Abstract
Background and objective
The mechanism by which periodontal pathogens dominate at disease sites is not yet understood. One possibility is that these late colonizers antagonize quorum-sensing systems of early colonizers and render those early colonizers less resistant to environmental factors. In this study, we utilized Streptococcus mutans, a well-documented oral Streptococcus with many quorum-sensing dependent properties, as an example of targeted earlier colonizers that are antagonized by periodontal pathogens.
Material and Methods
S. mutans NG8, LT11, and BM71 were used in this study for assessment of transformation and bacteriocin production, respectively. The effects of Porphyromonas gingivalis and Treponema denticola on these competence-stimulating peptide (CSP)-dependent properties were evaluated in mixed broth assays.
Results
Both P. gingivalis (either live bacteria or membrane vesicles) and T. denticola antagonized transformation in S. mutans NG8 and LT11. S. mutans BM71 bacteriocin production was also inhibited by P. gingivalis and T. denticola. Boiling of these late colonizers before mixing the broth cultures abolished their ability to inhibit S. mutans transformation and bacteriocin production. P. gingivalis and T. denticola inactivated S. mutans exogenous CSP, whereas the boiled bacteria did not.
Conclusions
This study demonstrated that periodontal pathogens antagonized S. mutans quorum-sensing properties. This may render S. mutans less virulent and less resistant to environmental antibacterial factors.
Keywords: periodontal pathogen, Porphyromonas gingivalis, Treponema denticola, Streptococcus mutans, quorum sensing
Periodontal diseases are among the most common chronic infectious diseases occurring in humans. According to the National Institute of Dental and Craniofacial Research, periodontal diseases affect the majority of the American population (1). Oral chronic infectious diseases not only lead to tooth loss but may also act as a risk factor for systemic diseases such as cardiovascular diseases, respiratory diseases, and pre-term birth (2-4).
Quorum sensing involves the control of gene expression in response to cell density when a minimum population unit, or quorum, is reached (5). In streptococci such as Streptococcus mutans, one of the quorum sensing signaling systems depends on a competence-stimulating peptide (CSP, encoded by the comC gene) and a two-component signal transduction system which is encoded by the comD and comE genes, corresponding to a histidine protein kinase (receptor for CSP) and a response regulator, respectively (6-8). Quorum sensing systems modulate a variety of virulence activities in S. mutans. Disruption of the Com quorum sensing system in S. mutans results in attenuated virulence activities such as biofilm formation (9, 10), acid tolerance (11), bacteriocin production (12) and genetic competence (13, 14). In addition, Matsumoto-Nakano and Kuramitsu (15) have recently demonstrated that the comC gene of strain GS5 also modulates the sensitivity of S. mutans to a variety of antimicrobial agents such as triclosan, fluoride, antibiotics and histatin-5. Therefore, CSP represents a potential target for control of S. mutans infection.
Some bacteria such as S. mutans and Streptococcus gordonii are naturally transformable, being able to take up naked DNA from the extracellular environment (16). Homologous recombination of foreign DNA into the host chromosome following transformation is believed to play a major role in the evolution of bacteria. This was demonstrated by both the rapid emergence of penicillin resistance following the acquisition of low-affinity penicillin binding proteins (17) and evidence for the occurrence of frequent recombination events in the evolution of virulence factors in Streptococcus pneumoniae (18, 19). Experimental evidence also has shown that oral bacteria such as S. gordonii can take up free extracellular DNA from saliva in vitro (20). Furthermore, transformation of bacteria has been demonstrated after infection in animal hosts (21). Such events suggest that the expanded capacities conferred by acquired genes could result in more virulent bacterial species. Since bacteria can acquire resistance to compromising environments by taking up extracellular DNA, the ability to transfer genetic material among bacteria should be considered as a possible virulence attribute of bacteria involved in caries and periodontal disease.
The production of bacteriocins by microorganisms is also one of the important mechanisms used by bacteria for antagonistic interference (22). Although these peptide molecules are not required for growth, they may help the microorganisms that produce them to compete for the limited nutrients in their environment (23).
