOI] [PubMed] [Google Scholar]
- 27.Palmantier R, Borgeat P. Transcellular metabolism of arachidonic acid in platelets and polymorphonuclear leukocytes activated by physiological agonists: enhancement of leukotriene B4 synthesis. Adv Exp Med Biol. 1991;314:73–89. doi: 10.1007/978-1-4684-6024-7_4. [DOI] [PubMed] [Google Scholar]
- 28.Herd CM, Page CP. Pulmonary immune cells in health and disease: platelets. EurRespir J. 1994;7:1145–1160. [PubMed] [Google Scholar]
- 29.Yeo EL, Sheppard JA, Feuerstein IA. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model) Blood. 1994;83:2498–2507. [PubMed] [Google Scholar]
- 30.Lalor P, Nash GB. Adhesion of flowing leucocytes to immobilized platelets. Br J Haematol. 1995;89:725–732. doi: 10.1111/j.1365-2141.1995.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 31.Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J Clin Invest. 2008;118:173–182. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]