Some late colonizers of dental plaque such as Porphyromonas gingivalis and Treponema denticola have been implicated as pathogens causing chronic periodontitis (24, 25). Colonization by these pathogens can result in a proportional decrease in the population of early colonizers such as streptococci leading to the domination of sites of periodontal diseases by the former (24). How these periodontal pathogens dominate in dental plaque is not yet understood. One possibility is that these late colonizers antagonize the quorum sensing of the early colonizers and therefore render the early colonizers less virulent and less resistant to endogenous antimicrobial agents such as histatins, peroxide, and lysozyme. The objective of the present study was to determine if these late colonizers interfere with quorum sensing in S. mutans.
The Com quorum-sensing system has been identified in several early colonizers of dental plaque (8, 26, 27) and may be generalized targets for bacterial antagonism by late colonizers. In this study, we used S. mutans, a well-documented oral streptococcus with many CSP-dependent quorum-sensing properties, as an example of an earlier colonizer which is antagonized by periodontal pathogens such as P. gingivalis and T. denticola.
Materials and methods
Bacterial strains and media
S. mutans BM71, S. mutans NG8, and their com mutants were maintained on Tryptic Soy Agar (TSA) plates supplemented with erythromycin (10 μg/mL) where indicated. Bacteria were routinely cultured in Todd Hewitt broth (THB). A group C streptococcal strain RP66 was used as an indicator for assays of S. mutans bacteriocin activity (28). P. gingivalis 381 was grown anaerobically in enriched tryptic soy broth (TSB) medium (containing, per liter, 40 g of TSB, 5 g of yeast extract, 0.5 g of cysteine, 10 mg of hemin, and 1 mg of vitamin K1) and maintained on TSA blood agar plates (containing, per liter, TSB plus 15 g of agar and 50 mL of sheep blood). T. denticola 35405 was routinely maintained in tryptone yeast extract-gelatin-volatile fatty acids-serum (TYGVS) medium under anaerobic conditions. Bacteria used in this study are listed in Table 1.
Table 1.
Bacteria used in this study
| S. mutans NG8 | WT, for genetic transformation assays |
| S. mutans LT11 | WT, for genetic transformation assays |
| S. mutans BM71 | WT, for bacteriocin assays |
| S. mutans comC mutants | No CSP production due to inactivation of the comC gene |
| RP66 | Group C Streptococcus, an indicator strain that is sensitive to S. mutans bacteriocin |
| P. gingivalis 381 | WT, late colonizer |
| T. denticola 35405 | WT, late colonizer |
Natural genetic transformation
Recipient S. mutans NG8 or LT11 in the stationary phase were diluted twenty-fold in THB with 10% horse serum (THB-HS) and cultured at 37°C for 30 minutes to induce competence. For the transformation of mixtures of S. mutans with other periodontal pathogens, bacteria at the stationary phase were diluted twenty-fold in THB-HS and mixed in 1:1 ratio for competence induction. For some experiments, the periodontal pathogens were boiled for 10 minutes before mixed culturing. Competent S. mutans recipient cells (100 μL) were exposed to exogenous donor plasmid or chromosomal DNA [1 μg of pTet (29) or 10 μg of DNAgtfD (30)] for 2 hours. Transformants were then sonified and selected on TSA agar plates supplemented with tetracycline (10 μg/mL). Transformation frequencies were determined after 48-72 hours of anaerobic incubation at 37°C.
Isolation of membrane vesicles from P. gingivalis 381
Membrane vesicles (MVs) from a culture of P. gingivalis 381 in early stationary growth phase in TSB were isolated from the supernatants of the cultures by filtrating through 0.22-μm-pore-size filters. Vesicles were recovered from the resulting filtrates by ultra-centrifugation at 150,000 X g for 2 hours at 4°C with a Ti 75 rotor (Beckman Instruments, Inc., Fullerton, CA). Isolated MVs were resuspended in PBS (100-fold concentrated from the original cultures) and stored at -70°C for further transformation experiments. For transformation experiments, MVs (40 μL) were added to 100 μL twenty-fold diluted S. mutans NG8 for competence induction.
Bacteriocin production and assays
S. mutans BM71 cells (107 cfu) or mixtures with P. gingivalis 381 or T. denticola 35405 (107 or 5 × 107 cfu) were cultured in 1.0 mL of THB supplemented with 3% yeast extract (THBY) at 37°C for 24 hours. For some experiments, the periodontal pathogens were boiled for 10 minutes before mixed culturing. Supernatants containing bacteriocin from the cultures were neutralized to pH 7.0 with NaOH, filtered through 0.22 μm pore size filters, and either assayed immediately or frozen at -20°C for subsequent assays. RP66 cells (2 × 105 cfu) in 0.7 mL THB were grown in the presence of the above-mentioned supernatant fluids (300 μL) and incubated at 37°C for 5-6 hours. OD600nm measured thereafter indicated the presence or the absence of bacteriocin.
Effects of periodontal pathogens on the CSP of S. mutans
Supernatants from P. gingivalis 381 or T. denticola 35405 grown to the stationary phase in TSB or TYGVS respectively were filtered through 0.22 μm filters to eliminate cellular components. The supernatants were then neutralized with NaOH to pH 7.0-7.5. Exogenous synthetic S. mutans CSP [amino acid sequence: SGSLSTFFRLFNRSFTQALGK (13), synthesized by Sigma-Genosys, The Woodlands, TX, 2.5 μg/mL final concentration] was incubated with the supernatants at 37°C for 2 hours. CSP thus treated was added to S. mutans BM71 comC mutant cells (107 cfu) in 0.9 mL of THBY to a final concentration of 0.25 μg/mL. After 24-hour incubation at 37°C, the supernatants from the cultures were passed through 0.22 μm filters and the bacteriocin level produced by the S. mutans BM71 comC mutant was determined by the agar well assay.
Agar well assays
The supernatant fluids containing bacteriocin from the S. mutans BM71 comC mutant cultures were obtained as described above. The supernatants were added into pre-cut wells in THB agar plates and incubated at 37°C for 24 hours to facilitate the absorption of the supernatants into the agar surrounding the wells. The wells were then filled using THB with 1% low melting temperature agarose and the plates were overlaid with the indicator strain RP66 (106 cfu) in 3 mL THB with 1% low melting temperature agarose. After 24 hours of further incubation at 37°C under anaerobic conditions, the diameters of the inhibition zones surrounding the wells were measured.
Statistical analysis
Student's t-Test was performed to determine significance. A difference was considered significant when a p value < 0.05 was obtained.
Results
Attenuation of S. mutans natural transformation by P. gingivalis
Experiments were performed to determine the efficiencies of S. mutans natural transformation. S. mutans natural transformation is mediated by CSP and the comC knockout mutant exhibited minimum transformation efficiency, relative to its parent strain (Table 2). A late colonizer, P. gingivalis, either live bacteria or MVs, significantly antagonized natural transformation in S. mutans NG8.
Table 2.
Competent S. mutans NG8 and mixtures with P. gingivalis or MVs from P. gingivalis were transformed with exogenous chromosomal DNA gtfD and the transformation frequencies are indicated as numbers of tetracycline-resistant colonies/100 μL. Assessments were performed in triplicate in two independent experiments. Data presented are the means ± standard deviation (n=6).
| Species | Transformation Efficiency (colonies/100 μL) |
|---|---|
| S.m. NG8 | 437.5 ± 53.9 |
| S.m. NG8 + P.g. 381 | 11.6 ± 4.7 |
| S.m. NG8 + P.g. 381 MVs | 14.6 ± 3.8 |
| S.m. NG8 comC- | 0.3 ± 0.5 |
Boiling of periodontal pathogens abolished their antagonism for S. mutans natural transformation
We then compared the ability to antagonize S. mutans natural transformation in different periodontal pathogens. As shown in Table 3, the transformation efficiency of S. mutans LT11, like S. mutans NG8, was significantly antagonized by P. gingivalis. Another periodontal pathogen, T. denticola 35405, also significantly antagonized the natural transformation of S. mutans strain LT11. Boiling of periodontal pathogens before mixed incubation with S. mutans abolished their antagonism for S. mutans natural transformation.
Table 3.
Competent S. mutans LT11 and mixtures with P. gingivalis 381 or T. denticola 35405 were transformed with pTet, which transforms only S. mutans. P. gingivalis and T. denticola cells were boiled for 10 minutes before mixed culturing in the experimental groups 3 and 5. The transformation frequencies are indicated as numbers of tetracycline resistant colonies/100 μL. Assessments were performed in triplicate in two independent experiments. Data presented are the means ± standard deviation (n=6).
| Species | Transformation Efficiency (colonies/100 μL) |
|---|---|
| S.m. LT11 | 691.8 ± 67.0 |
| S.m. LT11 + P.g. 381 | 0.5 ± 0.8 |
| S.m. LT11 + Boiled P.g. 381 | 682.0 ± 90.9 |
| S.m. LT11 + T.d. 35405 | 13.5 ± 3.5 |
| S.m. LT11 + Boiled T.d. 35405 | 703.8 ± 62.9 |
Periodontal pathogens abolished S. mutans bacteriocin
To investigate interactions among oral bacteria relative to another CSP-dependent property in S. mutans, we performed broth assays to determine bacteriocin production by S. mutans BM71. Strain BM71 was chosen for the study, since it produces bacteriocin that kills an indicator strain, RP66, whereas NG8 and LT11 do not. Both periodontal pathogens, P. gingivalis 381 and T. denticola 35405, completely abolished bacteriocin production by S. mutans BM71 in the mixed cultures, which was demonstrated by no inhibition of RP66 growth (Table 4).
Table 4.
Bacteria were cultured in THB broth at 37°C for 24 hours. Supernatants containing bacteriocin from the cultures were neutralized to pH 7.0, filtered through 0.22 μm pore size filters. RP66 cells were grown in the presence of the supernatants and incubated at 37°C for 5-6 hours. Assessments were performed in triplicate in two independent experiments. Data presented are the means ± standard deviation (n=6).
| Supernatant Sources | RP66 Growth (OD600) |
|---|---|
| S.m. BM71 | 0.020 ± 0.009 |
| S.m. BM71 + P.g. 381 | 0.601 ± 0.071 |
| S.m. BM71 + Boiled P.g. 381 | 0.019 ± 0.011 |
| S.m. BM71 + T.d. 35405 | 0.598 ± 0.062 |
| S.m. BM71 + Boiled T.d. 35405 | 0.020 ± 0.009 |
| None | 0.586 ± 0.046 |
We did titration to determine the amount of bacteriocin from S. mutans BM71 monocultures for the complete inhibition of RP66 growth. <50 μL bacteriocin supernatants did not consistently inhibit RP66 growth, while >100 μL volumes did. We therefore utilized 300 μL bacteriocin supernatants in the bacteriocin assays, which ensured that any growth of RP66 is due to the decrease in bacteriocin, not from experimental error. These volumes of bacteriocin supernatants (300 μL) completely inhibited the growth of RP66 and did not change over time such as after overnight incubation. However, the growth of RP66 without bacteriocin (either with or without the supernatants from the mixed cultures of periodontal pathogens) did increase over time.
Periodontal pathogens inactivated exogenous S. mutans CSP
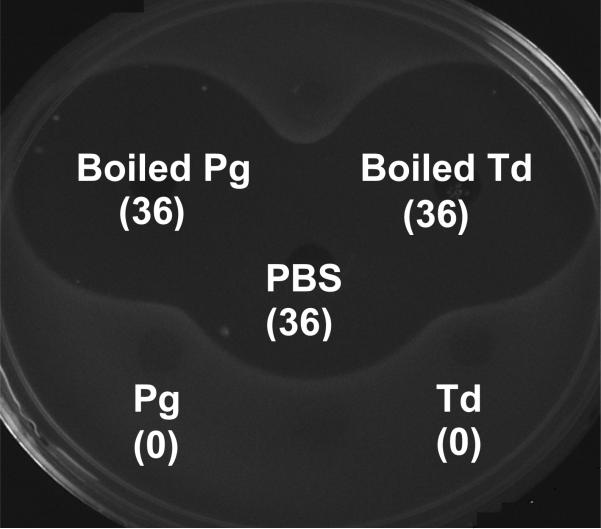
We have demonstrated previously that S. gordonii inactivated S. mutans CSP, which in turn interfered with bacteriocin production, a Com quorum-sensing-dependent phenomenon. Since P. gingivalis 381 and T. denticola 35405 interfered with two of the CSP-dependent properties in S. mutans and boiling of these two periodontal pathogens abolished the interference (Tables 2, 3, and 4), we carried out experiments to determine the ability of P. gingivalis 381 and T. denticola 35405 to inactivate S. mutans CSP. As shown in Fig. 1, the supernatant fluids from both P. gingivalis 381 and T. denticola 35405 inactivated S. mutans CSP. Boiling of the supernatants before S. mutans CSP inactivation abolished such inhibition.
Figure 1.
The supernatants from broth cultures of P. gingivalis 381 or T. denticola 35405 (with or without boiling) were incubated with exogenous S. mutans CSP for 2 h and added to the comC mutant cells of S. mutans BM71. The bacteriocin activity was assayed by inoculating the supernatants into pre-cut wells in THB plates. After absorption of the supernatants, the plates were overlaid with RP66 indicator cells and incubated at 37°C overnight. The presence of bacteriocin in the culture supernatants of the comC mutant would inhibit RP66 growth, which indicated the presence of functional exogenous CSP. The diameters of the inhibition zones surrounding the wells (mm) were shown in brackets.
We carried out additional experiments to exclude the possibility that the supernatant fluids from P. gingivalis 381 and T. denticola 35405 inactivated the bacteriocin produced by the S. mutans, instead of inactivating S. mutans CSP per se. The supernatant fluids from P. gingivalis 381 or T. denticola 35405 were mixed with existing S. mutans bacteriocin (produced by the S. mutans comC mutant in the presence of CSP) at the same ratio (1:10 in volume) as in the above-mentioned well assays for the CSP activity assessment. After 2 h incubation at 37°C, S. mutans bacteriocin in the mixtures was still functional and inhibited RP66 growth (data not shown).
Discussion
Periodontal pathogens such as P. gingivalis and T. denticola can dominate at the sites of periodontal diseases. It is not yet understood how these late colonizers antagonize the earlier dental plaque colonizers. The comCDE operon has been demonstrated in multiple oral streptococci such as S. mutans, S. gordonii, Streptococcus mitis, Streptococcus oralis, and Streptococcus sanguinis (26, 27) and may be generalized targets for bacterial antagonism. We chose S. mutans as an example of oral streptococci in the present study, since it has several CSP-dependent virulence properties. However, other oral streptococci do not have demonstrable CSP-dependent properties in vitro, despite the presence of CSP-dependent quorum sensing pathways. We realize that S. mutans is not commonly found in subgingival sites and therefore our findings may or may not be applicable to other oral streptococcal species mentioned above. However, our preliminary data has demonstrated that the Com-dependent natural transformation in S. gordonii was also abolished by P. gingivalis in mixed broth assays (data not shown). This observation suggests that our findings may be relevant for other oral streptococci as well.
We have previously reported that proteases produced by other species of oral streptococci such as S. gordonii interfere with the quorum sensing properties of S. mutans (12). It has been well documented that some of the periodontal pathogens produce proteases. Using BANA assays, we confirmed the presence of proteases in P. gingivalis 381 and T. denticola 35405 (data not shown). Our studies were designed to determine if periodontal pathogens such as P. gingivalis or T. denticola interrupt quorum sensing in S. mutans and if multiple CSP-dependent quorum-sensing properties in S. mutans are antagonized by these late colonizers as did at least one early colonizer.
Our results indicated that both P. gingivalis and T. denticola can attenuate some of the virulence properties of S. mutans by altering the quorum-sensing-dependent properties of the organism. Tables 2, 3, and 4 demonstrated that both P. gingivalis and T. denticola antagonized two quorum-sensing dependent properties in S. mutans. In transformation assays, strains NG8 and LT11 were used for transformation efficiency assessment since S. mutans NG8 and LT11 exhibit high CSP-dependent transformation efficiencies. However, S. mutans NG8 or LT11 do not produce bacteriocin that kills the indicator strain, RP66, while S. mutans BM71 does.
The THB medium and static aerobic culturing conditions utilized in our experiments do not support the growth of periodontal pathogens. In addition, the levels of periodontal pathogens in the mixed cultures are relatively low (107 or 5 × 107 cfu). We have also carried out experiments to determine S. mutans viability in monocultures and its mixtures with P. gingivalis under the conditions used for both natural transformation and bacteriocin production. There was no decrease in viability of S. mutans in the mixed cultures relative to its monocultures (data not shown).
Our results indicated that antagonism of S. mutans quorum sensing is not restricted to a particular species of oral bacteria and periodontal pathogens affected multiple quorum-sensing properties in several S. mutans strains. Since it is well-documented that both P. gingivalis and T. denticola produce multiple proteases, our results indicated that heat-sensitive proteins, speculated to be proteases, from periodontal pathogens are responsible for inactivation of S. mutans CSP.
It has been reported that a large number of Gram-negative bacteria form and release MVs during growth (31). Because of their small dimensions (approximately 50-150 nm), MVs might more easily reach inaccessible areas such as the interior of biofilms, relative to their whole bacteria counterparts. Furthermore, MVs could have bacteriolytic effects on both Gram-positive and Gram-negative bacteria (32). Since P. gingivalis generates MVs, we compared live bacteria and MVs of P. gingivalis for their ability to antagonize S. mutans natural transformation. As shown in Table 2, P. gingivalis MVs exhibited a similar capacity to antagonize S. mutans transformation, relative to live bacteria.
Taken together, our results suggest that the presence of periodontal pathogens in dental plaque could modulate the virulence properties of S. mutans by interfering with its Com quorum sensing system. Since the Com quorum sensing system exists in many species of earlier dental plaque colonizers, this interference of quorum sensing by periodontal pathogens such as P. gingivalis and T. denticola could, at least in part, be a mechanism of bacterial antagonism in periodontal diseases.
Acknowledgements
We thank M. M. Vickerman for helpful discussions. These studies were supported in part by grant DE017708 from the National Institute of Dental and Craniofacial Research.
References
- 1.NIDCR, CDC Oral Health US, 2002. NIDCR/CDC Dental, Oral and Craniofacial Data Resource Center. 2002 [Google Scholar]
- 2.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, Moss K, Elter J, Offenbacher S. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005;112:19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 3.Scannapieco FA. Periodontal inflammation: from gingivitis to systemic disease? Compend Contin Educ Dent. 2004;25:16–25. [PubMed] [Google Scholar]
- 4.Offenbacher S. Maternal periodontal infections, prematurity, and growth restriction. Clin Obstet Gynecol. 2004;47:808–821. doi: 10.1097/01.grf.0000141894.85221.f7. discussion 881-882. [DOI] [PubMed] [Google Scholar]
- 5.Withers H, Swift S, Williams P. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr Opin Microbiol. 2001;4:186–193. doi: 10.1016/s1369-5274(00)00187-9. [DOI] [PubMed] [Google Scholar]
- 6.Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM. Quorum sensing by peptide pheromones and two-component signal- transduction systems in Gram-positive bacteria. Mol Microbiol. 1997;24:895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 7.Morrison DA. Streptococcal competence for genetic transformation: regulation by peptide pheromones. Microb Drug Resist. 1997;3:27–37. doi: 10.1089/mdr.1997.3.27. [DOI] [PubMed] [Google Scholar]
- 8.Cvitkovitch DG, Li YH, Ellen RP. Quorum sensing and biofilm formation in Streptococcal infections. J Clin Invest. 2003;112:1626–1632. doi: 10.1172/JCI20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida A, Kuramitsu HK. Multiple Streptococcus mutans Genes Are Involved in Biofilm Formation. Appl Environ Microbiol. 2002;68:6283–6291. doi: 10.1128/AEM.68.12.6283-6291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YH, Tang N, Aspiras MB, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J Bacteriol. 2002;184:2699–2708. doi: 10.1128/JB.184.10.2699-2708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YH, Lau PC, Tang N, Svensater G, Ellen RP, Cvitkovitch DG. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J Bacteriol. 2002;184:6333–6342. doi: 10.1128/JB.184.22.6333-6342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang BY, Kuramitsu HK. Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii. Appl Environ Microbiol. 2005;71:354–362. doi: 10.1128/AEM.71.1.354-362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuramitsu HK, Wang BY. Virulence properties of cariogenic bacteria. BMC Oral Health. 2006;6(Suppl 1):S11. doi: 10.1186/1472-6831-6-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto-Nakano M, Kuramitsu HK. Role of bacteriocin immunity proteins in the antimicrobial sensitivity of Streptococcus mutans. J Bacteriol. 2006;188:8095–8102. doi: 10.1128/JB.00908-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz MG, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowson CG, Coffey TJ, Spratt BG. Origin and molecular epidemiology of penicillin-binding-protein- mediated resistance to beta-lactam antibiotics. Trends Microbiol. 1994;2:361–366. doi: 10.1016/0966-842x(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen K, Reinholdt J, Jespersgaard C, Boye K, Brown TA, Hauge M, Kilian M. A comprehensive genetic study of streptococcal immunoglobulin A1 proteases: evidence for recombination within and between species. Infect Immun. 1998;66:181–190. doi: 10.1128/iai.66.1.181-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowson CG, Barcus V, King S, Pickerill P, Whatmore A, Yeo M. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. J Appl Microbiol. 1997;83:42S–51-S. doi: 10.1046/j.1365-2672.83.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 20.Mercer DK, Scott KP, Bruce-Johnson WA, Glover LA, Flint HJ. Fate of free DNA and transformation of the oral bacterium Streptococcus gordonii DL1 by plasmid DNA in human saliva. Appl Environ Microbiol. 1999;65:6–10. doi: 10.1128/aem.65.1.6-10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottolenghi E, Macleod CM. Genetic transformation among living pneumococci in the mouse. Proc Natl Acad Sci U S A. 1963;50:417–419. doi: 10.1073/pnas.50.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brook I. Bacterial interference. Crit Rev Microbiol. 1999;25:155–172. doi: 10.1080/10408419991299211. [DOI] [PubMed] [Google Scholar]
- 23.Vining LC. Functions of secondary metabolites. Annu Rev Microbiol. 1990;44:395–427. doi: 10.1146/annurev.mi.44.100190.002143. [DOI] [PubMed] [Google Scholar]
- 24.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 25.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 26.Havarstein LS, Hakenbeck R, Gaustad P. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J Bacteriol. 1997;179:6589–6594. doi: 10.1128/jb.179.21.6589-6594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whatmore AM, Barcus VA, Dowson CG. Genetic diversity of the streptococcal competence (com) gene locus. J Bacteriol. 1999;181:3144–3154. doi: 10.1128/jb.181.10.3144-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul D, Slade HD. Production and properties of an extracellular bacteriocin from Streptococcus mutans bacteriocidal for group A and other streptococci. Infect Immun. 1975;12:1375–1385. doi: 10.1128/iai.12.6.1375-1385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie H, Lin X, Wang BY, Wu J, Lamont RJ. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology. 2007;153:3228–3234. doi: 10.1099/mic.0.2007/009050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda O, Kato C, Kuramitsu HK. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990;136(Pt 10):2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- 31.Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. Interactions between biofilms and the environment. FEMS Microbiol Rev. 1997;20:291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 32.Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]